Soil water repellency and its implications for organic matter decomposition – is there a link to extreme climatic events?
Abstract
Earth system models associate the ongoing global warming with increasing frequency and intensity of extreme events such as droughts and heat waves. The carbon balance of soils may be more sensitive to the impact of such extremes than to homogeneously distributed changes in soil temperature (Ts) or soil water content (θs). One parameter influenced by more pronounced drying/rewetting cycles or increases in Ts is the wettability of soils. Results from laboratory and field studies showed that low θs, particularly in combination with high Ts can increase soil water repellency (SWR). Recent studies have provided evidence that the stability of soil organic matter (SOM) against microbial decomposition is substantially enhanced in water repellent soils. This review hypothesizes that SWR is an important SOM stabilization mechanism that could become more important because of the increase in extreme events. We discuss wettability-induced changes in soil moisture distribution and in soil aggregate turnover as the main mechanisms explaining the reduced mineralization of SOM with increasing SWR. The creation of preferential flow paths and subsequent uneven penetration of rainwater may cause a long-term reduction of soil water availability, affecting both microorganisms and plants. We conclude that climate change-induced SWR may intensify the effects of climatic drought and thus affects ecosystem processes such as SOM decomposition and plant productivity, as well as changes in vegetation and microbial community structure. Future research on biosphere–climate interactions should consider the effects of increasing SWR on soil moisture and subsequently on both microbial activity and plant productivity, which ultimately determine the overall carbon balance.
Introduction
Combustion of fossil fuels and changes in land use are strongly affecting the global carbon cycle (Le Quéréet al., 2009) and thus global climate. Global temperature has already increased by 0.6 °C since the late 19th century (Meehl et al., 2007). Depending on the climate model and scenario used, mean global temperature is projected to increase by another 2.4–5.6 °C by the end of the 21st century. In addition, changing climate is associated with increasing temporal variability and occurrence of extremes (Meehl & Tebaldi, 2004; Heimann & Reichstein, 2008). Longer drought periods and more extremely dry years (Meehl et al., 2007) as well as heat waves (Ganguly et al., 2009; Fischer & Schär, 2010) are predicted to become more frequent in the future. The change in global climate may lead to a global shift of vegetation and ecological zones (Peñuelas & Boada, 2003; Mueller et al., 2005) and is expected to have various adverse direct and indirect impacts on human health (Haines et al., 2006; Rabie et al., 2008; Kurane, 2009). Improving the capacity of soil to act as carbon sink is one option to mitigate global climate change due to elevated atmospheric CO2 (Lal et al., 2007; Lal, 2008).
Soil organic matter (SOM) as a major component of the world's surface carbon reserves plays an important role in the global carbon cycle (Gruber et al., 2004). Soil respiration accounts for approximately two-thirds of total carbon loss from terrestrial ecosystems (Luo & Zhou, 2006) and is generally sensitive to changes in temperature and precipitation (Davidson & Janssens, 2006). Besides soil temperature (Ts) and soil water content (θs), aerobic heterotrophic decomposition of SOM is controlled by the availability of nutrients (Hadas et al., 1998) and oxygen (Paul, 2007), as well as by SOM stabilization processes (von Lützow et al., 2006). SOM stability is determined by (i) its chemical composition, (ii) interactions with particle surfaces and metal ions and (iii) its spatial accessibility (Sollins et al., 1996). The latter depends on soil physical properties like particle and pore size distribution, pore continuity and structure.
In addition to the architecture of soils, determined by texture and aggregation (Young et al., 1998; Six et al., 2000, 2002; Strong et al., 2004), the spatial accessibility and degradability of SOM also depends on the availability and spatial distribution of water in the soil matrix (Young & Ritz, 2000). Water governs the advective and diffusive transport of nutrients, substrates and enzymes, microbial motility, the aeration status of the soil (Or et al., 2007), soil thermal diffusivity (Ghuman & Lal, 1985), and is crucial for all microbial uptake mechanisms (Marschner & Kalbitz, 2003). Bachmann et al. (2008) showed that the availability and distribution of water in the soil matrix depends on soil particle wettability. In strongly water repellent soils water tends to form droplets rather than continuous water films on the particle surfaces (Goebel et al., 2007). Even if water films are present, their thickness can be reduced significantly with increasing soil water repellency (SWR) (Churaev, 2000). Since microbes (Wong & Griffin, 1976), solutes and enzymes have to move through this water film, they are highly sensitive to changes in water film thickness (Derjaguin & Churaev, 1986).
An additional mechanism in water repellent soils is that the reduced infiltration capacity favors the protection of aggregates (Hallett & Young, 1999), thus enhancing the stability of occluded SOM (Tisdall, 1996). On the other hand, reduced infiltration capacity may also enhance surface runoff and soil erosion (Doerr et al., 2000), which can increase the mineralization of formerly protected SOM (Lal, 2003).
SWR was shown to be affected by θs and Ts (Doerr et al., 2000). In general, the longer a soil is dry and the higher is Ts, the larger its wetting resistance will be. The dependence of SWR on θs and Ts makes it susceptible to changing climatic conditions. Thus, it is likely that increasing mean global temperatures and a more frequent occurrence of droughts and heat waves will have an effect on the extent and time-dependent dynamics of SWR.
So far, there has been limited discussion on the role of SWR as an important climate change-sensitive soil property that affects the carbon sink strength of soils. The aim of this paper is to analyze the potential relationship between changing climate and the dynamics of SWR by considering the recently published literature. Specific objectives are (i) to give a short overview of the origin and significance of SWR phenomena, (ii) to discuss the impact of SWR on important mechanisms relevant for carbon mineralization, (iii) to assess the potential impact of extreme climatic events on the development and dynamics of SWR, and finally, (iv) to combine these insights and discuss the potential consequences of climate change-induced repellency changes for SOM decomposition and soil carbon sequestration.
SWR – its origin and significance
If a solid is not completely wettable by water, it is considered to be water repellent. Water beads up when it makes contact with the surface of a water repellent solid and a solid–water contact angle (αsw) can be measured at the three-phase (i.e. gas–liquid–solid) boundary line (Bachmann et al., 2003). Here, we define SWR to occur if αsw>0°. Soils with 0°<αsw<90° show reduced wettability, that is, infiltration of water into the soil matrix is decreased. Values of αsw>90° indicate extreme SWR, which is also termed soil hydrophobicity (Fig. 1).
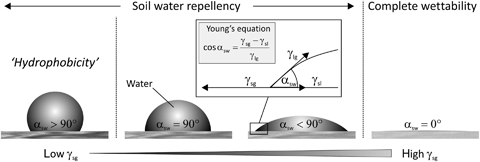
Schematic showing the solid–water contact angle (αsw) as a measure of soil water repellency. The contact angle is related to the interfacial free energies (γ) of the three-phase (gas–liquid–solid) system by Young's equation (Young, 1805), where the indices g, l and s denote the gas, liquid and solid phase, respectively. A value of αsw=0° indicates complete wettability of a surface, values of αsw>0° indicate soil water repellency, which is also termed hydrophobicity for αsw>90°.
The existence of water repellent soils has been known for many years and there are indications that under certain conditions most soils show SWR to some degree. At first, SWR was reported mostly from semiarid regions (see Doerr et al., 2000), but in the last two decades it has also been observed in humid regions (e.g. Jaramillo et al., 2000; Johnson et al., 2005; Doerr et al., 2006). This suggests that at least low levels of repellency are more the rule than the exception (de Jonge et al., 2009). For instance, about 75% of grassland and arable soils in the Netherlands (Dekker & Ritsema, 1994) and about 2 million ha of sandy soil in southern and western Australia (Franco et al., 2000) are affected by SWR. The phenomenon was primarily ascribed to sandy soils, but it has also been observed in loam, clay, peat and volcanic ash soils (Wallis & Horne, 1992; Ellies & Hartge, 1994; Ritsema et al., 1997; Jaramillo et al., 2000; Mataix-Solera & Doerr, 2004).
SWR is caused by low solid surface free energy (γsg) resulting in a weak attraction between particles and the liquid phase (Roy & McGill, 2002). Generally, pure soil minerals have high-energy surfaces (Lewin et al., 2005), but under natural conditions they are often covered by organic substances with low surface free energy (Doerr et al., 2000). This may result in a large number of nonpolar sites on the particle surfaces (Tschapek, 1984; Drehlich, 1997). Several studies showed that SWR is positively correlated with the soil organic carbon (SOC) content (e.g. Mataix-Solera & Doerr, 2004; Moral Garcia et al., 2005; Varela et al., 2005). However, others have found a more complex relationship (Ellerbrock et al., 2005; Goebel et al., 2008), no general relationship (Rumpel et al., 2004; Woche et al., 2005; Doerr et al., 2006), or even an inverse relationship (Eynard et al., 2006) between SWR and SOC. This indicates that the composition of organic matter and its overall effectiveness to influence the wetting properties of soil particles can be more important than the total amount of SOC (Ellerbrock et al., 2005). The amount of hydrophobic C–H groups relative to that of hydrophilic C=O, C–OH and C–NH2 groups at the solid–liquid interface of a few Ångström thickness determines the wettability of SOM (Ferguson & Whitesides, 1992).
Another important parameter affecting SWR is soil pH (Wallis & Horne, 1992). For example, Woche et al. (2005) found a slight tendency of increasing SWR with decreasing pH for a series of arable and forest soils. In line with this, de Jonge et al. (1999, 2007) and Hurraß & Schaumann (2006) reported a negative correlation between pH and SWR at least for some of their investigated soils. This was explained by a decreasing polarity of SOM functional groups due to protonation at low pH, as was shown, for instance, by Terashima et al. (2004) for amphiphilic SOM molecules. However, by investigating the reaction of SWR to artificially induced changes in pH, Diehl et al. (2010) recently found that maximum SWR occurred for soil at natural pH when the density of charged sites was minimal and may decrease with both increasing and decreasing pH along with the development of (negative or positive) surface charge.
The organic compounds suggested to cause SWR comprise waxes (Franco et al., 2000), alkanes (Ma'shum et al., 1988; Horne & McIntosh, 2000), as well as fatty acids (Mainwaring et al., 2004; Morley et al., 2005; Graber et al., 2009). These compounds may originate from fungal hyphae (McGhie & Posner, 1980; Sun et al., 1999; Feeney et al., 2006), microbial biomass (Bond & Harris, 1964; Chan, 1992) or decomposed plant materials (McGhie & Posner, 1981). Also evergreen trees (e.g. Eucalyptus spp., Pinus spp.) (de Blas et al., 2010) with a considerable amount of resins, waxes or essential oils, but also fungi (York & Canaway, 2000; Zhang et al., 2007) and certain shrubs and grass species were shown to be associated with the occurrence of SWR (Doerr et al., 2000). SWR can also be caused by the presence of hydrophobic interstitial particulate organic matter (Franco et al., 1995), but to a lesser extent as compared with repellency due to organic coatings (Bisdom et al., 1993). In a recent study, de Blas et al. (2010) found evidence that SWR is mainly governed by the concentration of free lipids, which are considered to be incorporated in hydrophobic coatings. In addition, the proportions and composition of humic substances and the free particulate SOM were found to be relevant to explain the extent of SWR, whereas their relative importance differed between vegetation and, to a lesser extent, the geologic substrate (de Blas et al., 2010).
Impact of SWR on processes relevant for SOM decomposition
SWR affects hydrological processes and potentially all processes where water is involved (de Jonge et al., 2009). Three processes, which are relevant at different spatial scales can be identified as being potentially important for microbial decomposition of SOM: (i) water infiltration into the soil, (ii) water distribution within the soil matrix and (iii) microscopic arrangement of water on particle surfaces. In general, SWR reduces the water infiltration capacity (Doerr et al., 2000). This can reduce the water availability for microorganisms and promotes the stability of soil aggregates against water slaking (Zhang et al., 2007), which in turn can reduce the accessibility of aggregate-occluded SOM. On the other hand, the low infiltration capacity of water repellent soils can enhance runoff flow and erosional processes (Shakesby et al., 2000), which may result in the loss of large amounts of SOM (Lal, 2003). Moreover, SWR affects the distribution and continuity of the liquid phase in the soil matrix (Goebel et al., 2007), which can be crucial for the accessibility of SOM and the availability of water, oxygen and nutrients. The relevance of these SWR-mediated effects on carbon mineralization is illustrated in Fig. 2 and will be discussed in the following sections.
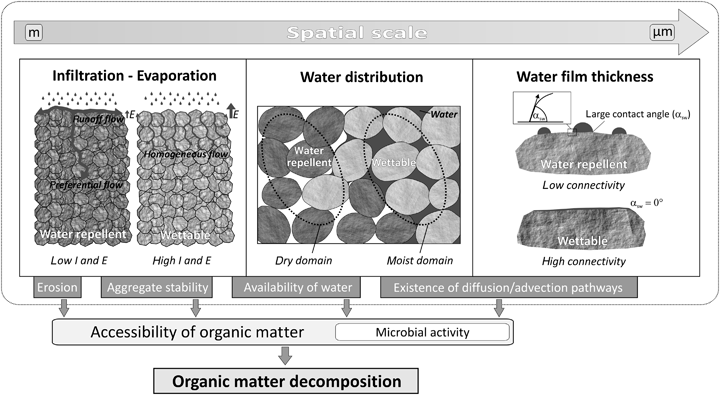
Schematic showing the impact of soil water repellency on processes relevant for organic matter decomposition. It illustrates how water repellency affects infiltration and evaporation as well as the distribution and connectivity of the water phase in the soil, which in turn influence the accessibility of organic matter as well as the conditions for microbial activity as the most important factors controlling the decomposition of soil organic matter.
Wettability-induced changes in water distribution and availability – an important factor in controlling microbial decomposition processes
The activity of all microorganisms in soil is governed by the availability of water and was found to be linearly related to θs (Orchard & Cook, 1983). Hence, any change in water availability will have fundamental consequences for biological activity (Feeney et al., 2006). Diffusion rates of extracellular enzymes to SOM and of dissolved substrates back to the microbial cells are proportional to the thickness of the water film surrounding soil particles (Or et al., 2007). Increasing SWR has been found to reduce water film thickness on particle surfaces (Churaev, 2000). For various mineral–water and glass–water systems, Derjaguin & Churaev (1986) reported a considerable decrease of water film thickness with increasing solid–water contact angle (αsw). Measured isotherms of water films on quartz surfaces indicated that at 98.5% relative humidity water film thickness on slightly water repellent quartz particles (αsw=10°) is reduced by a factor of 5 as compared with completely wettable quartz particles (αsw=0°) (Churaev, 2000). This suggests that already small differences in wettability may have a strong impact on water film thickness and thus on microbial decomposition of SOM.
The impact of wettability on microscopic water distribution and connectivity of the liquid phase is illustrated in Fig. 3 showing environmental scanning electron microscopy (ESEM) images of water condensed on hydrophobic (αsw=158°) and slightly water repellent glass beads (αsw=48°). On the hydrophobic particle surfaces, the water condensed as small drops. Consequently, there was a reduced water connectivity between individual particles confined by the diameter of water drops formed between them. Contrastingly, the slightly water repellent surfaces were uniformly wetted which led to a higher water connectivity between the particles. The liquid menisci formed between the slightly water repellent particles had a larger cross-sectional area as compared with the water drops formed between the hydrophobic particles. This illustrates that for strongly water repellent material the water connectivity on individual particles as well as between the particles can be markedly reduced, which can have important consequences for diffusion processes (Or et al., 2007; Young et al., 2008).
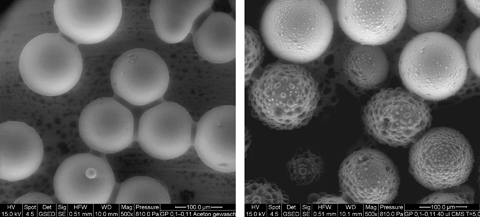
Environmental scanning electron micrographs (Quanta 200, FEI Company, Eindhoven, the Netherlands) showing water condensed on slightly water repellent glass beads (αsw=48°) washed with acetone (left) and hydrophobic glass beads (αsw=158°) treated with 40 μL dichlorodimethylsilane per 100 g (right). The glass beads (B. Braun Biotech International GmbH, Melsungen, Germany) have a mean diameter of 105 μm.
It was shown by Poll et al. (2008) that the extent and intensity of the active zone of microbial decomposition was directly related to diffusive and advective solute transport, with slow solute transport at low θs. Generally, bacterial utilization of labile carbon compounds seems limited mainly by short-distance advective and diffusive transport processes (Ekschmitt et al., 2008). At a water film thickness of <1 μm microbial movement was negligible (Kieft et al., 1993), and solute diffusion rate was reduced by more than 50% relative to saturated conditions in a loamy soil (Griffin, 1981). The reduction of substrate and enzyme diffusion with decreasing θs and water film thickness can be caused by several mechanisms, such as film straining in thin water films (Zevi et al., 2005), attachment to the air–water interface (Wan et al., 1994; Torkzaban et al., 2006a), increasing attachment to the solid–water interface (Torkzaban et al., 2006b) and retention at the air–water–solid interface (Steenhuis et al., 2006). Both the obstruction of microbial movement and the reduction in diffusion result in a physical separation of microorganisms from substrates and nutrients and may cause dormancy and long-term starvation (Kieft et al., 1993).
As shown by Hallett et al. (2004) water repellent properties could induce very high levels of small-scale variability in soil water movement. The exclusion of water from water repellent soil domains can, however, also be effective at larger scales (see Fig. 2). The formation of preferential flow paths, for example, is favored in soils with water repellent properties (Dekker & Ritsema, 1994). Preferential flow paths may result in irregular wetting patterns with wet and dry soil domains (Täumer et al., 2005; Zavala et al., 2009). For instance, in the top layer of a silt loam soil, Dekker & Ritsema (1995) measured differences in θs of up to 37% by volume. In the dry soil domains, which can persist for months (Dekker & Ritsema, 1996), residing SOM may effectively be protected. Similarly, SOM residence times within preferential flow paths penetrating deeper soil horizons may occur in the order of decades, whereas it can amount to millennia in adjacent bulk soil just decimeters away (Chabbi et al., 2009). These findings suggest that SWR can contribute to the formation of biologically nonpreferred soil domains at different spatial scales where decomposition rates are slow or where decomposition is frequently interrupted. On the other hand, it is important to note that the reduction in conducting liquid pathways with increasing SWR is accompanied by increasing soil air content and liquid–vapor interfacial area resulting in enhanced gaseous diffusion and exchange with the atmosphere (Or et al., 2007), which may improve the conditions for aerobic microbial decomposition in wet soil.
Recently, a series of papers (Muhr et al., 2008, 2010; Muhr & Borken, 2009) discussed SWR as a possible explanation for the slow regeneration of CO2 fluxes after experimental drought treatments. Nevertheless, only a few studies have directly investigated the role of SWR for carbon mineralization processes. A study by Goebel et al. (2005) in which carbon mineralization from different topsoil horizons was related to soil wettability, revealed decreasing CO2 efflux rates with increasing SWR. However, the authors pointed out that the measured effect was not necessarily due to the impact of SWR on water distribution and availability but could also result from different chemical stability of the SOM itself. To exclude these effects, Goebel et al. (2007) mixed natural topsoil material with a series of carbon-free silt particle mixtures, containing different portions of artificially hydrophobized grains. Different mixing ratios of the untreated (i.e. wettable) and the hydrophobized silt particles created a gradient in wettability by ensuring same amounts and composition of SOM in the resulting three-component (natural soil+wettable silt+hydrophobic silt) mixtures. Subsequent laboratory incubation experiments carried out on these three-component soils adjusted to same bulk θs revealed decreasing CO2 efflux with increasing SWR of the material (Goebel et al., 2007). In one particular three-component mixture, addition of 25% hydrophobic silt particles resulted in a moderate increase of αsw from 0° to 22°, but caused a pronounced reduction in cumulative CO2 release of approx. 75% (Goebel et al., 2007). Recently, Lamparter et al. (2009) stressed the importance of the wetting history (i.e. whether a soil is drying or wetting) for the effectiveness of SOM stabilization by SWR. After rewetting initially dry soil to a water potential of −31.6 kPa the authors found decreasing CO2 release rates with increasing SWR, whereas no significant relation could be observed for the same soils coming from a moist state and dried to −31.6 kPa. These relations between SWR and carbon mineralization indicate that the strength and duration of soil drying and pattern of wetting and drying events can be important for the stabilization of SOM by SWR and that global change-induced changes in the frequency and intensity of climate extremes may affect soil nutrient cycling and carbon sequestration.
Impact of SWR on the stability of SOM occluded in aggregates
Another important feature of SWR with respect to carbon mineralization is its influence on aggregate stability. Dynamics of SOM in aggregate fractions correlate well with the lifetime of the aggregates themselves (Besnard et al., 1996), and thus any change in aggregate stability can be expected to affect SOM decomposition rates. A number of studies have shown that increasing SWR favors the stability of aggregates (e.g. Sullivan, 1990; Zhang & Hartge, 1992; Cosentino et al., 2006; Arcenegui et al., 2008), which may increase the protection of occluded organic substances against microbial decomposition (Tisdall & Oades, 1982; Tisdall, 1996). The protection of aggregates due to SWR is mainly related to the reduction of the initial wetting rate, which diminishes the build-up of air pressure in soil pores, thus reducing the slaking stress (Zhang et al., 2007).
Capriel et al. (1990) and Capriel (1997) found that a decrease of aggregate stability was accompanied by a decrease of hydrophobic SOM compounds, such as lipids. This was also confirmed by the findings of Piccolo & Mbagwu (1999), who demonstrated that aggregate stability increased after the application of hydrophobic substances. Goebel et al. (2005) showed that a difference in αsw of only 13° can significantly reduce water uptake rates and enhance aggregate stability of air-dry aggregates immersed in water. The authors concluded that wettability was a better predictor of the initial aggregate breakdown dynamics than the total SOC content, particularly at very low SOC contents. Mataix-Solera & Doerr (2004) also demonstrated for soils with similar SOC contents that aggregate stability is enhanced with increasing SWR.
Evidence that aggregation physically protects SOM from decomposition due to a reduced accessibility for microorganisms has been provided by a multitude of aggregate disruption studies (Powlson, 1980; Elliott, 1986; Gupta & Germida, 1988; Gregorich et al., 1989; Hassink, 1992; Goebel et al., 2009). Huygens et al. (2005), for example, found a negative relationship between carbon mineralization and aggregate stability and suggested that physical protection of SOM in soil aggregates was an important SOM stabilization process in Andisols from southern Chile. A recent study by Lamparter et al. (2009) found evidence that the SOM-stabilizing effect of aggregates depends on soil water potential. At a water potential of −31.6 kPa, where oxygen diffusion is not limited, mineralization from crushed aggregates exceeded that of intact aggregates sevenfold, highlighting the physical protection of SOM in aggregates. Conversely, at a water potential of −6.3 kPa, where oxygen diffuses less freely, the authors found larger mineralization rates from intact aggregates as compared with corresponding crushed aggregates. This was explained by the larger amount of macropores and thus the better oxygen supply in soils with intact aggregates.
Effects of SWR on soil erodibility and its implications for SOM decomposition
A more indirect impact of SWR on SOM decomposition may be due to its role in exacerbating soil erosion. In this context, it has been suggested that SOM displaced by erosion can be particularly susceptible to mineralization (Lal, 2003), provided that the eroded SOM is deposited in an environment favoring decomposition. Because of their low infiltration capacity water repellent soils have often been associated with the occurrence of soil erosion (Shakesby et al., 2000). Several authors reported a positive relationship between the degree of SWR and soil erosion (e.g. Benavides-Solorio & MacDonald, 2005; Leighton-Boyce et al., 2007; Jordan et al., 2009). On the other hand, enhanced aggregate stability often associated with SWR (Arcenegui et al., 2008) can increase the resistance to erosion (Barthes & Roose, 2002).
As pointed out by Doerr & Moody (2004) it is often difficult to differentiate the effects of SWR from other covarying factors. Particularly the existence or lack of a vegetation and litter cover was shown to be decisive for whether or not SWR will have an effect on runoff flow and soil erosion (Coelho et al., 2005; Cerdà & Doerr, 2007; Leighton-Boyce et al., 2007; Badia & Marti 2008; Doerr et al., 2009). Also the spatial heterogeneity of SWR itself can be important for its impact on runoff flow and erosion (Ferreira et al., 2000; Shakesby et al., 2000). Thus, although SWR can induce high runoff locally, the effects at larger scales can be strongly attenuated (Imeson et al., 1992; Prosser & Williams, 1998; Coelho et al., 2004).
The disruptive energy of erosive forces can lead to the breakup of soil aggregates and exposure of previously protected SOM (Lal, 2003). Accordingly, a number of studies have shown that erosion can induce accelerated decomposition of SOM (e.g. Jacinthe et al., 2002; Van Hemelryck et al., 2010). A field study of Polyakov & Lal (2008) showed that up to 15% of SOC displaced by erosion can be mineralized. Whether or not the potential for mineralization is achieved depends on where the eroded SOM is deposited. Burial of SOM at deposition sites that are less accessible to microbes can also increase its stability against decomposition (Gregorich et al., 1998; McCarty & Ritchie, 2002).
Because of the difficulty in quantifying the net effect of these interacting processes at larger scales (Van Oost et al., 2007; Kuhn et al., 2009) there is a controversial discussion about the question whether erosion represents a major sink or source of carbon (Lal et al., 2004; Renwick et al., 2004; Van Oost et al., 2004; Lal & Pimentel, 2008; Van Oost et al., 2008). A recent literature analysis (Quinton et al., 2010) concluded that erosion can increase both the emission and the sequestration of carbon. Which process is dominating must be addressed in relation to a specific ecosystem (Polyakov & Lal, 2008). It is therefore concluded that, in contrast to the direct effects of SWR that tend to decrease the microbial accessibility of SOM, the tendency of the erosion-mediated effect of SWR is far less clear.
Role of abiotic environmental factors in the dynamics of SWR
Besides pH and the amount and composition of SOM as important influencing factors, SWR is affected primarily by θs and Ts, resulting in a coupled dynamic behavior. The mechanisms and consequences of changes in θs and Ts for SWR are illustrated in Fig. 4 and will be discussed in the following sections.
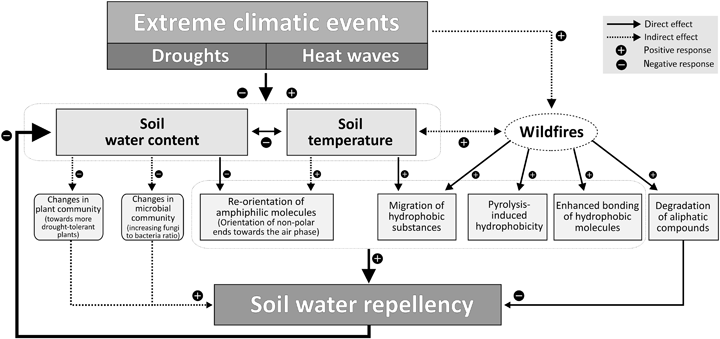
Schematic showing the role of extreme climatic events in the development of soil water repellency. It demonstrates the potential effects of droughts, heat waves and wildfires on processes affecting the wettability of soil and illustrates the feedback mechanism between soil water repellency and soil moisture.
Impact of soil moisture on the dynamics of SWR
Many authors have reported a negative relationship between θs and SWR and concluded that air-dry soils repel water most strongly (e.g. Dekker & Ritsema, 1994; Dekker et al., 1998; Coelho et al., 2005; Keizer et al., 2005; Leighton-Boyce et al., 2005; Thwaites et al., 2006). This behavior is explained by a rearrangement of soil organic molecules during wetting and drying (Ma'shum & Farmer, 1985; Valat et al., 1991; Roy & McGill, 2000; Douglas et al., 2007; Graber et al., 2009). Horne & McIntosh (2000) attributed the changes of SWR with θs to the existence of multiple, functionally different layers of SOM molecules on mineral particles, where an inner layer of more hydrophobic compounds is covered by an outer layer of amphiphilic compounds. This is in line with the zonal model suggested by Kleber et al. (2007). When the soil is moist, the hydrophobic portions of inner layer molecules are effectively screened by these amphiphilic molecules, orienting their polar functional groups towards the soil solution. Conversely, when the soil is drying out, the polar ends of amphiphilic molecules may associate with each other and interact through hydrogen bonds, thereby contracting and exposing parts of the interior hydrophobic layer (Horne & McIntosh, 2000). Consequently, rewetting of dry water repellent soil requires an activation energy to induce a cooperative rearrangement restoring the configurations favoring hydrogen bonds to water (Todoruk et al., 2003; Diehl & Schaumann, 2007).
In general, with increasing rainfall, the wetting resistance of the soil is reduced when θs reaches a threshold, called the ‘critical θs’ (θcrit) (Dekker & Ritsema, 1994). Values of θcrit can range from 2% by volume for a dune sand (Dekker et al., 2001) to about 40% by volume for a SOM-rich silt loam soil (Dekker & Ritsema, 1995) (see Table 1).
| Country | Soil type | Depth (cm) | Texture | SOC (mass%) | θ crit (vol%) | References |
|---|---|---|---|---|---|---|
| Australia | Lithosol | 0.0–30.0 | Sand | – | 6.0–8.0 | Kramers et al. (2005) |
| Germany | Luvisol | 0.0–10.0 | Sand | 2.8–5.0* | 7.4–10.3* | Buczko et al. (2007) |
| Germany | Arenosol | 0.0–7.8 | Sand | – | 14.0–16.0 | Greiffenhagen et al. (2006) |
| >7.8 | Sand | – | 5.0–6.0 | |||
| Greece | – | 0.0–5.0 | Sand | – | 9.3–15.0 | Ziogas et al. (2005) |
| – | 7.0–12.0 | Sand | – | 7.3–15.0 | ||
| The Netherlands | Anthrosol | 45.0–70.0 | Sand | 5.2–5.5* | ∼11.0* | Dekker et al. (1998) |
| The Netherlands | Gleysol | 0.0–2.5 | Sand | 18.0* | 18.0–23.0* | Dekker et al. (2001) |
| 2.5–5.0 | Sand | 10.0* | 14.0–20.0* | |||
| 7.0–12.0 | Sand | 0.5* | 3.0–8.5* | |||
| 14.0–19.0 | Sand | 0.5* | 2.0–5.5* | |||
| 25.0–30.0 | Sand | – | ∼2.5 | |||
| 35.0–40.0 | Sand | – | ∼2.0 | |||
| Portugal | Leptosol | 0.0–20.0 | Loamy sand | 5.0–17.6* | 14.0–27.0* | Leighton-Boyce et al. (2005) |
| The Netherlands | Fluvisol | 0.0–5.0 | Silt loam | – | ∼40.0 | Dekker & Ritsema (1995) |
| 10.0–15.0 | Silt loam | – | ∼24.0 | |||
| 20.0–35.0 | Silt loam | – | ∼20.0 | |||
| Japan | Cambisol | 0.0–50.0 | Clay loam | – | ∼29.0 | Kobayashi & Shimizu (2007) |
| The Netherlands | Histosol | 10.0–15.0 | Clayey peat | 40–70* | 34.0–38.5* | Dekker & Ritsema (1996) |
- * Data used for preparation of Fig. 5.
In reality, θcrit is not a sharp threshold but rather a transition zone (Dekker et al., 2001), which is a consequence of the hysteretic nature of SWR, breaking down and reestablishing at different θs (Leighton-Boyce et al., 2005). Investigations of Täumer et al. (2005) have shown that θcrit of a sandy Anthrosol linearly increased with increasing SOC content. This is consistent with Fig. 5 showing θcrit as a function of the SOC content for a set of soils with different texture. For the sandy soils, θcrit is linearly related to the SOC content in the range from 0.5% to 18% by mass, independently of the soil type (see Table 1). When including a clayey peat soil with very high SOC contents from 40% to 70% by mass the relationship can be described by an exponential function.
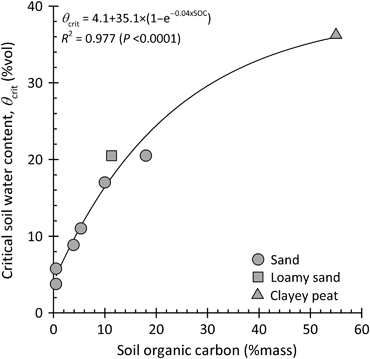
Critical soil water content (θcrit) as a function of the soil organic carbon (SOC) content for soils with different texture. The data originate from Dekker & Ritsema (1996), Dekker et al. (1998, 2001), Leighton-Boyce et al. (2005) and Buczko et al. (2007) (see Table 1).
Although some authors reported a more complex behavior of SWR in relation to θs (King, 1981; de Jonge et al., 1999, 2007; Goebel et al., 2004; Regalado & Ritter, 2005, 2009a, b), a general behavior can be derived (Fig. 6). For θs near field capacity a soil can be expected to be wettable (Bachmann et al., 2007). As the soil dries out, wettability will not change until θcrit is reached, where the soil becomes water repellent to some degree. The values of θcrit can be expected to be close to, but above the θs at the permanent wilting point (Bachmann et al., 2007) and depend on the SOC content (see Fig. 5). When the soil dries out further, wettability will decrease until a maximum of SWR is reached, which is mainly related to the SOC content, soil texture and pH (Doerr et al., 2000). When a dry soil is wetted again, wettability will not change until θcrit is reached. From this point onward, a further increase of θs will gradually decrease SWR. Both the increase of SWR upon drying and the decrease of SWR upon wetting are time-dependent processes due to conformational changes of SOM (Horne & McIntosh, 2000), and their rates depend on the composition of the SOM, the prevailing Ts and the present θs. As Ts during drying is usually higher than during rewetting, the conformational changes during drying may proceed faster as during wetting (Bayer & Schaumann, 2007), as indicated by the steeper slope of the drying branch in Fig. 6. The time dependence of these processes may in part explain the hysteretic nature of the SWR–θs relationship.
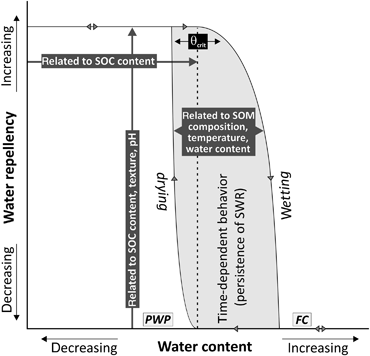
Schematic diagram illustrating the dynamics of soil water repellency as a function of the water content. θcrit, critical soil water content; PWP, permanent wilting point; FC, field capacity.
Impact of soil temperature on the development and variation of SWR
SWR is known to be induced by heat (Doerr et al., 2000, 2005). For instance, Dekker et al. (1998) reported increasing SWR of organic matter-rich sandy topsoil samples after heating at 65 °C for 3 days. Crockford et al. (1991) found increased SWR after exposing sandy clay loam soils to 43 °C for 2 h. A heating experiment with A horizon material from a gleyic Podzol revealed continuously increasing αsw with increasing heating temperature. As shown in Fig. 7, we found an increase in αsw of more than 40° as temperature increased from 25 to 60 °C demonstrating that SWR can be substantially enhanced by heat within the range of ecologically relevant soil surface temperatures. Repeated measurements carried out 4 months after the heat treatment revealed no significant changes in αsw showing the persistence of the heat-induced effect on soil wettability. In a recent study on anthropogenically influenced sandy soils, Diehl et al. (2009) found that initially wettable and slightly water repellent samples became considerably more water repellent after drying for 3 days at 65 °C.

Contact angle (αsw) as a function of heating temperature for a gleyic Podzol (A horizon). Subsamples of the soil were heated in an oven for 24 h at the respective temperature. Contact angles were measured immediately after equilibration with room temperature using the sessile drop method (see Bachmann et al., 2003). Data points are the mean of six replicates with error bars indicating the standard deviation.
It is generally assumed that the input of thermal energy promotes the rearrangement of SOM components during the drying process (Doerr et al., 2000). An alternative mechanism might be that at high Ts, waxes originating from particulate organic matter migrate onto mineral surfaces, thereby inducing or increasing SWR (Franco et al., 1995; see also Fig. 4). The relevance of the latter mechanism has been supported by micromorphological investigations showing increased formation of organic coatings on sand particle surfaces after exposing an organic matter-rich soil to 85 °C for 3 days (Dekker et al., 1998). Heat-induced enhancement of SWR may thus be an important mechanism, particularly in areas where Ts reaches high levels, such as in arid and semiarid climate zones (Doerr & Thomas, 2000). For instance, Rose (1968) reported a surface Ts of more than 50 °C for a loamy sand soil in central Australia and Garratt (1992) noticed surface Ts of more than 60 °C for a sandy loam soil located in a coastal area near Melbourne (Australia).
In this context, the occurrence of wildfires, which are likely to be promoted by the increasing drought and heat extremes (Mouillot et al., 2002; Goetz et al., 2005; Kasischke & Turetsky, 2006) can be very important (Shakesby & Doerr, 2006). Several studies have indicated that wettable soils became water repellent by the impact of fire (Huffman et al., 2001; Moral Garcia et al., 2005; Varela et al., 2005; Arcenegui et al., 2008; Zavala et al., 2009). Particularly wildfires occurring after long drought periods may have a pronounced impact on SWR (Novak et al., 2009) as the cooling effect of evaporating water (Chandler et al., 1983) will be reduced. The heat during a fire is thought to enhance the bonding of hydrophobic organic molecules to soil particles (Savage et al., 1972) and chemically intensify their hydrophobicity by pyrolysis (González-Pérez et al., 2004; Knicker, 2007). DeBano et al. (1970) showed that burning can cause hydrophobic substances to be translocated from the surface litter layer downward, rendering the subsoil water repellent (see Fig. 4). However, fire-induced reduction and even destruction of SWR in topsoils have also been reported for many parts of the world (Giovannini & Lucchesi, 1983; Garcia-Corona et al., 2004; Simkovic et al., 2008). This has been attributed to the selective degradation of aliphatic structures during fire (Almendros et al., 1988). In laboratory experiments, Doerr et al. (2005) showed for initially water repellent soils with SOC contents between 0.4% and 6.8% that exposure to temperatures from 20 to 200 °C tended to increase SWR, followed by a decline in SWR at exposure to 250 °C and destruction of SWR at 300 °C.
Significance of extreme climatic events for the future dynamics and development of SWR
The discussion above clearly demonstrates that θs and Ts are important abiotic factors determining the dynamics of SWR. Both factors are prone to be affected by climate change in various ways (Gerten et al., 2007; Mellander et al., 2007), and particularly by extreme events such as drought and heat waves. In general, θs and Ts are closely related to each other (e.g. Davidson et al., 1998; Almagro et al., 2009). During heat waves, high Ts is usually associated with low θs (De Boeck et al., 2011), while at higher moisture content Ts is typically dampened. The projected increasing intensity of coinciding drought and heat waves (Zampieri et al., 2009) underpins the assumption that the impact of SWR could become more pronounced in coming decades (Lichner et al., 2007; Hallett, 2008; Gordon & Hallett, 2009).
Several studies reported a seasonal variation of SWR with high levels in summer and low levels in winter (Buczko et al., 2005; Keizer et al., 2005; Leighton-Boyce et al., 2005; Keizer et al., 2007), which is typically attributed to changes in θs and Ts. Findings of Buczko et al. (2007) suggest that changes in SWR can be triggered within short time scales (approx. 3 days), and moreover, that SWR can be affected even by moderate changes in precipitation and slightly elevated temperatures. Particularly under relatively dry conditions, small changes in θs can result in strong changes in SWR (Doerr et al., 2002; Goebel et al., 2004; Leelamanie & Karube, 2007). Changes in SWR due to moderate fluctuations in precipitation and temperature are usually reversible. Extreme events, however, may induce semipermanent changes in SWR. Long drought periods may lead to a disruption of thin water films on the particle surface, which could result in difficult-to-reverse conformational changes or even condensation reactions (Bayer & Schaumann, 2007). During prolonged heat and drought periods, it is therefore conceivable that soils dry out to such an extent that severe SWR can develop in certain parts of the soil, such that previous water storage capacity cannot be restored during subsequent wetter periods. This effect was reported by Imeson et al. (1992) who found that a water repellent soil remained dry even during winter months in northeastern Spain. Similarly, Sowerby et al. (2008) reported persistently reduced moisture levels for rainfall exclusion plots even outside the experimental drought periods and proposed increased SWR as a likely mechanism to explain the nonrecovery of soil moisture. Consequently, if soil moisture is already reduced at the onset of a subsequent dry summer period, soil moisture level may decrease to even lower levels, thus promoting a self-amplification of SWR (see Fig. 4).
These effects are likely to become more important in the future because heat waves and drought periods are predicted to become more intense, more frequent and longer lasting (Meehl & Tebaldi, 2004; Meehl et al., 2007; Fischer & Schär, 2010). In principle, where global warming is expected to be accompanied by summer drying, SWR can become enhanced.
Consequences of climate-induced changes in soil wettability for carbon sequestration
In general, increased SWR can intensify climatic drought events by reducing rainwater infiltration, thus amplifying and extending periods with low water availability (Imeson et al., 1992; Sowerby et al., 2008; Muhr & Borken, 2009). As shown in many studies, low water availability reduces soil microbial activity (e.g. Emmett et al., 2004; Borken et al., 2006; Sotta et al., 2007), although in wet, oxygen-limited ecosystems, a sustained reduction in soil moisture can also stimulate microbial decomposition of SOM (Sowerby et al., 2008). In general, however, there is strong evidence that prolonged summer droughts will typically reduce cumulative carbon mineralization at the annual scale (Borken & Matzner, 2009).
Such a reduction in annual SOM mineralization cannot be explained by mere short-term delays in decomposition. Instead, long lasting effects on SOM decomposition can be explained in part by long lasting changes in SOM stability, such as the development of semipermanent SWR in certain soil domains, initialized during the drought. Consequently, enhanced SWR could be expected to reinforce future, drought-induced reductions in carbon mineralization.
On the other hand, a SWR-induced reduction in water availability will also affect plant productivity (Reichstein et al., 2007). In a recent study, De Boeck et al. (2011) showed that photosynthesis, and consequently, plant growth and biomass production of experimental plant communities were significantly reduced by imposed summer drought, particularly when combined with high temperatures. Low water availability causes a decreased leaf carbon fixation due to stomatal closure and a reduction in total carbon uptake is due to inhibition of growth (Chaves & Oliveira, 2004). Another important factor reducing plant productivity during drought is the amount of plant available nitrogen, which was shown to decline with increasing duration and intensity of drought (Borken & Matzner, 2009) and may be affected by SWR (Hentschel et al., 2007).
The creation of preferential water flow paths and subsequent uneven penetration of rainwater into water repellent soil was shown to decrease the germination of annual plants (Bond, 1972). It is therefore conceivable that on the long term, SWR can accelerate drought-induced changes in vegetation structure. A theoretical study by Ursino & Rulli (2010) investigating the effects of fire and water availability on vegetation patterns suggested that postfire vegetation patterns can be significantly affected by SWR. In this context, it is important to note that a change in vegetation structure may have substantial consequences for SWR itself, because increasing soil dryness can favor a drought resistant vegetation, whose litter is typically rich in oils and waxes (Doerr et al., 2000). On the long term, this can further enhance SWR. As fungi can survive drought stress better than bacteria (Wilson & Griffin, 1975), intensive and long-lasting drying during drought can also cause a shift in soil microbial community structure towards fungal dominance (Jensen et al., 2003). Because of their chemical nature, the dominance of fungi can potentially increase SWR (York & Canaway, 2000; Zhang et al., 2007) and may give rise to a feedback loop: if soil moisture remains at a lower level, this promotes a further shift to fungal dominance and increases SWR.
Therefore, comparable to climatic drought effects, the SWR-induced reduction of water availability can have positive and negative consequences with respect to carbon sequestration as both carbon loss by microbial decomposition of SOM and carbon input by plants are likely to become smaller. However, an important difference between climate-induced and SWR-induced decrease in water availability is that the former leads to an actual reduction in the total amount of available water, whereas the latter only leads to a different distribution of the water. Except for the situation after a wildfire, when the water repellent layer may have developed at greater depth (Doerr et al., 2000), during drought SWR will develop initially within a thin soil surface layer, because the soil dries out from the surface and Ts is highest directly at the surface. When a water repellent surface layer is developed, infiltration is reduced and rain water may pond or runoff on the soil surface and eventually infiltrate via preferential flow paths (e.g. root holes) (Doerr et al., 2000). If the water infiltrates through preferential flow paths into the subsoil (e.g. Dekker & Ritsema, 1995, 1996; Täumer et al., 2005) it will by-pass large domains of the topsoil, which can lead to dry surface soil and higher soil moisture in the subsoil as reported, for instance, by Burch et al. (1989) and Imeson et al. (1992). This would have important consequences with respect to the response of microorganisms and plants, because microorganisms, which are primarily located in the topsoil (Nunan et al., 2001) depend strongly on the moisture conditions in the topsoil, whereas plants can actively explore deeper soil domains for water (Hsiao & Xu, 2000).
In wettable soils, even small amounts of precipitation may cause short-term respiration pulses (Birch, 1958; Austin et al., 2004; Huxman et al., 2004; Lee et al., 2004; Cisneros-Dozal et al., 2007), but such effects may be suppressed in water repellent soils (van Straaten et al., 2010). Even heavy rainfalls following prolonged drought periods can be virtually ineffective for microbial activity in water repellent soils, as most water will runoff on the soil surface and infiltrate via preferential flow paths with only small amounts directly infiltrating into the topsoil (Cerdàet al., 1998). The development of a water repellent surface layer can also reduce subsequent drying of deeper soil layers by preventing evaporation and upward capillary movement of water (Imeson et al., 1992). Even a thin water repellent surface layer (<1 cm) was shown to reduce evaporation considerably (Bachmann et al., 2001). This reduced water vapor loss to the atmosphere may keep soils longer above wilting point, potentially avoiding severe plant water stress.
Different from wettable soils, where even small amounts of water can be used by microorganisms, in water repellent soil the microscopically heterogeneous distribution of water and the reduction in water film thickness on particles can effectively reduce diffusion pathways for enzymes and solutes as well as the mobility of microorganisms, thus leading to a reduced accessibility of SOM. Experimental field evidence for this effect was possibly provided by Muhr & Borken (2009) who reported a slow regeneration of soil moisture and an even slower recovery of CO2 emission from throughfall exclusion plots in a Norway spruce forest during the rewetting phase. The authors found reduced CO2 emissions up to 6 weeks after differences in matric potential between drought plots and control plots disappeared, which was attributed in part to SWR inhibiting a homogeneous rewetting of the organic horizons.
It can be concluded that an increase in SWR may cause a substantial reduction in soil water availability, affecting both microorganisms and plants. In the short term, different from a climate-induced water shortage, the SWR-induced reduction in water availability may reduce microbial decomposition processes relatively more than plant productivity, which could temporarily increase the carbon sink strength of soils. However, in the long term, the reduced availability of water will also reduce plant productivity and is likely to induce changes in vegetation composition, thus offsetting the short-term positive effects of SWR on carbon sequestration. While in laboratory and small-scale field studies the effects of SWR can be relatively easily assessed, on the ecosystem level the existence of many covarying factors strongly complicates the assessment of the effects directly related to SWR. However, a particular situation where SWR can play a distinctive role in affecting the carbon balance at the ecosystem scale can occur after wildfires, when SWR is often heterogeneously distributed both spatially and vertically. Thus, after a wildfire, changes in soil wettability may induce patterns with lower and higher soil moisture availability, which then may have important consequences for postfire vegetation recovery, seed germination and microbial activity.
Conclusions
In this paper, we highlighted the impact of extreme climatic events on SWR and discussed possible climate change-triggered variations in wettability and their significance for carbon sequestration. We put forward that SWR may have important consequences for carbon mineralization due to changes in soil water distribution and availability and its impact on aggregate stability and erosion. While the first two effects can potentially reduce the microbial accessibility of SOM, the net effect of erosion remains unclear.
Furthermore, it was shown that SWR is potentially sensitive to extreme climatic events, such as droughts and heat waves. Particularly after long drought events in combination with high temperatures it is conceivable that a formerly wettable soil will not regain complete wettability, but remains water repellent to some degree. Such scenarios are likely to become more important where global warming is expected to be accompanied by summer drying. The reduced infiltration capacity of water repellent soils can lead to a general reduction and more heterogeneous partitioning of available water, with low water input into the topsoil and high infiltration into the subsoil via preferential flow paths. This may cause long-term effects on soil moisture dynamics with reduced water availability even outside of the dry season.
We conclude that there is a potential impact of extreme climatic events on SWR, and that SWR in turn could reduce SOM decomposition and thus temporarily increase the carbon sink strength of soils. However, in the long term, the reduced water availability will also reduce plant productivity and induce changes in vegetation composition, which may override the short-term positive effects of increasing SWR on carbon sequestration. Future research on biosphere–climate interactions should consider the response of SWR to climate change and its consequences for microbial activity, decomposition rates and plant productivity. Consequently, there is a need for systematic manipulation experiments investigating the effects of dryness and heat on both SWR and the turnover times of different SOM fractions via isotope tracers. Generally, for the interpretation of lag effects in water manipulation experiments, SWR should always be a considered factor.
Acknowledgements
The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7/2007–2013) under Grant Agreement no 226701 (CARBO-Extreme). The authors would like to thank Susanne K. Woche for performing laboratory work and three anonymous reviewers for their suggestions and comments that helped to improve the manuscript.




