Potential effects of interaction between CO2 and temperature on forest landscape response to global warming
Abstract
Projected temperature increases under global warming could benefit southern tree species by providing them the optimal growing temperature and could be detrimental to northern species by exposing them to the supra optimal growing temperatures. This benefit-detriment trade-off could increase the competitive advantage of southern species in the northern species range and cause the increase or even dominance of southern species in the northern domain. However, the optimum temperature for photosynthesis of C3 plants may increase due to CO2 enrichment. An increase in the optimum temperature could greatly reduce the benefit-detriment effect. In this study, we coupled a forest ecosystem process model (PnET-II) and a forest GAP model (LINKAGES) with a spatially dynamic forest landscape model (LANDIS-II) to study how an optimum temperature increase could affect forest landscape response due to global warming. We simulated 360 years of forest landscape change in the Boundary Water Canoe Area (BWCA) in northern Minnesota, which is transitional between boreal and temperate forest. Our results showed that, under the control scenario of continuing the historic 1984–1993 mean climate (mainly temperature, precipitation and CO2), the BWCA will become a spruce-fir dominated boreal forest. However, under the scenario of predicted climatic change [the 2000–2099 climates are predicted by Canadian Climate Center (CCC), followed by 200 years of continuing the predicted 2090–2099 mean climate], the BWCA will become a pine-dominated mixed forest. If the optimum temperature increases gradually with [CO2] (the increase in optimum temperature is assumed to change gradually from 0 °C in year 2000 to 5 °C in year 2099 when [CO2] reaches 711 ppm and stabilizes at 5 °C after year 2099), the BWCA would remain a fir-dominated boreal forest in areas with relatively high water-holding capacity, but not in areas with relatively low water-holding capacity. Our results suggest that the [CO2] induced increases in optimum temperature could substantially reduce forest landscape change caused by global warming. However, not all tree species would be able to successfully adapt to future warming as predicted by CCC, regardless of optimum temperature acclimations.
Introduction
We are in a time of unprecedented climatic warmth in the context of the past 1200 years due to anthropogenic disturbances (Osborn & Briffa, 2006). According to the Intergovernmental Panel on Climate Change (IPCC), the global mean temperature will continue to increase by 1.4–5.8 °C in the next 100 years (IPCC, 2001). This unprecedented global warming may affect plant species physiology, phenology and distribution (Hughes, 2000). For forest ecosystems, the global warming may affect tree species abundance, distribution, forest community characteristics (e.g. species richness and diversity) and biome distributions (Hansen et al., 2001). Forest ecosystems near the southern and northern ranges of their distributions are expected to have the strongest responses to climate warming (Pastor & Post, 1988; He et al., 2005). Because the northern limits of tree species are mainly determined by the species' cold hardiness (in terms of both frost tolerance and growth at low temperatures) (Loehle, 1998), the coldness reduction caused by current global warming may cause a shift of the northern forest limit. An altitudinal shift of forests has already been observed in alpine forests in European (Pauli et al., 1994; Kullman, 2001) and North American (Peterson, 1994). The polarward shift of forests has also been suggested by the reconstruction of tree-line vegetation based on tree rings and fossils (Payette et al., 1989).
A species' southern limit is assumed to be mainly determined by the competition between the northern species and southern species (Woodward, 1987; Loehle, 1998). Under global warming, the temperature increase could benefit southern species by providing them their optimal growing temperatures and could be detrimental to northern species by putting them in a state of supra optimal growing temperatures. This benefit-detriment effect can greatly affect the competitive performance of southern species in the northern species range, and may cause the increase in extent and/or dominance of southern species in the northern domain and the retraction of northern species. This has been suggested by results from forest GAP models (Urban et al., 1993; Sykes & Prentice, 1995), spatially dynamic forest landscape models (He et al., 1999, 2002b, 2005; Scheller & Mladenoff, 2005), statistical analysis of current species distributions against climate variables by regression tree analysis (Iverson & Prasad, 1998, 2001, 2002; Iverson et al., 1999, 2004a, b; Prasad et al., 2006) and by response surface analysis (Flannigan & Woodward, 1994; Shafer et al., 2001). Pollen data have shown that there were large changes in the distribution of tree species, forest ecosystems, and biomes due to the historical quaternary climatic change (Davis, 1981; Overpeck et al., 1992; Jacobson & Dieffenbacherkrall, 1995; Davis & Shaw, 2001). However, until now there are no specific reports of forest landscape change on the boundaries between southern species and northern species, which can be specifically attributed to current global warming. This is because the southern species can only gain competitive advantage when the current northern forest stands are removed by death or disturbances, which can take hundreds of years (Loehle, 1998), except for very shade tolerant species (e.g. sugar maple) which could quickly gain a competitive advantage in the understory. In addition, the low abundance of the species near the boundary may slow down the northward migration due to global warming (Iverson et al., 2004a).
Another possible reason for lack of observed change on the boundary between southern species and northern species is that the physiological change caused by the accompanied CO2 enrichment may facilitate the ability of a forest ecosystem to adapt to current global warming. The previous modeling studies based on the benefit-detriment effect omitted an important benefit for northern species from the accompanied increase of CO2 concentration under global warming. The elevated [CO2] can stimulate the photosynthesis of C3 plants and inhibit photorespiration (Long, 1991; Saxe et al., 2001; Long et al., 2004). Owing to the photorespiration inhibition effect of CO2, there could be an increase in the optimum temperature for photosynthesis of C3 plants. This has been validated through experimental results (McMurtrie et al., 1992; Battaglia et al., 1996; Wayne et al., 1998; Roberntz, 2001). According to Long (1991), the optimum temperature could increase by 5 °C under a doubling of [CO2]. According to Idso et al.'s review (2003), the observational mean increase of optimum temperature is about 4.6 °C for 300 ppm increase of [CO2]. The increase in optimum temperature could have large effects on forest landscape change under the global warming. Some researchers have already pointed out that the results from previous forest simulation models may overestimate the forest response to global warming as they did not take into consideration the possible physiological change caused by CO2 enrichment (Loehle & LeBlanc, 1996; Wayne et al., 1998; Idso et al., 2003).
Although the importance of the optimum temperature increase in tree species' response to global warming has been acknowledged recently, there are no specific studies quantifying the effects of optimum temperature increase on forest landscape response to global warming at large spatial and long temporal scales. In this study, we couple a forest ecosystem process model (PnET-II) and a forest GAP model (LINKAGES) with a spatially dynamic forest landscape model (LANDIS-II). The coupled models are used to quantitatively study the potential effects of optimum temperature increase on the forest landscape change in a transitional ecosystem of boreal and temperate forest.
Material and methods
Study area description
Our study area is part of Boundary Waters Canoe Area (BWCA). BWCA Wilderness is located in northern Minnesota, USA (Fig. 1). BWCA forests are transitional between boreal forests and Great Lakes north temperate forests. The BWCA is a protected wilderness area within the Superior National Forest in the north central United States. The 438 000 ha tract is no longer logged although fire suppression and limited prescribed burning continues. The BWCA contains almost all of the presettlement flora and fauna native to the area (Heinselman, 1973; Scheller et al., 2005). In BWCA forests, the main tree species are red pine (Pinus resinosa), jack pine (Pinus banksiana), white pine (Pinus strobus), black spruce (Picea mariana), white spruce (Picea glauca), balsam fir (Abies balsamea), white cedar (Thuja occidentalis), quaking aspen (Populus tremuloides) (Heinselman, 1973; Baker, 1989). Red maple (Acer rubrum), white ash (Fraxinus americana), sugar maple (Acer saccharum), paper birch (Betula papyrifera) and balsam poplar (Populus balsamifera) are also present (Heinselman, 1973; Scheller et al., 2005).
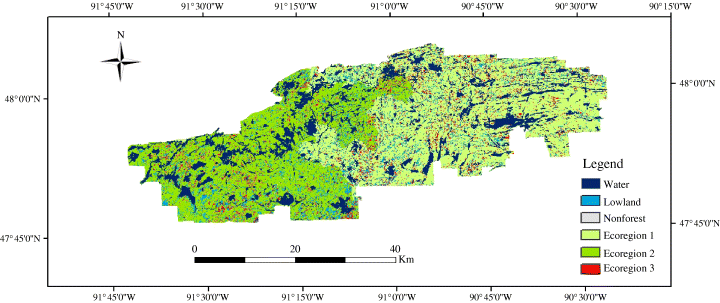
Study area within the Boundary Waters Canoe Area Wilderness, Minnesota, USA.
Historically, due to the high fire frequency, the BWCA forests were dominated by even-aged stands of two species which are fire-adapted through opportunistic reproduction: jack pine, which is serotinous, and quaking aspen (Scheller et al., 2005). Since 1910, due to fire suppression, fire has largely been absent (fire rotation period > 1000 years) (Baker, 1992; Frelich & Reich, 1995). Nowadays, much of the forest is transitioning toward a shade-tolerant mixed-age composition (Scheller et al., 2005). The shade-intolerant, early- to mid-successional species are being replaced by shade-tolerant species including white spruce, black spruce, balsam fir and white cedar.
The climate is cold temperate continental and the glacial geology is typical of the Laurentian continental shield (Heinselman, 1973). Soils are generally thin due to glacial scouring, although some till, outwash, and lacustrine deposits exist. Soil type and depth to bedrock are highly heterogeneous at fine spatial scales (Heinselman, 1973).
Model description
LANDIS models are cell-based spatially dynamic forest landscape models of disturbance, succession and management (Mladenoff et al., 1996; Mladenoff & He, 1999; Scheller et al., 2007). LANDIS models simulate species-level forest dynamics by tracking species age cohorts under natural and anthropogenic disturbances including fire, windthrow, insects and diseases, harvesting and fuel management. The forest landscape is divided into environmentally homogenous landtypes/ecoregions within which species establishment probabilities (SEPs) and disturbances regimes are the same. The LANDIS models include different succession and disturbance extensions, including the fire disturbances extension (He & Mladenoff, 1999b), harvesting extension (Gustafson et al., 2000), biological disturbances extension (Sturtevant et al., 2004a) and biomass succession extension (Scheller & Mladenoff, 2004).
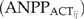 is adjusted for the available growing space and maximum net primary production capability for species of interest,
is adjusted for the available growing space and maximum net primary production capability for species of interest,
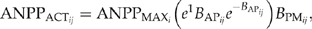 (1)
(1)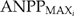 are maximum aboveground net primary production for species i (Mg/Ha),
are maximum aboveground net primary production for species i (Mg/Ha), 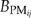 is the ratio of actual biomass to maximum biomass for species i with age of j, and
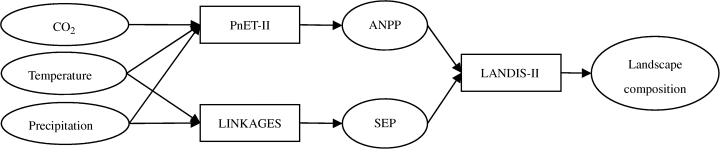
is the ratio of actual biomass to maximum biomass for species i with age of j, and 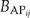 is the ratio of actual biomass to potential biomass for species i with an age of j. The mortality of biomass is related to the previous biomass and the age of a certain cohort. Biomass related mortality (
is the ratio of actual biomass to potential biomass for species i with an age of j. The mortality of biomass is related to the previous biomass and the age of a certain cohort. Biomass related mortality (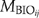 ) for cohort of species i and age of j is modeled as follows:
) for cohort of species i and age of j is modeled as follows:
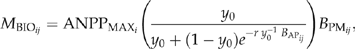 (2)
(2)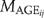 ) for cohort of species i and age j is modeled as follows:
) for cohort of species i and age j is modeled as follows:
 (3)
(3)Seed dispersal and seedling establishment process in LANDIS-II are simulated as three steps: dispersal (Ward et al., 2004), light condition checking, and site condition checking (He & Mladenoff, 1999a; Mladenoff & He, 1999). For light condition checking, species with shade tolerance classes 1–4 can only be established when the available relative living biomass (ratio of available living biomass to maximum living biomass) is < 0.247, 0.326, 0.428 and 0.588, respectively (Scheller & Mladenoff, 2004). For species of shade class 5, it can establish if the relative living biomass is higher than 0.247. The site condition checking is determined by comparing SEPs, which are homogenous within each ecoregions, to a random number generator.
Model parameterization
For LANDIS-II, the main parameters include the initial species age cohort map, landtype/ecoregion map, species life historical attributes, disturbances regimes, maximum ANPP, and SEPs. The initial species age cohort information are based on the TM imageries interpretation and forest stand age maps (Scheller et al., 2005). The BWCA is divided into three forested ecoregions (Fig. 1). Ecoregions 1 and 2 are based on State Soil Geographic (STATSGO) Data Base (STATSGO, 1994; Scheller et al., 2005). Ecoregion 3 is limited to areas designated as pure black spruce forests. Species life historical attributes are based on Scheller et al. (2005) (Table 1). As already mentioned, due to the fire suppression, fire is mainly absent from BWCA. For this reason, we simulate without fire disturbances. For wind throw disturbance, we simulate a wind throw rotation period of 500 years for all scenarios (Scheller et al., 2005).
| Species | LONG | MTR | ST | ED | MD | VP | MINVP | MAXVP | GGDMin | GDDMax |
|---|---|---|---|---|---|---|---|---|---|---|
| Aspen | 160 | 25 | 1 | 200 | 5000 | 0.9 | 0 | 90 | 743 | 2900 |
| Paper birch | 230 | 30 | 2 | 200 | 5000 | 0.5 | 0 | 70 | 484 | 2036 |
| Balsam poplar | 150 | 25 | 1 | 200 | 5000 | 0.4 | 0 | 150 | 555 | 2491 |
| Red maple | 150 | 10 | 3 | 100 | 200 | 0.5 | 0 | 150 | 1260 | 6600 |
| Sugar maple | 300 | 40 | 5 | 100 | 200 | 0.1 | 0 | 240 | 1222 | 3100 |
| White ash | 200 | 30 | 4 | 70 | 140 | 0.1 | 0 | 70 | 1298 | 5993 |
| Red pine | 300 | 40 | 2 | 12 | 275 | 0 | 0 | 20 | 1100 | 2035 |
| White pine | 350 | 40 | 3 | 100 | 250 | 0 | 0 | 0 | 1100 | 3165 |
| Jack pine | 200 | 15 | 1 | 20 | 40 | 0 | 0 | 200 | 830 | 2216 |
| White spruce | 250 | 40 | 4 | 30 | 200 | 0 | 0 | 0 | 280 | 1911 |
| Black spruce | 200 | 20 | 3 | 80 | 300 | 0 | 0 | 200 | 247 | 1911 |
| Balsam fir | 150 | 25 | 5 | 30 | 160 | 0 | 0 | 0 | 560 | 2386 |
| White cedar | 300 | 35 | 4 | 45 | 60 | 0 | 0 | 300 | 1000 | 2188 |
- LONG, longevity (year); MTR, age of maturity (year); ST, shade tolerance (1 least tolerant and 5 most tolerant); ED, effective seeding distance (m); MD, maximum seeding distance (m); VP, vegetative reproduction probability; MINVP, minimum age of vegetative reproduction (year); MAXVP, maximum age of vegetative reproduction (year). Data are from Scheller et al. (2005). GDDMax, maximum grow degree days; GGDMin, minimum growing degree days. Data are from Pastor & Post (1985).
The ANPP and SEP inputs for LANDIS-II are initialized using the PnET-II model (Aber & Federer, 1992; Aber et al., 1995) and LINKAGES model (Pastor & Post, 1985), respectively (Fig. 2).
Flow diagram of model coupling. The ovals represent input/output variables for a certain model. The rectangles represent models.
ANPP
PnET-II is used to provide maximum ANPP (ANPPmax) for each species within each ecoregion (Scheller & Mladenoff, 2005). PnET-II is a process based model for carbon and water dynamics in forest ecosystems (Aber & Federer, 1992; Aber et al., 1995). The core relationship in the model is a linear response of maximum net photosynthetic rate to foliar N concentration. Stomata conductance varies linearly with photosynthesis, such that transpiration is a function of CO2 gain and atmospheric vapor pressure deficit.
 (4)
(4)| Species | T opt | FNC | MLMA | LRY |
|---|---|---|---|---|
| Aspen | 20.0 | 2.5 | 83 | 1.0 |
| Paper birch | 18.8 | 2.3 | 100 | 1.0 |
| Balsam poplar | 17.7 | 2.5 | 80 | 1.0 |
| Red maple | 25.1 | 2.4 | 75 | 1.0 |
| Sugar maple | 25.0 | 2.5 | 85 | 1.0 |
| White ash | 25.5 | 2.1 | 76 | 1.0 |
| Red pine | 21.5 | 1.5 | 250 | 2.3 |
| White pine | 22.5 | 2.2 | 175 | 3.0 |
| Jack pine | 19.9 | 2.3 | 244 | 1.6 |
| White spruce | 17.8 | 1.5 | 286 | 4.0 |
| Black spruce | 17.7 | 1.5 | 286 | 4.0 |
| Balsam fir | 19.6 | 1.6 | 204 | 4.0 |
| White cedar | 21.0 | 1.3 | 222 | 2.0 |
- T opt, optimum temperature for photosynthesis (°C); FNC, foliage nitrogen content (%); MLMA, maximum leaf mass area (g m−2); LRY, leaf retention years (years). Topt is derived based on Eqn (4). The data for FNC, MLMA and LRY are from Scheller & Mladenoff (2005).
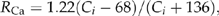 (5)
(5)SEPs
SEPs of each species are initialized using LINKAGES (He et al., 1999; Scheller et al., 2005). LINKAGES is a forest GAP model simulating the diameter growth of individual trees at the forest stand level (Pastor & Post, 1985). The abiotic factors affecting tree growth includes soil carbon, soil nitrogen, soil moisture and growing degree days (GDD). Competition between trees is mainly controlled by the light available. LINKAGES can be used to parameterize the SEPs at specific ecoregions for LANDIS-II (He et al., 1999; Scheller et al., 2005). The SEPs are based on 100 replicates of LINKAGES simulation for 10 years on a stand of 200 seedlings. If the biomass of the stand increases over the 10 years, then the species is assumed to have successfully established. If there is positive biomass increase in all replicates, the species is assigned with an establishment probability of 1.0. If there is no positive biomass increase in all replicates, the species is assigned with a small establishment probability value of 0.01 instead of 0.00, in view that all species listed in the paper are present in our study area and northern species can generally be successfully planted much farther south than their southern range limits (125–625 km) (Woodward, 1987, 1988).
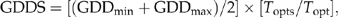 (6)
(6)Climatic change
The historical and future climatic change inputs for PnET-II and LINKAGES are derived from the Canadian Climate Center (CCC) projections based on 0.5 °C grid in the Phase-II Vegetation-Ecosystem Modeling and Analysis Project (VEMAP) (Kittel et al., 2000). For PnET-II, the climatic change inputs include monthly minimum and maximum temperature; precipitation; CO2 concentration and total solar incident radiation. For LINKAGES, the climatic change inputs include monthly mean temperature, monthly mean precipitation, and standard deviation of monthly mean temperature and mean precipitation.
According to the climate prediction by CCC, there is about 6 °C of annual mean temperature increase from 1994 to 2099 (Fig. 3a). The temperature increase in the winter is relatively higher than in the summer (Fig. 3b). As both LIKAGES and PnET model control the photosynthesis by the GDD (there will be no photosynthesis activity if the GDD are ≤0), the impact of shifts in optimal temperature will only be of relevance when trees are photosynthetically active in spring and summer. The temperature increase in the winter will not have much effect on tree photosynthesis except for very warm winters. According to IPCC (2001), there will be greater night-time warming than daytime warming. As the night-time warming will not have an effect on tree photosynthesis, the PnET-II model calculates the daytime temperature and night-time temperature from the monthly maximum and minimum temperature to address the nonuniform diurnal temperature increase. In the LINKAGES model, it does not distinguish nonuniform diurnal temperature increase. This may increase the uncertainty in our simulation results. However, it should not affect the basic result as the uncertainty due to the diurnal difference is relatively small compared to the overall temperature increase.
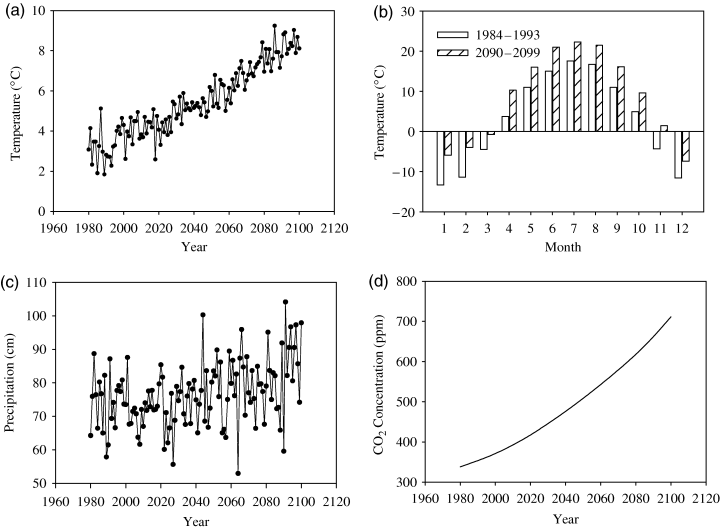
Climate change inputs for PnET-II and LINKAGES. (a) annual mean temperatures (°C); (b) monthly mean temperature differences between the historic 1984–1993 climate and the predicted 2090–2099 climate; (c) annual precipitation (cm); (d) annual mean CO2 concentration (ppm). Data after 1994 is based on prediction by Canadian Climate Center (CCC) in the Phase-II Vegetation-Ecosystem Modeling and Analysis Project (VEMAP). Data before 1994 is historical data. We assume the climate stabilizes after year 2099.
According to CCC predictions, the precipitation is not substantially increased (Fig. 3c). The CCC prediction is based on the IS92a CO2 emission scenario (Leggett et al., 1992) (Fig. 3d). At year 2100, the CO2 concentration is assumed to reach 711 ppm.
Scenario analysis
In this study, we run LANDIS-II for 360 years (1990–2350) under three scenarios: (1) the continuing of the historic 1984–1993 mean climate; (2) the CCC-predicted climates with no optimum temperature increase and (3) the CCC-predicted climates with a gradual optimum temperature increase. The scenario 1 is used as a control scenario. For scenarios 2 and 3, we assume that the climate stabilizes after year 2099. The predicted climates in scenarios 2 and 3 refer to the CCC-predicted 2000–2099 climates followed by 200 years of the predicted 2090–2099 mean climate. As most greenhouse gas emission scenarios predict that CO2 will continue to rise after 2099 (IPCC, 2001), our predictions after 2099 are conservative. However, this would not affect the basic results of the effects of optimum temperature increase. For scenario 3, the increase in optimum temperature is assumed to change gradually from 0 °C in year 2000 to 5 °C in year 2099 when [CO2] reaches 711 ppm. The increase is assumed to stabilize at 5 °C after year 2099.
Using PnET-II and LINKAGES, we calculated ANPPmax and SEPs for 13 species present in the BWCA under the three scenarios for Ecoregions 1 and 2. For Ecoregion 3, the ANPPmax and SEPs are based on average values from Ecoregions 1 and 2. For scenarios 2 and 3, we calculate ANPPmax and SEPs for every 10 years from 2000 to 2099. ANPPmax and SEPs before year 2000 are based on the historic 1984–1993 climates. After year 2099, ANPPmax and SEPs are set to be same as that under the predicted 2090–2099 climates.
For LANDIS-II output, we classify the forest into five forest types: aspen-birch (aspen, paper birch and balsam poplar), maple-ash (red maple, sugar maple and white ash), pine (red pine, white pine and jack pine), spruce-fir (white spruce, black spruce and balsam fir), and cedar (white cedar). For a specific cell, the assignment of forest type is based on the maximum biomass. We use a landscape metric analysis software –apack (Mladenoff & DeZonia, 2000) – to calculate the percentage area of different forest types in the forested ecoregions.
Results
SEP
Results from LINKAGES simulations show that all coniferous species, except for white pine, have relatively high establishment probabilities under the historic 1984–1993 climates (Table 3). However, under the CCC-predicted 2090–2099 climates without an optimum temperature increase, their establishment probabilities are all reduced to very small values. Red maple, white ash and white pine which have very low SEPs under the historic 1984–1993 climates begin to have relatively high SEPs under the 2090–2099 climates without the optimum temperature increase. Under the predicted 2090–2099 climates with an optimum temperature increase of 5°, the establishment probabilities of red maple, sugar maple and white pine remain high compared with that without optimum temperature increase. For both white spruce and black spruce, their establishment probabilities remain very low. However, the establishment probabilities of balsam fir, paper birch, jack pine have substantially increased.
| Species | 1984–1994 climate | 2090–2099 climatewithout Topt increase | 2090–2099 climate with5 °C Topt increase | |||
|---|---|---|---|---|---|---|
| 1* | 2† | 1 | 2 | 1 | 2 | |
| Aspen | 0.99 | 1.00 | 0.84 | 0.99 | 0.86 | 0.99 |
| Paper birch | 0.97 | 1.00 | 0.01 | 0.01 | 0.10 | 0.21 |
| Balsam poplar | 0.99 | 1.00 | 0.37 | 0.58 | 0.85 | 0.99 |
| Red maple | 0.02 | 0.02 | 0.62 | 0.98 | 0.28 | 0.69 |
| Sugar maple | 0.23 | 0.24 | 0.28 | 0.60 | 0.25 | 0.67 |
| White ash | 0.01 | 0.01 | 0.69 | 0.97 | 0.02 | 0.05 |
| Red pine | 0.55 | 0.75 | 0.01 | 0.01 | 0.02 | 0.03 |
| White pine | 0.06 | 0.06 | 0.22 | 0.55 | 0.25 | 0.53 |
| Jack pine | 0.96 | 1.00 | 0.01 | 0.01 | 0.63 | 0.85 |
| White spruce | 1.00 | 1.00 | 0.01 | 0.01 | 0.01 | 0.01 |
| Black spruce | 0.62 | 0.70 | 0.01 | 0.01 | 0.01 | 0.01 |
| Balsam fir | 0.85 | 0.93 | 0.01 | 0.01 | 0.10 | 0.37 |
| White cedar | 0.58 | 0.73 | 0.01 | 0.01 | 0.02 | 0.15 |
- Note: Topt, optimum temperature for photosynthesis (°C).
- * Ecoregion 1.
- † † Ecoregion 2.
ANPP
Results from PnET-II simulations show that the species maximum ANPP pattern based on the historic 1984–1993 climates is much different from that under the predicted 2090–2099 climates without the optimum temperature increase (Fig. 4a and b). Under the historic 1984–1993 climates, mean ANPP for spruce-fir is relatively higher than that for other forest types (Fig. 4c and d). Under the predicted 2090–2099 climates, the mean ANPP for pines and maple-ash become much higher than that for spruce-fir and aspen-birch. However, if there is an optimum temperature increase, then the relative differences in mean ANPP among spruce-fir, pine, maple-ash and aspen-birch are much reduced.
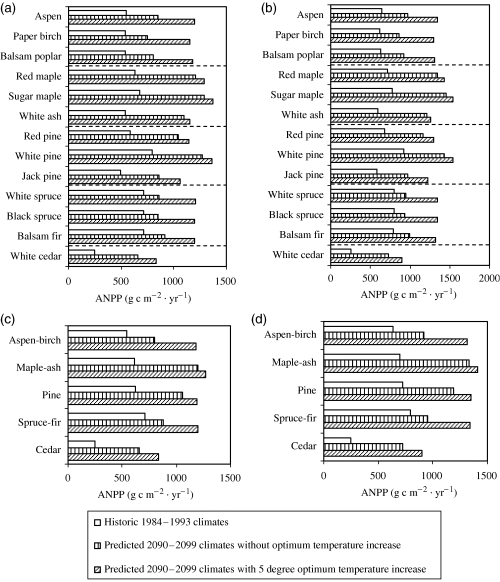
Aboveground net primary production (ANPP) simulated by PnET-II under the historic 1984–1993 climates and the predicted 2090–2099 climates with or without a 5 °C increase in optimum photosynthetic temperature. (a) ANNP for individual species in Ecoregion 1; (b) ANNP for individual species in Ecoregion 2; (c) grouped mean ANNP for species in individual forest types in Ecoregion 1; (d) grouped mean ANNP for species in individual forest types in Ecoregion 2.
Forest landscape composition
LANDIS-II simulation results show that, due to the fire suppression in BWCA, the spruce-fir forest will become the dominant forest type in BWCA area under the control scenario of continuing the historic 1984–1993 mean climate (5, 6). Under the scenario of CCC-predicted climates without an optimum temperature increase, pine forest becomes the dominant forest type after approximately 200 simulation years (5, 6). The percentage area of aspen-birch also increases after approximately 150 years.
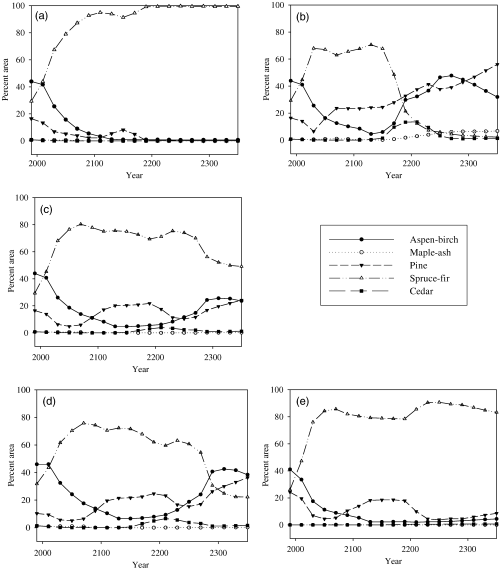
Species forest type percentage area change for ecoregions under different scenarios. (a) Entire study area (Ecoregion 1–3) under the control scenario of continuing the historic 1984–1993 mean climate; (b) entire study area under the scenario of Canadian Climate Center (CCC)-predicted climates without an optimum temperature increase; (c) entire study area under the scenario of CCC-predicated climates with a gradually increase of optimum temperature (the increase in optimum temperature is assumed to change gradually from 0 °C in year 2000 to 5 °C in year 2099 when [CO2] reaches 711 ppm and stabilizes at 5 °C after year 2099); (d) Ecoregion 1 under the scenario of CCC-predicated climates with a gradually increase of optimum temperature; and (e) Ecoregion 2 under the scenario of CCC-predicated climates with a gradually increase of optimum temperature. For (d) and (e), Ecoregion 3 is merged into Ecoregion 1 or 2 based on its spatial location.
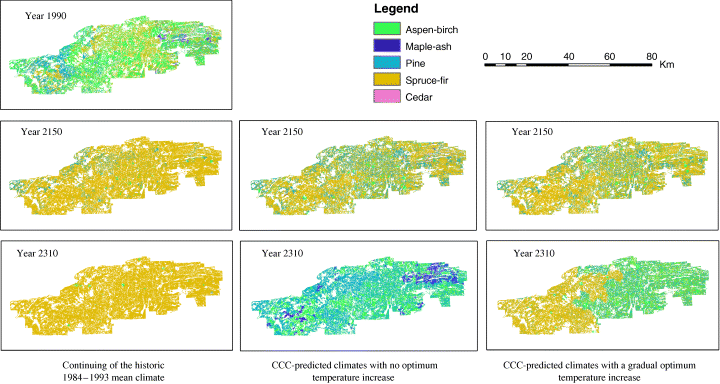
Forest landscape changes simulated by LANDIS-II.
If there is a gradually optimum temperature increase under CO2 enrichment, the forest landscape will not become pine dominated mixed forest in 360 simulation years (Fig. 5c). Instead, the forest will remain largely dominated by fir and spruce. However, the dominance of fir is lower than that under the control scenario of continuing the historic 1984–1993 mean climate and there is a higher percentage of pine and aspen-birch forest. This suggests that if the optimum photosynthetic temperature increases, the BWCA forests may not transition to pine dominated mixed forest in 360 years. However, the forest landscape composition will still be much altered by the global warming.
Further examinations shows that, after year 2300, the spruce-fir percentage area in Ecoregion 2 is much higher than that in Ecoregion 1 (5, 6). This is because there is higher fir establishment probability in Ecoregion 2 (Soils in Ecoregion 2 have a higher water-holding capacity). In Ecoregion 2, spruce-fir has pronounced dominance after year 2300. However, in Ecoregion 1, aspen-birch and pine have relatively higher percentage area than spruce-fir (5, 6). The forest landscape composition differences between the two ecoregions suggest that the soil moisture change under global warming may also be an important factor affecting forest landscape response to global warming.
Discussion
Result implications
The establishment probability changes under climatic change without optimum temperature increases are in agreement with previous studies. For example, the decrease of establishment probability in BWCA for spruce-fir is in agreement with the results from Iverson & Prasad (1998, 2001) and Shafer et al. (2001). The decrease of red pine establishment probability around BWCA is in agreement with the results from Flannigan & Woodward (1994). The decrease of paper birch establishment probability agrees with the predicted results from Shafer et al. (2001). These agreements suggest that the LINKAGES model is valid for predicting the change in the probability of establishment.
Our results from LANDIS-II show that the spruce-fir forest percentage area will decrease and pine forest percentage area will increase under the scenario of CCC-predicted climates without optimum temperature increase. This is in agreement with previous studies suggesting that that white pine would be favored, while spruce-fir would be reduced under climatic change (Jacobson & Dieffenbacherkrall, 1995; Iverson & Prasad, 1998, 2001; Shafer et al., 2001). The reduction in areas dominated by spruce-fir is also in agreement with that based on forest simulation results from China (He et al., 2005) and Europe (Sykes & Prentice, 1995). However, our results show that the optimum temperature increase could keep BWCA as fir dominated boreal forest for Ecoregion 2. This suggests that an optimum temperature increase could substantially reduce forest landscape change caused by global warming. Our results also show that two coniferous species (white spruce and black spruce) which are the dominant species under the control scenario of continuing the historic 1984–1993 mean climate have very low establishment probabilities even with a 5 °C increase of optimum temperature. This suggests that not all tree species would be able to successfully adapt to future warming as predicted by CCC, regardless of optimum temperature acclimations.
Our results show that, in areas with low water-holding capacity (Ecoregion 1), the forest may not be fir-dominated forest even with an optimum temperature increase. This suggests that available water capacity can be an important factor affects forest landscape change. This finding is in agreement with previous studies suggesting that drought caused by global warming may negatively affect forest growth (Pastor & Post, 1988; Koerner et al., 2005). However, in this study, we do not include the effect of elevated [CO2] on the stomata conductance (Saxe et al., 1998; Medlyn et al., 2001; Long et al., 2004), which may relieve the effect of draught. In addition, the stomata conductance response to increased [CO2] is significantly stronger in deciduous trees than coniferous trees (Medlyn et al., 2001). This may increase the competition performance of deciduous trees in case of draught.
Assumptions and caveats
The response of a forest ecosystem to climatic change (including temperature, precipitation and CO2) is a multiscale complex process, which is difficult to model and predict. Our study is not trying to accurately predict the future forest landscape, but to explore the potential effects of optimum temperature increase on the forest landscape composition, based on a series of reasonable assumptions.
First, our study is based on the assumption that species life history attributes (maximum age, shade tolerance, seed dispersal and age of maturity) and the relationship between SEPs and environmental factors (soil nitrogen, soil moisture and frost) will be unaffected by global warming. Under climatic change, the relationship between SEPs and these environmental factors may be altered due to the species modification through physiological change (e.g. stomata conductance change) (Hughes, 2000) or even genetic change (Bradshaw & Holzapfel, 2006). The magnitude of the modification could affect forest landscape response to climatic change.
Second, in our study, the effect of temperature on species performance occurs mainly through the vegetative growth processes. However, the temperature increase may also affect other developmental processes (such as pollen and seed production or early seedling establishment) (Greenwood et al., 2002), and subsequently the species performance under climatic change. That means, even if the growth process were able to adapt to the future climatic change due to the [CO2] enrichment, individual species may still not be able to fully adapt to the new conditions. In order to be more accurate, more-detailed models with other important development processes are needed. Until now, there are few forest landscape models that incorporate other developmental processes.
Finally, our study is based on the homogeneous increase of optimum temperature for every species. The forest landscape change may be much different from our simulation if there is a highly different optimal temperature increase. However, there are currently only a few reports of tree species on the effect of [CO2] enrichment on the optimum temperature increase (McMurtrie et al., 1992; Battaglia et al., 1996; Wayne et al., 1998; Roberntz, 2001). In order to be able to more accurately assess the global warming effects on forest landscape change, more-detailed study results as to optimum temperature increase for individual tree species are needed.
Method implications
Forest landscape response to climatic change is a very complex process, which may take decades or hundreds of years to identify at large spatial scales. It will be very difficult to study the forest landscape's response at large spatial and long temporal scales based only on traditional experimental and observational studies. Spatially dynamic forest landscape models can be a very useful tool to study the forest landscape response to climatic change (He et al., 1999, 2005; Scheller & Mladenoff, 2005; Schumacher & Bugmann, 2006). As Davis & Shaw (2001) pointed out, adaptation and migration are both important for species' response to global warming. Compared with the empirical models such as logistic regression and regression tree analysis method, forest landscape models are more realistic as they take into consideration the spatial and competitive interaction among species. They are also more flexible since they can be coupled with other models (e.g. GAP models and process models) to study the effect of species adaptation on forest's response to climatic change. The validity of coupling forest landscape model with other forest models to study the forest landscape's response to climatic change have been illustrated in He et al. (1999, 2002b, 2005) and Scheller & Mladenoff (2005). In addition, using a spatially dynamic forest landscape model, it is possible to assess the effect of climate induced disturbance regime change on forest landscape change (He et al., 2002b; Schumacher & Bugmann, 2006), which may be a very important consequence of global climatic change (Dale et al., 2001).
For forest landscape models, it is always difficult to validate simulation results due to the lack of detailed forest type data at large spatial and long time scale. However, it is still possible to partially verify the model simulation results by expert knowledge. The validity of the LANDIS model assumptions is illustrated in many simulation studies from different forest types including various temperate deciduous ecosystems of the Midwestern United States (He & Mladenoff, 1999b; Gustafson et al., 2000, 2004; Sturtevant et al., 2004a, b; Scheller & Mladenoff, 2005) and China (He et al., 2002a); boreal forest ecosystems of North America (Mehta et al., 2004; Pennanen et al., 2004), Finland (Pennanen & Kuuluvainen, 2002) and China (Xu et al., 2004; Wang et al., 2006); coastal chaparral of Southern California, USA (Franklin et al., 2001), transitional areas between boreal forest and temperate forest (Scheller et al., 2005) and high elevation coniferous forests of Switzerland (Schumacher et al., 2004).
Acknowledgements
US Department of Agriculture McIntire-Stennis funds are used to support this study. We thank Dr Yinyan Guo and Prof. Ronald Amundson from University of California, Berkeley, for providing us the soil carbon content data in Minnesota. We thank Prof. Stephen P. Long and Dr Lisa A. Ainsworth for their excellent instructions on plant responses to climatic change. We also thank Prof. John Pastor and another anonymous reviewer for their helpful comments which greatly improve this paper. We thank the VEMAP data group within the Ecosystem Dynamics and the Atmosphere Section, Climate and Global Dynamics Division, National Center for Atmospheric Research for providing us the access to the VEMAP climatic database.




