Growth of Eastern Cottonwoods (Populus deltoides) in elevated [CO2] stimulates stand-level respiration and rhizodeposition of carbohydrates, accelerates soil nutrient depletion, yet stimulates above- and belowground biomass production
Abstract
We took advantage of the distinctive system-level measurement capabilities of the Biosphere 2 Laboratory (B2L) to examine the effects of prolonged exposure to elevated [CO2] on carbon flux dynamics, above- and belowground biomass changes, and soil carbon and nutrient capital in plantation forest stands over 4 years. Annually coppiced stands of eastern cottonwoods (Populus deltoides) were grown under ambient (400 ppm) and two levels of elevated (800 and 1200 ppm) atmospheric [CO2] in carbon and N-replete soils of the Intensive Forestry Mesocosm in the B2L. The large semiclosed space of B2L uniquely enabled precise CO2 exchange measurements at the near ecosystem scale. Highly controllable climatic conditions within B2L also allowed for reproducible examination of CO2 exchange under different scales in space and time. Elevated [CO2] significantly stimulated whole-system maximum net CO2 influx by an average of 21% and 83% in years 3 and 4 of the experiment. Over the 4-year experiment, cumulative belowground, foliar, and total aboveground biomass increased in both elevated [CO2] treatments. After 2 years of growth at elevated [CO2], early season stand respiration was decoupled from CO2 influx aboveground, presumably because of accelerated fine root production from stored carbohydrates in the coppiced system prior to canopy development and to the increased soil carbohydrate status under elevated [CO2] treatments. Soil respiration was stimulated by elevated [CO2] whether measured at the system level in the undisturbed soil block, by soil collars in situ, or by substrate-induced respiration in vitro. Elevated [CO2] accelerated depletion of soil nutrients, phosphorus, calcium and potassium, after 3 years of growth, litter removal, and coppicing, especially in the upper soil profile, although total N showed no change. Enhancement of above- and belowground biomass production by elevated [CO2] accelerated carbon cycling through the coppiced system and did not sequester additional carbon in the soil.
Introduction
The partitioning of ecosystem respiration between above- and belowground components of the vegetation, and the effects of elevated atmospheric [CO2] on soil respiration and carbon storage are poorly understood (Norby et al., 1999). Progress has been made in some areas of fundamental importance. For example, there has been a long-standing discussion of direct and indirect effects of elevated [CO2] on respiratory activities in plants and ecosystems. We now know that many leaf-level studies purporting to show immediate inhibitory effects of elevated [CO2] on plant respiration were confounded by artifacts (Amthor et al., 2001; Jahnke, 2001). Although now it seems we can be confident that growth in elevated [CO2] stimulates leaf respiration on leaf area or leaf mass bases (Davey et al., 2004), at the system level we still do not understand the implications for respiration in other plant organs, especially in terms of respiration of fine roots and soil respiration (Wang et al., 1998). Clearly, a better understanding of system-level responses of forests to elevated [CO2] will make an important contribution to improved models of global climate change.
Most tree species have shown a positive response to elevated [CO2], in terms of aboveground biomass production (Norby et al., 1999; Sigurdsson et al., 2001; DeLucia et al., 2002; Dilustro et al., 2002; Marissink et al., 2002) and belowground biomass production (Norby, 1994; Hungate et al., 1997; DeLucia et al., 1999; Pregitzer et al., 2000; Martin-Olmedo et al., 2002). Fewer studies, however, have examined the longer-term effects of elevated [CO2] on plant biomass production and the influence that might have on belowground processes and feedback responses (i.e. root exudation, mineralization, and depletion of soil nutrients; Ceulemans et al., 1999).
Plant and ecosystem responses to elevated [CO2] concentrations are variable and sometimes transitory (Rogers & Dahlman, 1993), in part because factors other than carbon acquisition may limit plant production and system-level responses. For example, tree growth at elevated [CO2] can be increased by inputs of mineral fertilizer (Conroy et al., 1992), and soil fertility constrains C sequestration in forests exposed to elevated [CO2] in FACE experiments (Oren et al., 2001). Previous studies of soil respiration response to elevated [CO2] levels provide many examples of variable results. Some experiments have shown increased soil respiration under elevated atmospheric [CO2] (Johnson et al., 1994; Vose et al., 1995; Hungate et al., 1997) and others have shown no effect or variable responses (Prior et al., 1997; Johnson et al., 2001). Discrepancies between these studies have been attributed to variations in the effects of elevated [CO2] on N uptake, microbial activities, root allocation patterns or soluble organic C. Some plants respond to increased [CO2] by increasing labile C in the rhizosphere (Zak et al., 1993). Large-scale girdling experiments in forests have shown a 37% decrease in soil respiration within 5 days, suggesting that soil respiration may be driven by rhizodeposition of current photosynthesis (Högberg et al., 2001).
Because forests have been estimated to contain up to 80% of all aboveground C and approximately 40% of all belowground (i.e. soils, roots, and litter) terrestrial organic C (Dixon et al., 1994), the influence of elevated CO2 on biomass production and the resulting effects on soil properties are extremely important. An increase in biomass production could lead to increased root exudates in the form of labile C. Increased C exudates could lead to increased microbial mineralization and a resulting depletion in soil nutrients. The interactive effects of these processes could potentially increase soil respiration and limit net carbon gain and productivity of a stand.
Elevated [CO2] studies have also uncovered variable effects on soil nutrient availability and N mineralization. Experiments have shown no change or variable results (Johnson et al., 2001; Martin-Olmedo et al., 2002), an increase (Hungate et al., 1997; Prior et al., 1997), or a decrease (Berntson & Bazzaz, 1997) in total N concentration because of growth under elevated [CO2]. Similar discrepancies exist in terms of the availability of other nutrients (i.e., phosphorous, potassium). Many studies have also shown a variety of responses in N mineralization to elevated [CO2], ranging from a variable response or no change (Prior et al., 1997; Martin-Olmedo et al., 2002) to an increase (Billes et al., 1993; Zak et al., 1993) or a decrease (Hungate et al., 1997).
In a review of root and soil responses to elevated [CO2], Norby (1994) emphasized nutrient acquisition, roots as components of soil carbon storage, and the root turnover component of system respiration as three important issues for future research. We used a continual litter removal and annual coppicing protocol to monitor biomass and to simplify the interpretation of system respiratory components in a Populus deltoides agriforest. We report here the results of stand-level, controlled environment experiments that took advantage of the uniquely large, semiclosed facility of B2L, which enabled gas exchange measurements at a near ecosystem scale to address these issues. We controlled the concentration and isotopic composition of injected CO2, temperature, precipitation, and humidity. The rates of change in system CO2 exchange were measured with precision during drawdown following brief periods of closure. In contrast, respiratory measurements in flux-tower studies are limited to periods of air turbulence, and less easily monitored with precision throughout the night. Experimental conditions were replicated in time over 4 years. Thus, the enclosed P. deltoides stands could be reproducibly and comprehensively examined at different scales in space and time under elevated and ambient [CO2], controlled temperature, and precipitation. For example, soil respiration was measured in substrate saturated laboratory experiments, in situ using soil collars, and in the 500 m−3 soil bed during system closure. Data presented here also serve as a point of reference for a range of other controlled environment experiments, including soil and atmospheric water stress (Murthy et al., 2005), plant isoprene emission and soil isoprene uptake (Rosenstiel et al., 2003; Pegoraro et al., 2005a, b), and soil microbial ecology (Lipson et al., 2005), conducted from time to time in the agriforest mesocosm.
Materials and methods
Site and stand characteristics
This experiment was conducted at the B2L located in Oracle, Arizona, USA, using the Intensively managed Forest Mesocosm (IFM). The 2000 m2 footprint IFM is divided roughly equally into three individual bays (sections) separated by thick translucent plastic curtains, with growing areas of 34 × 18 m2 in a north–south orientation, a maximum height of 24 m, a soil volume of 550 m3, and an estimated total air volume of 11 873 m3. Further details on climate control and the structure of the IFM can be found elsewhere (Lin et al., 1998; Dempster, 1999; Marino & Odum, 1999; Zabel et al., 1999). Cuttings of an eastern cottonwood (P. deltoides Bartr.) clone were planted in May 1998. At the end of each growing season, air temperature was lowered to 15–20°C (day)/7–12°C (night) to send the trees into dormancy. Each January or February (2000–2002), the main stem of each tree was coppiced to 30 cm above the soil. Each spring, air temperature was increased to approximately 25/20°C (day/night) to allow the trees to begin resprouting, and by May or June all trees were pruned to have a single leader. The stands were not coppiced in 2003 in an effort to accelerate canopy development for a range of other experimental observations. All leaf litter was removed from the mesocosm each month, and fallen biomass resulting from coppicing was removed at the end of the growing season. This 4-year study began with newly coppiced trees sprouting in May 2000 when CO2 treatments were implemented. Trees were harvested from time to time in connection with other studies in the IFM; the number of trees and cuttings remaining at the start of each growing season is shown in Table 1. Details of other experiments conducted on these stands can be found elsewhere (Griffin et al., 2002a, b; Murthy et al., 2003; Rosenstiel et al., 2003).
| CO2 growth concentration | ||||||
|---|---|---|---|---|---|---|
| 400 | 800 | 1200 | 400 | 800 | 1200 | |
| Beginning | End | |||||
| Trees | ||||||
| 2000 | 35 | 31 | 34 | 35 | 31 | 34 |
| 2001 | 35 | 31 | 34 | 35 | 31 | 34 |
| 2002 | 29 | 25 | 28 | 25 | 22 | 25 |
| 2003 | 25 | 22 | 25 | 25 | 22 | 25 |
| Cuttings | ||||||
| 2000 | 13 | 13 | 12 | 13 | 12 | 12 |
| 2001 | 13 | 12 | 12 | 13 | 12 | 12 |
| 2002 | 13 | 12 | 12 | 6 | 7 | 7 |
| 2003 | 6 | 7 | 7 | 6 | 7 | 7 |
- ‘Trees’ refer to the Populus deltiodes trees originally planted in 1998 that remained after thinning in 2000, and ‘cuttings’ refer to P. deltiodes replacement cuttings planted in 2000.
- IFM, intensively managed forest mesocosm.
Soil depth throughout the stands averages 1 m. The constructed soil, approximating a rich agricultural loam, contains approximately 30% sand, 36% silt, and 24% clay (Tolbert & Johnson 2001). The soil profile has evolved in place over 12 years and now shows normal bulk density distribution with depth and N concentration of 2–3 mg g−1 (Table 2) with a C/N ratio of about 10 (Table 3). Some other nutrient profiles are described below. The C/N ratio of the soil within the IFM is approximately that of the San Pedro River Riparian area in southeastern Arizona (Martens & McLain, 2003). In fact, the soil in the B2L had been cropped for the previous 7 years, and also resembles some old-field soils commonly used to establish agro-forest systems in southeast USA. The soil microflora was examined in detail and found to be typical of agricultural and natural soils (Lipson et al., 2005).
| Depth (cm) | CO2 growth concentration (ppm) | ||
|---|---|---|---|
| 400 | 800 | 1200 | |
| Bulk density (g cm−3) | |||
| 0–25 | 0.91 | 0.97 | 0.95 |
| 25–50 | 1.06 | 1.11 | 1.11 |
| 50–75 | 1.16 | 1.24 | 1.23 |
| 75–100 | 1.22 | 1.27 | 1.24 |
| Total N (mg kg−3) | |||
| 0–25 | 2002 (90) | 1824 (77) | 1937 (100) |
| 25–50 | 1857 (71) | 1902 (67) | 1784 (81) |
| 50–75 | 1637 (69) | 1756 (84) | 1577 (112) |
| 75–100 | 1583 (80) | 1912 (70) | 1815 (124) |
- Values given are averages (with standard deviations in parentheses) of 12 samples collected at each of four depths throughout the soil profile.
- IFM, intensively managed forest mesocosm.
| 1995 | 1999 | 2000 | 2001 | 2002 | 2003 | |
|---|---|---|---|---|---|---|
| Organic carbon (mg g−1) | 29.0 (0.6) | 24.8 (0.6) | 29.0 (0.5) | 25.4 (0.4) | ND | 20.0 (0.3) |
| Carbohydrates (mg g−1) | 5.3 (1.1) | 4.3 (0.9) | 4.3 (1.4) | 4.1 (0.9) | 3.5 (1.0) | 3.5 (0.3) |
| Organic nitrogen (mg g−1) | 3.0 (0.3) | 2.1 (0.2) | 2.1 (0.5) | 3.2 (1.0) | nd | 2.3 (0.2) |
| Amino acids (mg g−1) | 1.8 (0.4) | 1.7 (0.3) | nd | nd | nd | nd |
| C/N | 9.67 | 11.8 | 13.8 | 7.94 | nd | 8.7 |
- Standard deviations are given in parentheses.
- nd, not determined
Experimental protocol
Throughout each growing season, the bays were operated in a closed state during the day and an open flow mode during the night. In this way, it proved possible to maintain ambient (∼400 ppm) or elevated (800 and 1200 ppm) [CO2] in the day by CO2 injection, and at night by managing fan speeds to stabilize respiratory CO2 build up. The three [CO2] treatments were maintained throughout the year, not solely during the most active portion of the growing seasons. After leaf fall and coppicing at the conclusion of each growth season, the bays were maintained in open flow mode and the different [CO2] treatments were maintained by manipulating fan speeds. On-demand drip irrigation, automatically connected to soil moisture probes, was used during 2000–2001. Unfortunately, very uneven soil moisture patterns developed in 2001, exposing stands to unpredictable soil moisture stress that had strong effects on CO2 uptake. Stand CO2 uptake and respiration data from 2000 and 2001 are not presented here. Weekly overhead irrigation was introduced throughout the growing seasons of 2002–2003 to simulate an 18.6 mm rainfall on each occasion.
Short-term experimental perturbations of [CO2], temperature, and drought (end of growing season 2002, start of growing season 2003) were applied in the course of other studies in the IFM, but all system-level measurements reported here are the average of at least 5 days of measurements representative of the monthly steady states for the years in question. Temperature was controlled throughout the growing seasons to match temperatures experienced by eastern cottonwoods in their natural environments. Figure 1 shows the steady day/night temperature regimes in growing seasons 2002 and 2003. The growing season in 2002 began late because coppicing had been delayed until February, and budburst was delayed until testing of the overhead irrigation system had been completed. Stands were not coppiced in winter 2002–2003, facilitating an early growth season, which was brought to an early end to accommodate closure of the facility in December of 2003. Overall, steady day/night temperature regimes were maintained for 5-month growing seasons June–October 2002 and May–September 2003. Relative humidity, temperature, atmospheric vapor pressure deficit, and PFD were measured at three canopy heights at four locations within the stand every 15 s, averaged, and stored every 15 min for each measurement point using data loggers (Campbell-CR10x, Campbell Scientific Inc., Logan, UT, USA). Volumetric soil moisture was monitored continuously using 12 probes (CS615, Campbell Scientific Inc.) that had been installed at three depths at four locations throughout the stand.
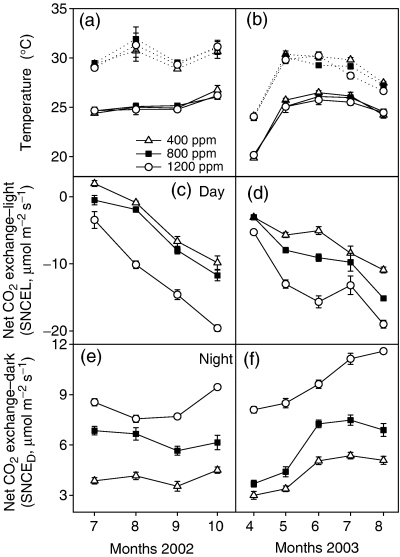
Average monthly daytime and night-time temperatures (°C) throughout (a) 2002 and (b) 2003 growing seasons; average monthly whole, closed-system net CO2 exchange in the light (SNCEL, μmol m−2 s−1) throughout the (c) 2002 and (d) 2003, and average monthly closed-system net CO2 exchange in the dark (SNCED, μmol m−2 s−1) throughout (e) 2002 and (f) 2003 growing seasons for a stand of Populus deltoides grown under ambient (400 ppm) and elevated (800 and 1200 ppm) atmospheric [CO2]. Data are treatment means (n=5) of five representative days/month, and vertical bars represent the standard error of the mean. SNCE, system net CO2 exchange.
Continuous measures of stand gas exchange
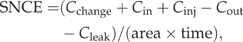
Leaks between the bays and to the outside environment were calculated monthly by tracking the dilution of an injected trace gas sulfahexafluroride (SF6), and system [CO2] was corrected based on the leak rate.
Spot measurements of soil respiration
Soil respiration was measured monthly at 28 locations within each [CO2] treatment area (bay) using a closed-chamber gas exchange system (LiCor 6200, Li-Cor Inc., Lincoln, NE, USA). The 28 PVC rings were installed at a uniform depth (10 cm) in the soil and oriented in two ‘L’ formations in each bay. Seven rings made up either a north–south or east–west gradient between two adjacent trees to reveal variability because of distance from the stem. [CO2] was recorded every 5 s, and CO2 efflux was calculated from a linear regression of increasing [CO2] in the chamber with time. Measurements were conducted between 10:00 and 16:00 hours. Preliminary studies did not reveal any detectable pattern of diurnal CO2 efflux. In an additional subexperiment in 2002, three 1 m diameter, 1 m deep cylindrical pits were dug within each bay. Roots were removed by passing all the excavated soil through a 1 mm sieve. The nine pits were lined with a strong plastic barrier, refilled with the homogeneously mixed soil, and planted with a single cutting of P. deltoides. Each pit was fitted with a vertical 1 m × 7.5 cm diameter PVC pipe filled with the same soil as the rest of the pit to allow measurements of soil respiration without the influence of roots. Other 7.5 cm PVC collars were installed at the soil surface to allow for measurement of soil respiration in the pits, including roots and exudates from the P. deltoides cutting as it grew in the three different [CO2]. Measurements were conducted between 10:00 and 16:00 hours, and diurnal patterns of CO2 efflux were calculated as above using the same closed-chamber gas exchange system. Additional measurements of soil respiration were made on soils collected in October 2002 under average growing season temperatures and volumetric soil water contents. Substrate-induced respiration (SIR) experiments using glucose were conducted as described in Lipson et al. (1999).
Stand biomass
The stands developed a leaf area index of 3–5 by the end of the growing season 2000 (Murthy et al., 2005), with elevated [CO2] yielding the highest values. Aboveground biomass was determined by coppicing the trees at the end of each growing season. Fresh- and dry-weight biomass values of foliage, branches, and stems were obtained separately for all trees within each of the three stands. Belowground biomass was determined in 2002 by conducting a full-tree harvest on a subset of trees within each bay at the beginning and end of the growing season (six trees in April and three in November). The aboveground portion of the tree was cut, and all root biomass was collected to a 1 m depth and within a 1.5 m radial area around the stem. Roots were sieved into >20, 2–20, and <2 mm fractions, and fresh- and dry-weight obtained.
Soil nutrients and carbon fractions
A soil probe (AMS Inc., American Falls, ID, USA) was used to collect soil samples at 0–25, 25–50, 50–75, and 75–100 cm depths (n=12 for each depth for each bay) at monthly intervals during 2000–2002, and biweekly in 2003. Each soil sample was homogenized, split in half, either air- or ovendried, and then ground. Soil total N concentration was determined on oven-dried samples by micro-Dumas combustion (NA1500 C/H/N Analyser, Carlo-Erba, Milan, Italy). Soil C content was determined by dry combustion interfaced with a Europa Hydra 20/20 isotope ratio mass spectrometer (Europea Scientific, Crewe, UK). Air-dried samples were treated via double acid extraction (Mehlich No.1) as described by Mehlich (1953) and Jones (1990) for an extensive macro- and micro-nutrient analysis, and analyzed using an inductively coupled argon plasma emission spectrometer (ICP; Thermo Jarrell-Ash 965, Franklin, MA, USA) at the Chemical Analysis Laboratory, University of Georgia. Further details on the soil collection and extraction methodology are given in Kudeyarov et al. (2002).
Carbohydrate determination
Total carbohydrate content of the soil was measured on H2SO4 extractions coupled with ion chromatography and pulsed amperometric detection of individual monosaccharides (Martens & Frankenberger, 1990). The protocol estimated total soil carbohydrate fractions, either released as exudates or accumulated as cell debris, which could serve as substrates for soil microbial respiration. Soil adjacent to two trees from each bay, chosen at random during the years 2000–2003, was sampled at 0–20, 20–40, and 40–60 cm depths. Soil samples (100 mg; air-dried) from the different years were sieved to pass a 2 mm and then a 1 mm sieve so all visible root material was removed before treatment with 800 μL 6 m H2SO4 for 30 min and digestion in an autoclave (1 m H2SO4, 30 min) to release hemicellulose monosaccharides (Martens & Loeffelmann, 2002). An aliquot of neutralized extract (pH 4.0–5.0) was diluted to 10 mL and injected via a 25 μL injection loop, separated by a CarboPac PA-10 (Dionex Corp., Sunnyvale, CA, USA) and detected with an ED 40 (Dionex Corp.) set to the integrated amperometry mode. Data are presented as percentage change in total C or carbohydrate concentration each year compared with the carbohydrate values in soils collected in 1999 before initiation of the elevated CO2 study.
Statistical analysis
Data comparison of whole-system net CO2 uptake and efflux, soil respiration, cumulative biomass production, and soil nutrient depletion between [CO2] treatments were performed using a two-factor analysis of variance (anova) for repeated measures within the SAS statistical software package (1996; SAS Institute Inc., Cary, NC, USA). Within-treatment comparisons over time was performed using a single-factor anova for repeated measures.
Results
System carbon exchange
The seasonal patterns of canopy development in response to elevated [CO2] are evident in the average monthly rates of maximum stand net CO2 exchange in the light (SNCEL, 12:00–14:00 hours) and in the dark (SNCED; Fig. 1). Throughout the 2002 and 2003 growing seasons, the 800 and 1200 ppm treatments were taking up an average of 21% and 83%, respectively, more CO2 than the ambient 400 ppm treatment, and the 1200 ppm treatment was taking up 51% more CO2 than the 800 ppm treatment (Fig. 1c, d). Throughout these same growing seasons, SNCED was clearly greater in elevated [CO2] treatments (Fig. 1e, f). Comparisons of the seasonal courses of SNCEL and SNCED in 2002 and 2003 suggest that system CO2 efflux in the dark was accelerated well before the full canopy developed, and the implications of this are discussed below.
In 2002, we compared whole-system net CO2 exchange in the dark (respiration) with independent spot measurements of soil respiration in situ, and with SIR of soil samples taken to the laboratory. Averaged over the whole growing season in 2002, SNCED values from the 800 and 1200 ppm treatment areas were on average 45% and 102% greater, respectively, than in the ambient treatment, and the 1200 ppm was an average of 39% greater than the 800 ppm treatment (Fig. 2a). As observed previously (Murthy et al., 2003), whole system net CO2 exchange rates (Fig. 3a) were lower than spot soil respiration measurements (Fig. 3b). In situ soil respiration measurements from soil collars were significantly greater in the elevated [CO2] treatments (Fig 2b; P<0.001), and substrate (glucose)-induced soil respiration in vitro was also significantly greater in these treatments (Fig. 3c; P=0.403). Clearly, 3 years of growth under elevated [CO2] resulted in significantly increased belowground respiration, as indicated by all three methods of measurement. Averages of belowground respiration was 42% and 80% greater in the 800 and 1200 ppm treatments, respectively, compared with the 400 ppm treatment, and the 1200 ppm treatment was 26.9% greater than the 800 ppm treatment.

Average (a) closed-system net CO2 efflux in the dark (SNCED; μmol m−2 s−1), (b) spot measurements of soil respiration (μmol m−2 s−1) using portable gas exchange equipment (LiCor), and (c) substrate-induced respiration (μg C g soil−1 h−1) for a soil block on which a stand of Populus deltoides have been grown under ambient (400 ppm) and elevated (800 and 1200 ppm) [CO2] for 4 years. Data are treatment means for the 2003 growing season, and vertical bars represent the standard error of the mean. SNCE, system net CO2 exchange.

Average soil respiration (μmol m−2 s−1) for a smaller block of soil (1 m diameter circle, 1 m deep) of a single eastern cottonwood after 1 year of growth under ambient (400 ppm) or elevated (800 and 1200 ppm) [CO2]. Without-roots measurements represent soil respiration alone, while with-roots measurements represent soil+root respiration. Vertical bars represent the standard error of the mean.
Data from the subexperiment in which a single cutting was grown in homogeneously mixed soil in 2002 illustrate that elevated [CO2] increased belowground respiration after only a single year of growth (Fig. 3). There was no significant change of respiration in the soil cylinders from which roots and exudates were excluded. This experiment confirmed the stimulation of belowground respiration by P. deltoides exposed to elevated atmospheric [CO2] that had been observed by Murthy et al. (2003, 2005) during growing season 2000.
Stand biomass
It is difficult to partition the stimulation of stand-level respiration between the contributions of increased above-, belowground biomass, and soil microbial metabolism. Elevated [CO2] resulted in increased cumulative root, foliar, and total aboveground biomass and by the end of the 4-year experiment, the 800 and 1200 ppm [CO2] treatments produced 27% and 28% more cumulative foliar and total aboveground biomass, respectively, than the 400 ppm. Despite the variable bacterial community composition, heterotrophic biomass and potential activity (measured by glucose SIR) also increased with increasing [CO2] (Lipson et al., 2005).
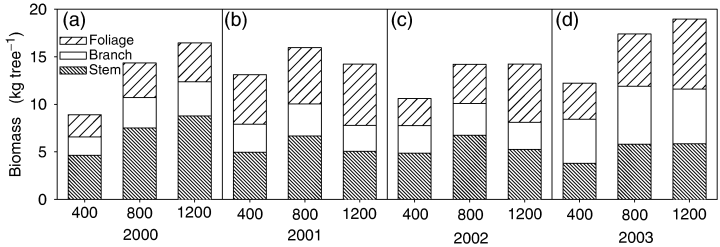
Trees in the 800 and 1200 ppm [CO2] treatments produced more cumulative stem, branch, and foliar biomass than the 400 ppm control treatment in each of the 4 years of growth (Fig. 4). In the ambient [CO2] treatment, stem and branch biomass production in the coppiced plantation increased each year. Aboveground biomass production in the 800 ppm [CO2] treatment always exceeds that of the ambient treatment. Failure of the on-demand drip irrigation presumably contributed to the lower aboveground biomass of the 1200 ppm treatment (compared with 800 ppm) in 2001 and 2002 (Fig. 4b, c). Specific leaf area decreased with height in the canopy, with SLA averaging 16% and 23% lower within middle and upper canopies, respectively, than the lower canopy (Fig. 5) and was lower in trees grown in elevated [CO2] than in ambient [CO2].
Average annual stem, branch, and foliar biomass production per tree within each of the ambient and elevated (800 and 1200 ppm) [CO2] treatments after the (a) 2000, (b) 2001, (c) 2002, and (d) 2003 growing seasons. Vertical bars represent the standard error of the mean.
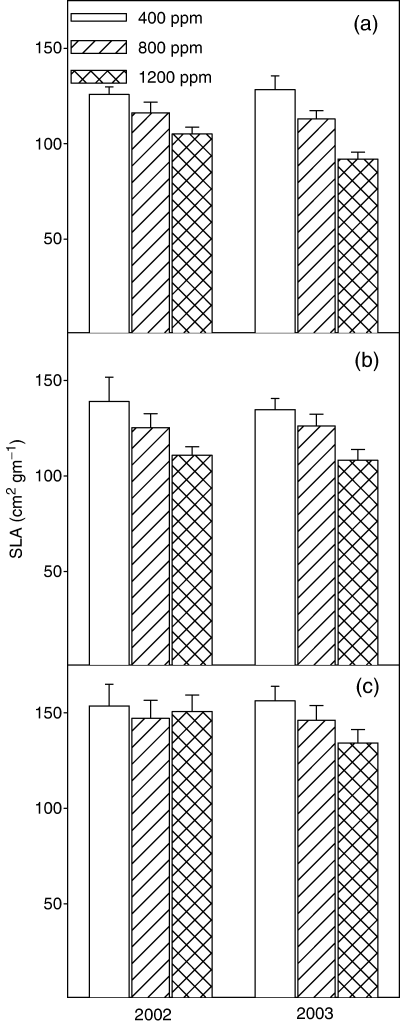
Average specific leaf area (SLA; cm2 g−1) for the 2002 and 2003 growing seasons within the (a) upper, (b) middle, and (c) lower canopy of a stand of eastern cottonwoods growing under ambient (400 ppm) and elevated (800 and 1200 ppm) [CO2]. Vertical bars represent the standard error of the mean.
Soil organic carbon fractions
Total soil organic carbon tended to decline throughout the experiment in all [CO2] treatments, continuing the trend observed in the mesocosm prior to the application of elevated [CO2] treatments (Table 3, Fig. 6). The 1200 ppm [CO2] treatment showed the least amount of soil organic C loss. A slight increase in soil organic C was noted in the 400 ppm bay in 2000 and in the 1200 ppm [CO2] treatment (top 40 cm) in 2000 and 2001 (Fig. 6a, b). The rate of decline in soil carbon presumably reflects the balance between losses because of belowground respiration and inputs in the form of belowground biomass (fine root fractions) and rhizodeposition of exudates. The soil carbohydrate pool was usually 16–18% of total soil organic C, and in the 400 ppm treatment soil carbohydrates declined throughout the 4-year experiment, at all levels in the profile (Fig. 6). In contrast, both elevated [CO2] treatments tended to show an increase in soil carbohydrates, especially in the first 2 years of the experiment. Compared with the levels prior to elevation of [CO2], the 800 ppm treatment had higher soil carbohydrate concentrations at all depths during 2000 and 2001, but lost carbohydrates during 2002 and 2003. The 1200 ppm [CO2] treatment also showed increased carbohydrate concentrations from 2000 to 2002, but lost carbohydrates in 2003.
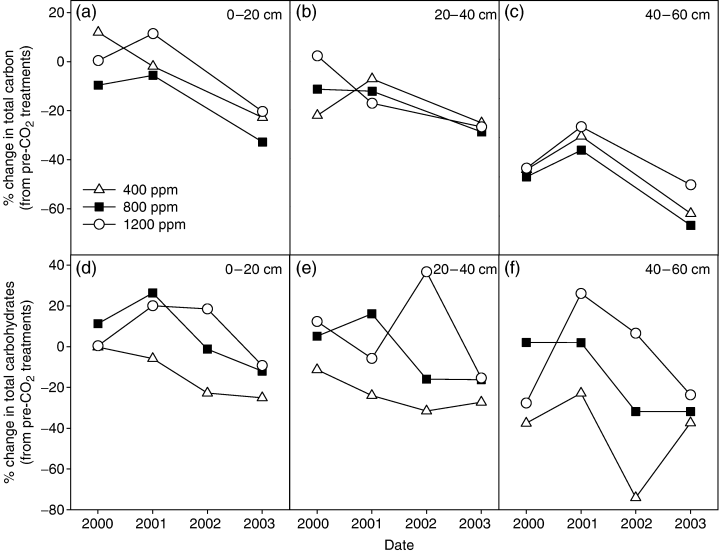
Average percent change in total soil carbon concentrations with time (from pre-CO2 treatments, 1999) within the (a) 0–20, (b) 20–40, and (c) 4–60 cm depths, and average percent change in total soil carbohydrate concentrations within the (d) 0–20, (e) 20–40, and (f) 4–60 cm depths, for a stand of coppiced eastern cottonwoods growing under ambient (400 ppm) and elevated (800 and 1200 ppm) CO2 for 4 years. Vertical bars represent the standard error of the mean.
Effects of [CO2] enrichment on root distribution and changes in soil nutrient capital
Most root biomass was found in the upper 25 cm of the soil profile (Fig. 7a; P<0.001), but there was no significant effect of [CO2] treatment on root biomass distribution. The greatest cumulative root biomass production from 2000 to 2002 was in the >20 mm size class. Trees in the 800 and 1200 ppm [CO2] treatments produced an average of 27% and 37%, respectively, more cumulative root biomass per tree than the 400 ppm control treatment after 3 years of growth, and the 1200 ppm [CO2] treatment produced 14% more belowground biomass per tree than the 800 ppm treatment (Fig. 7a). Although only a small proportion of total root biomass was in the 0–2 mm size class, this also increased 48–68% in response after 2 years growth in elevated [CO2] (Table 4). Whereas there was little change in the >20 mm size class between the root systems of coppiced trees before budburst and at the end of 2002, fine root biomass increased by 67–86% across all treatments, and at the end of this growing season the 1200 ppm treatment contained 60% more fine roots than the ambient [CO2] treatment. The 2–20 mm size class lost biomass in the 400 and 800 ppm treatments but was unchanged in the 1200 ppm.
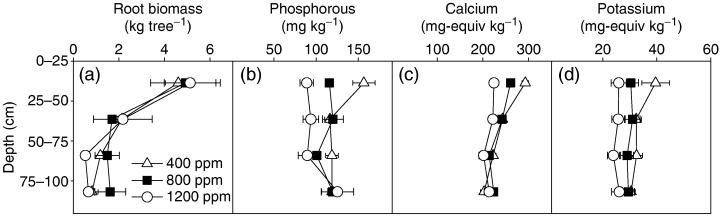
(a) Cumulative root biomass per tree by depth through the soil profile of a stand of coppiced eastern cottonwoods growing under ambient (400 ppm) and elevated (800 and 1200 ppm) [CO2] for 4 years. Average (b) phosphorous (mg kg−1), (c) calcium (mg equiv kg−1), and (d) potassium (mg equiv kg−1) concentrations within the soil block below the Populus deltoides. Data are means (n=12) of samples from the 2002 growing season, and vertical bars represent the standard error of the mean.
| Treatment; root size class | Root dry weight (kg tree−1) | |||||
|---|---|---|---|---|---|---|
| 400 ppm | 800 ppm | 1200 ppm | ||||
| Coppiced (April 2002) | Full canopy (November 2002) | Coppiced (April 2002) | Full canopy (November 2002) | Coppiced (April 2002) | Full canopy (November 2002) | |
| <2 mm | 0.52±0.15 | 0.97±0.21 | 0.77±0.24 | 1.29±0.22 | 0.87±0.35 | 1.55±0.27 |
| 2–20 mm | 1.87±0.38 | 0.81±0.09 | 2.18±0.51 | 1.38±0.24 | 1.46±0.20 | 1.43±0.20 |
| >20 mm | 4.89±0.55 | 4.48±0.28 | 6.68±0.62 | 6.20±2.05 | 7.21±0.37 | 8.22±1.87 |
- Soil was excavated for a 1.5 m radius around each of six coppiced trees in each treatment at the beginning of the growing season (April 2002) and each of three trees with full canopy at the end of the growing season.
In the coppiced stands annual removal of aboveground biomass, stimulation of plant growth in elevated [CO2] led to a marked depletion of soluble soil P, Ca2+ and K+ (Fig. 7b, c). The decrease in nutrient concentration was greatest in the upper 25 cm of the soil profile, where most of the fine root biomass was located (Fig. 7a). Concentrations of P in the 0–25 cm depth zone were 26% and 46% lower in the 800 and 1200 ppm treatments, respectively, than in the 400 ppm bay. In the 0–25 cm zone, concentrations of Ca2+ in the 800 and 1200 ppm treatments were 11% and 24% more depleted than in the 400 ppm, and K+ concentrations were 23% and 35% lower in the 800 and 1200 ppm treatments, respectively. Similar effects of elevated [CO2] on the distribution Mg2+, Na+, and NO3− in the upper profile were observed, whereas NH4+, total N, and total C profiles were unchanged (Kudeyarov et al., 2002).
Discussion
This study of coppiced plantations of the fast growing agriforestry tree P. deltoides examined the longer-term effects of elevated [CO2] on stand net CO2 exchange in the light and dark under controlled conditions. Above- and belowground plant biomass production was also measured, and the consequences for belowground processes such as root exudation, mineralization, and depletion of soil nutrients were assessed. The study is distinctive because it was done using stands of trees grown in nutrient-rich soil in a closeable flow-through system, which permitted direct and precise estimates of net ecosystem CO2 exchange; especially of system respiratory efflux at night. Four years of growth under elevated [CO2] increased stand net CO2 exchange in the light (SNCEL) and increased biomass production above- and belowground. Growth in elevated [CO2] also increased system CO2 exchange in the dark (SNCED), increased soil respiration and increased soil carbohydrate pools. Aboveground biomass was removed from the coppiced system in the first 2 years of the experiment, leading to faster depletion of soil concentrations of P, K+, and Ca2+ in the elevated [CO2] treatments.
This extended study confirms early indications that elevated [CO2] stimulated both the influx and efflux of CO2 in the P. deltoides stands established in B2L. After one season of growth (1999) at ambient CO2, Murthy et al. (2003) found no differences in system respiratory CO2 efflux from the three coppiced P. deltoides stands used in this study, yet by the end of the first season of growth under elevated [CO2], SNCED was much greater in the 1200 ppm treatment, due in part to the increased leaf area and biomass that developed under elevated [CO2] (Murthy et al., 2005). Our experiments show that increased SNCED in elevated [CO2] continued throughout 2001–2002, and in 2003 (with and without coppicing, respectively). In general, results from the 4-year enclosed agriforest system in B2L resembled those from other studies that reported increased soil respiration following growth under elevated atmospheric [CO2] (Johnson et al., 1994; Vose et al., 1995; Hungate et al., 1997).
The partitioning of the stimulation of stand respiration among component processes is not straightforward. If aboveground biomass dominated SNCED, one would expect a close relationship between SNCED and SNCEL throughout the growing season. However, it is clear that in 2002 and 2003, SNCED became uncoupled from SNCEL, implying that that the contributions of different component respiring systems varied throughout the growing season. With day and night temperatures and irrigation under control early in the 2002 growing season, SNCED at elevated [CO2] was two- to threefold higher than ambient [CO2], well before maximum canopy and SNCEL were attained (Fig. 1c, d). There was little subsequent change in SNCED in spite of the five- to 10-fold increase in SNCEL throughout the rest of the season. Belowground respiration showed the same response to elevated [CO2] when assessed in situ and in vitro in August 2002 (Fig. 3). Similar results were obtained in the absence of coppicing in May 2003, when SNCED in the 1200 ppm treatment was two- to threefold higher than that in ambient [CO2] while SNCEL was low and similar in all treatments. As the season progressed, SNCED in the 1200 ppm treatment increased by about 40% while SNCEL increased three- to fourfold. Canopy expansion and increased aboveground biomass in 800 and 1200 ppm treatments presumably contributed to increases in stand respiration as the season progressed. Enhanced production of leaves with a smaller SLA (evidently reflecting the higher starch content; Walter et al., 2005) was presumably responsible for enhanced canopy respiration (Davey et al., 2004).
The stand-level data suggest that soil warming to release dormancy stimulated belowground respiration early in the season either because of the production of strongly respiring fine roots or the acceleration of microbial metabolism of previously accumulated, readily respired C, or both. The role of accelerated production of new, carbohydrate rich, roots at elevated [CO2], and of C exudates from these roots, in accelerating soil respiration was clearly evident in the subexperiment conducted the same year (Fig. 4). Soil respiration was stimulated some 40–60% by growth of a single cutting of P. deltoides at elevated [CO2] in about 0.8 m3 of soil. Only a small proportion of belowground biomass was associated with fine roots (<2 mm) in the P. deltoides stands, but these showed a consistent 48% and 68% increase in biomass under 800 and 1200 ppm [CO2], respectively, after 2 years growth (Table 4). Thus, early in the growing season, the P. deltoides stands may resemble the ponderosa pine system investigated by Johnson et al. (1994), in which fine root respiration made a strong contribution to soil CO2 efflux under elevated [CO2]. Unlike the ponderosa pine system, root free soil from the P. deltoides stands at elevated [CO2] also showed clearly higher microbial activity.
There were clear increases in the fine root fraction during the growing season (Table 4). Fine root production is particularly strongly stimulated (60–240%) in other forest trees after exposure to elevated [CO2] (reviewed by Norby et al., 1999; Matamala & Schlesinger, 2003). More detailed analyses by Trueman & Gonzalez-Meler (2005) of the soil samples taken in the present experiments show that stimulation of fine root biomass production by elevated [CO2] at the start of each growing season was twofold greater than the residual coarse root content of the soil. These fine roots were lost on coppicing, and presumably the carbon of the decaying roots contributed to the high rates of system respiration in these treatments after warming, but before the canopy developed in the subsequent growing season (Fig. 1).
The importance of soil exudates as a component of ecosystem belowground respiration was demonstrated in the large-scale girdling experiments by Högberg et al. (2001). Our attempts to girdle P. deltoides at the time of coppicing were unsuccessful. However, a contribution of carbon exudates from roots to SNCED is suggested by the dynamics of soil carbohydrate pools. These increased in most seasons under elevated [CO2] but not in ambient [CO2] (Fig. 6). The increase in soil respiration followed a trend of higher rhizosphere carbohydrate concentrations in the upper two depths (0–20 and 20–40 cm; Fig. 6a–c) during the first 2 years, and is consistent with the majority of root biomass in this part of the profile. The lower carbohydrate levels found in the 40–60 cm depth reflect the fact that roots were not abundant at this depth. The rapid loss of soil C (0–60 cm) from the 400 ppm treatment suggests that the presence of living roots and exudates had a priming effect on decomposition of existing soil C pools. Elevated [CO2] increased soil C loss under the 800 ppm treatment, the ambient treatment was intermediate in C loss, and the 1200 ppm treatment had the highest soil C remaining over the 4-year experiment (compared with 1999 pre-CO2 treatment). It appears that the extra C, released by the 800 ppm treatment, may have increased the mineralization of the native soil C, while the higher carbohydrate release by the 1200 ppm treatment may have helped offset the higher C mineralization rates. The intimate contact of belowground organic carbon with soil minerals may have physically protected these relatively labile exudates from decomposition to some extent (Christensen, 1996; Jastrow & Miller, 1998). However, other studies of the stable isotope composition of respired soil carbon during our experiments confirmed that elevated [CO2] not only made more contemporary carbon available to the soil microbial flora, but also stimulated the respiration of old carbon in the soil (Trueman & Gonzalez-Meler, 2005). This is in contrast to studies of grasslands exposed to elevated [CO2] in which decomposition of old soil carbon was retarded as soil microorganisms switched to easily metabolized rhizodeposits from current photosynthesis (Hungate et al., 1997; Cardon et al., 2001).
Soil from the elevated [CO2] treatments showed higher SIR (glucose) and higher microbial metabolic capacity. Lipson et al. (2005) found that soil heterotrophic biomass and potential activity (measured by glucose SIR) increased linearly with increasing [CO2] in the IFM cottonwood stands, and specific glucose respiration (glucose SIR per unit microbial biomass C) was highest in the 1200 ppm treatment. In contrast, the SIR of other substrates responded nonlinearly to [CO2] and was significantly correlated to the abundance of the most frequent bacterial taxa in the soil. Increases in respiration of root free soil from elevated [CO2] treatments can be attributed to changes in the abundance of these major bacterial taxa.
Overall, total soil organic carbon declined throughout the experiment (Table 2; Fig. 6d–f), as confirmed in the more detailed analyses of the IFM soils by Trueman & Gonzalez-Meler (2005). These authors also estimated the residence time in the soil of new carbon as a matter of a few days (R. Trueman, personal communication). There was no evidence of increased C sequestration in the soils with increased [CO2] in our experiments. Rather, the system-level response of C cycling to elevated [CO2] in the P. deltoides agriforest was similar to that found with an inshore marine mesocosm in seawater in equilibrium with 500 ppm CO2. In this mesocosm in B2L, accelerated CO2 influx into organic fractions was almost instantaneously (within a day) reflected in accelerated [CO2] efflux attributed to benthic microbial activity (Langdon et al., 2003). The system-level responses differ in that harvestable biomass in the agriforest was stimulated by elevated [CO2], whereas in the marine system, carbonate deposition was drastically reduced.
Four years of stimulated aboveground growth under elevated [CO2], with removal of all foliar, stem, and branch biomass in the coppicing protocol, led to a significant decrease in soluble P, Ca, and K, particularly within the upper 25 cm of the soil profile. The decreased availability of soluble P after several years of growth under elevated [CO2] has been seen before in a 3-year study (Johnson et al., 2001). Total soil nitrogen (N) concentration did not change significantly after 4 years of tree growth, although concentrations did vary with depth within each [CO2] treatment. This lack of change in total soil N has been seen in other recent studies (Johnson et al., 2001; Martin-Olmedo et al., 2002), but it is surprising that biomass removal each growth season, in our experiments, did not lead to some depletion of soil N. Again, isotopic analyses show seasonal variations consistent with substantial N uptake (Trueman & Gonzalez-Meler, 2005), but perhaps mineralization processes were stimulated by carbohydrate availability in the N-replete soil and helped to obscure changes in total N over the course of the experiment.
Conclusion
Four years of growth of eastern cottonwoods under elevated [CO2] significantly stimulated whole system net CO2 influx and increased cumulative belowground, foliar, and total aboveground biomass. All components of stand respiration (total system respiration, soil respiration with and without roots) were also stimulated, and at elevated [CO2], early season stand respiration was decoupled from CO2 influx aboveground. Elevated [CO2] accelerated depletion of soil nutrients P, Ca, and K, and total soil carbon declined throughout the experiment. Enhancement of above- and belowground biomass production by elevated [CO2] accelerated carbon cycling through the coppiced system and did not sequester additional carbon in the soil. The net system CO2 fluxes, above- and belowground biomass pools, and soil nutrient pools reported here provide a background to more detailed studies of soil respiration using stable C isotope analyses (Trueman & Gonzalez-Meler, 2005) and of the microbial ecology of the soils in B2L (Lipson et al., 2005).
Acknowledgements
The authors thank Dr Barry Osmond for his assistance in the development and review of this manuscript and for leadership in the management of research activities within the Biosphere 2 Laboratory. We also thank Lane Patterson for pest management throughout the 4-year study, and the B2L research support and operations teams, led by Allen Wright and Jim Davis, for environmental control and monitoring in the course of these experiments. Most of all, we are grateful to Mr Edward P. Bass and the Office of the Executive Vice President Columbia University (Dr Michael Crow) for support and maintenance of research in B2L from 1996 to 2003.




