The effects of hormonal contraceptives on bone turnover markers and bone health
Summary
Sex hormones are important regulators of bone metabolism. As hormonal contraceptives contain either oestrogens or progestins, or a combination thereof, it is conceivable that these widely used agents have an effect on bone metabolism and bone health. The main users of hormonal contraceptives, adolescent girls and young women, are still building bone and accruing bone mass and may therefore be particularly susceptible to the effects of hormonal contraceptives on bone. Despite these concerns, the effects of hormonal contraceptives on bone health are still poorly understood. As biochemical markers of bone turnover have been proven useful tools in the assessment and monitoring of bone metabolism, we reviewed the effects of combined and gestagen-only hormonal contraceptives on bone turnover markers and related effects on bone mineral density and fracture risk in premenopausal women, as documented in the literature until January 2009.
Introduction
Hormonal contraceptives are widely used pharmaceuticals that contain either oestrogens or progestins, or a combination thereof. In addition to their contraceptive effects and associated health outcomes, many other health benefits such as the prevention of endometrial and ovarian cancer, cyclic pain relief and control of menstrual bleeding irregularities have been recognized.1 However, as with all active agents, there are also adverse effects such as an increased risk of cardiovascular events (venous thromboembolism, stroke, myocardial infarction) and breast cancer.1
As sex hormones are major regulators of bone metabolism,2–4 it is conceivable that the use of hormonal contraceptives affects bone health.1,5 Above all, adolescent girls and young women who are still in the process of building bone and accruing bone mass may be particularly susceptible to the effects of hormonal contraceptives on bone. As osteoporosis is one of the most frequent diseases in ageing women, causing enormous individual suffering and cost to health care systems worldwide, the effects of hormonal contraceptives on bone metabolism should be of some interest to the medical community.6,7
Biochemical markers of bone turnover are widely used tools for the assessment and monitoring of bone metabolism,8–10 and the actions of hormonal contraceptives on bone metabolism should be reflected by specific changes in these markers. We therefore reviewed the effects of hormonal contraceptives on biochemical markers of bone turnover and related effects on bone mineral density (BMD) and fracture risk in premenopausal women.
Forms of hormonal contraceptives
Amongst the many different hormonal contraceptives available, combined oral contraceptives (COC) containing oestrogens and progestins are the most widely used preparations. In addition, there are hormonal contraceptives based on progestins only. Apart from oral preparations, various delivery systems such as intramuscular injections, transdermal patches, vaginal rings and intrauterine devices have been developed to improve compliance or convenience.1
The main intended effect of all hormonal contraceptives is the inhibition of ovulation and implantation. With regards to COC, ethinyl estradiol is the most frequently used oestrogen, with the main advance over the past years being a significant dose reduction to as little as 20 μg of ethinyl estradiol per tablet. By contrast, there is a large number of mainly synthetic gestagens (synonym: progestins) in use, all of which have proven contraceptive effects.11,12 Chemically, the progestins can be classified into two main families: (i) progesterone derivatives (progesterone, retro-progesterone, 19-norprogesterone and 17alpha-hydroxyprogesterone) and (ii) 19-nortestosterone derivatives (norethisterone, levonorgestrel, desogestrel, gestodene, norgestimatepregnane).12 The majority of progestins contained in modern hormonal contraceptives are 19-nortestosterone derivatives although individual compounds may exhibit significant differences in potency and binding to related steroid hormone receptors such as the androgen receptor (AR), the glucocorticoid receptor (GR) or the mineralocorticoid receptor (MR).11,13,14 Most progestins bind to the AR but not all lead to receptor activation and significant androgenic effects (Table 1, e.g. dienogest, drospirenone). By competitive inhibition, these agents can prevent the action of endogenous androgens, which results in a net antiandrogenic effect. Other progestins (e.g. norethisterone, levonogestrel) are able to activate the AR and hence may induce androgenic effects. The androgenic and antiandrogenic effects of progestins are known to cause many of their side effects, such as acne, hypertrichosis/hirsutism, hair loss or weight gain14 and may also be of importance in regards to bone.
| Progestogens | Antiandrogenic | Androgenic | Glucocorticoid |
|---|---|---|---|
| Progesterone | (+) | − | + |
| Medroxyprogesterone acetate | − | (+) | + |
| Norethisterone | − | + | + |
| Levonorgestrel | − | + | − |
| Gestodene | − | + | (+) |
| Etonogestrel | − | + | (+) |
| Norgestimate | − | + | ? |
| Dienogest | + | − | − |
| Drospirenone | + | − | − |
| Nomegestrol acetate | + | − | − |
- +, effect; (+), potential effect; −, no effect; ?, unclear.
Combined oral contraceptive prevent pregnancy primarily by suppressing ovulation. At low doses, progestins mainly act through modifying the endometrial structure, thus preventing the implantation of the fertilized egg.11,14 Moreover, they cause thickening of cervical mucus and decrease tubal motility, creating a difficult passage for sperm. At higher concentrations, however, most progestins inhibit the pulsatile secretion of gonadotrophin releasing hormones from the hypothalamus, resulting in a suppression of the hypothalamic–pituitary–ovarian axis and profound hypogonadism.15,16 In addition, there seem to be direct effects on the ovary such as inhibition of ovarian steroid synthesis.14
The oestrogen component of COC augments contraceptive efficacy and improves cycle control.12,17 It increases COC efficacy by inhibiting the release of follicle-stimulating hormone from the pituitary, preventing the development of a dominant follicle, and simultaneously potentiates the progestin’s inhibition of the luteinizing hormone (LH) surge. Oestrogen improves cycle control by stabilizing the endometrium and minimizing irregular bleeding.
Effects of oestrogen and progestins on bone
Both oestrogens and progestins have profound effects on bone metabolism and hence bone health. For example, withdrawal of endogenous oestrogens leads to a significant increase in bone turnover and rapid bone loss, as almost invariably seen in early postmenopausal women18–20 or in premenopausal women undergoing GnRH therapy.21 Some of these effects are mediated through the action of oestrogens on the bone-resorbing activity of osteoclasts, as well as on osteoclastogenesis and osteoclast apoptosis.2 Furthermore, there is evidence from experimental and clinical studies that oestrogens directly affect osteoblast function. However, studies into the effects of oestrogens on the proliferative and synthetic activity of osteoblasts reported conflicting results, perhaps due to the diversity of the in vitro systems used.2In vivo, administration of oestrogens to postmenopausal women leads to a rapid decrease in both bone resorption and bone formation markers with a concomitant increase in apparent BMD.22–24
With regards to the effects of progestins on bone, several different mechanisms require consideration. First, there are the obvious and well-established effects of higher-dosed progestins on the hypothalamic–pituitary–ovarian axis, which may result in hypogonadism, accelerated bone turnover and rapid bone loss.18–20 Secondly, however, there is evidence of progestins having direct stimulatory effects on osteoblasts through the progesterone receptor (PR)25–29 and the AR.30–33 Both the PR and the AR are expressed in osteoblasts and osteoclasts, and activation of these two receptors has been shown to increase bone mass by increasing osteoblast activity and reducing osteoclastic bone resorption.25,26,30,31,34 Thirdly, progestins – and particularly medroxyprogesterone acetate (MPA) – bind to the GC receptor, which may also lead to the inhibition of osteoblast activity.13 While physiological concentrations of glucocorticoids are essential for osteoblast proliferation and differentiation,35,36 supraphysiological levels of GC are known to induce bone loss, mainly through the inhibition of osteoblast activity.37–40 Considering these complex interactions, the net effects of progestins on bone cells are likely to be determined by the degree of hypogonadism they induce and the stimulatory and inhibitory actions through the various hormone receptors. Obviously, dose is a major determinant of the net bone effects, as, for example, the glucocorticoid-like effects of progestins with high affinity to the GR (e.g. MPA) become much more relevant at higher doses.13
As a result of the bone-preserving effects of oestrogens, one might expect that the oestrogen component in COC would have beneficial effects on bone metabolism by counteracting the potential adverse effects of the progestins. However, with the lower and lower doses of ethinyl estradiol in modern COC, these potentially beneficial oestrogenic effects become less and less pronounced and there is considerable concern that doses of ethinyl estradiol as low as 20 μg are perhaps insufficient to counteract the negative effects of progestins. Of note, in a recent head to head comparison of two COC containing ethinyl estradiol at either 20 or 30 μg plus drospirenone 3 mg COC users showed lower bone turnover than nonusers with both ethinyl estradiol doses being equally effective (see also below).41 This result clearly shows that COC containing a very low dose of ethinyl estradiol do still have significant effects on bone metabolism and may therefore still be beneficial for bone health.
Biochemistry and clinical use of bone turnover markers
Bone is a metabolically active tissue and undergoes continuous remodelling, a process that largely relies on the activity of osteoclasts to remove bone and of osteoblasts to form bone. Under normal conditions, bone resorption and formation are coupled to each other, and the long-term maintenance of skeletal balance is achieved through the action of systemic hormones and local mediators. By contrast, metabolic bone diseases, states of increased or decreased mobility and therapeutic interventions are characterized by more or less pronounced imbalances in bone turnover.8,40,42,43 With the increasing awareness of disorders of bone and mineral metabolism in clinical practice, the interest in, and the need for effective measures to be used in the screening, diagnosis and follow-up of such pathologies has markedly grown. Along with clinical and imaging techniques, laboratory tests play an integral role in the assessment and differential diagnosis of metabolic bone disease.
In recent years, the isolation and characterization of cellular and extracellular components of the skeletal matrix have resulted in the development of biochemical markers that specifically reflect either bone formation or bone resorption (for review).44 These biochemical indices have greatly enriched the spectrum of analytes used in the assessment of skeletal pathologies. They are noninvasive, comparatively inexpensive and, when applied and interpreted correctly, helpful tools in the diagnostic and therapeutic assessment of metabolic bone disease. Although the various serum and urinary markers of bone turnover include both cellular derived enzymes and nonenzymatic peptides, they are usually classified according to the metabolic process they are considered to reflect. For clinical purposes, therefore, markers of bone formation are distinguished from indices of bone resorption (Fig. 1 and Table 2). It should be born in mind, however, that some of these compounds may reflect, at least in part, both bone formation and resorption (e.g. urinary hydroxyproline). Also, most marker components are present in other tissues than bone and may therefore be influenced by nonskeletal processes. Thirdly, changes in bone markers are usually not disease specific, but reflect alterations in skeletal metabolism independent of the underlying cause.
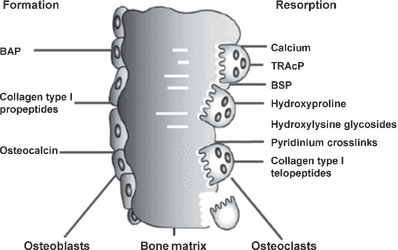
Biochemical markers of bone remodelling.87
| Marker | Tissue | Specimen required | Remarks |
|---|---|---|---|
| Markers of bone formation | |||
| Bone-specific alkaline phosphatase (BAP) | Bone | Serum | Specific product of osteoblasts. Some assays show up to 20% cross-reactivity with liver isoenzyme (LAP) |
| Osteocalcin (OC) | Bone, platelets | Serum | Specific product of osteoblasts; many immunoreactive forms in blood; some may be derived from bone resorption |
| C-terminal propeptide of type I procollagen (PICP) | Bone, soft tissue, skin | Serum | Specific product of proliferating osteoblasts and fibroblasts |
| N-terminal propeptide of type I procollagen (PINP) | Bone, soft tissue, skin | Serum | Specific product of proliferating osteoblast and fibroblasts; partly incorporated into bone extracellular matrix |
| Markers of bone resorption | |||
| Collagen-related markers | |||
| Hydroxyproline, total and dialysable (Hyp) | Bone, cartilage, soft tissue, skin | Urine | Present in all fibrillar collagens and partly collagenous proteins, including C1q and elastin. Present in newly synthesized and mature collagen; both collagen synthesis and tissue breakdown contribute to urinary hydroxyproline |
| Hydroxylysine-glycosides | Bone, soft tissue, skin, serum complement | Urine, serum | Hydroxylysine in collagen is glycosylated to varying degrees, depending on tissue type. Glycosylgalactosyl-OHLys in high proportion in collagens of soft tissues, and C1q; galactosyl-OHLys in high proportion in skeletal collagens |
| Pyridinoline (PYD) | Bone, cartilage, tendon, blood vessels | Urine, serum | Collagens, with the highest concentrations in cartilage and bone; absent from skin; present in mature collagen only |
| Deoxypyridinoline (DPD) | Bone, dentin | Urine, serum | Collagens, with the highest concentration in bone; absent from cartilage or skin; present in mature collagen only |
| Carboxyterminal cross-linked telopeptideof type I collagen (ICTP, CTX-MMP) | Bone, skin | Serum | Collagen type I, with the highest contribution probably from bone; may be derived from newly synthesized collagen |
| Carboxyterminal cross-linked telopeptide of type I collagen (CTX-I) | All tissues containing type I collagen | Urine (α/β) Serum (αα/ββ) | Collagen type I, with the highest contribution probably from bone. Isomerization of aspartyl to β-aspartyl occurs with ageing of collagen molecule |
| Aminoterminal cross-linked telopeptide of type I collagen (NTX-I) | All tissues containing type I collagen | Urine, serum | Collagen type I, with the highest contribution from bone |
| Collagen I alpha 1 helicoidal peptide (HELP) | All tissues containing type I collagen | Urine | Degradation fragment derived from the helical part of type I collagen (α1 chain, AA 620–633). Correlates highly with other markers of collagen degradation, no specific advantage or difference with regard to clinical outcomes |
| Noncollagenous proteins | |||
| Bone sialoprotein (BSP) | Bone, dentin, hypertrophic cartilage | Serum | Acidic, phosphorylated glycoprotein, synthesized by osteoblasts and osteoclastic-like cells, laid down in bone extracellular matrix. Appears to be associated with osteoclast function |
| Osteocalcin fragments (ufOC, U-Mid-OC, U-Long-OC) | Bone | Urine | Certain age-modified OC fragments are released during osteoclastic bone resorption and may be considered an index of bone resorption |
| Osteopontin (OPN) | Bone, kidney, placenta, dentin, cartilage, brain, muscle, blood vessels | Serum | Synthesized by a variety of tissue types. Synthesis in bone is stimulated by 1,25-dihydroxy-vitamin D3 |
| Osteoclast enzymes | |||
| Tartrate-resistant acid phosphatase (TRAcP) | Bone, blood | Plasma, serum | Six isoenzymes found in human tissues (osteoclasts, platelets, erythrocytes). Band 5b predominant in bone (osteoclasts). Enzyme identified in both the ruffled border of the osteoclast membrane and the secretions in the resorptive space |
| Cathepsins (e.g. K, L) (Cath K, Cath L) | K: primarily in osteoclasts L: macrophage, osteoclasts | Plasma, serum | Cathepsin K, cysteine protease, plays an essential role in osteoclast-mediated bone matrix degradation by cleaving helical and telopeptide regions of collagen type I. Cathepsin K and L cleave the loop domain of TRAcP and activate the latent enzyme. Cathepsin L has a similar function in macrophages. Tests for measurement of cathepsins in blood are under evaluation |
Effects of hormonal contraceptives on bone
Combined oral contraceptives
For obvious reasons, most studies on the effects of hormonal contraceptives on bone health focus on the use of COC. Three large cross-sectional studies in younger women consistently describe bone turnover markers to be significantly lower in COC users than in nonusers with concentrations ranging in the lower reference interval of nonusers.45–47 Garnero et al. measured different bone formation and resorption markers in 52 users and 156 nonusers of hormonal contraceptives.45 The overall age-range was 35–49 years, and 44 of 52 users were taking COC for a mean duration of 7 years. Without exception, levels of all bone turnover markers were significantly lower in users of hormonal contraceptives than in nonusers. The magnitude of the reduction in bone turnover ranged between 15 and 30% (Table 3). Very similar results were reported by de Papp et al., who compared 118 nonusers with 119 age-matched COC users (Fig. 2).46 Susan Ott et al. analysed urinary NTX-I and serum osteocalcin (OC) in 72 controls and 39 oral contraceptive users, most of which were assumed to be taking COC.47 Again, urinary aminoterminal cross-linked telopeptides of collagen I (uNTX-I) and serum OC were significantly lower in users of oral contraceptives than in nonusers. In a more recent study, Gargano et al. compared OC, pro-collagen type I N-terminal peptide (PINP) and CTX-I serum levels of 534 premenopausal women not taking oral contraceptives with those of 83 women using various oral contraceptives (mostly COC).48 Circulating levels of the bone formation markers OC and PINP were 14% and 26 % lower in oral contraceptive users than in nonusers (P < 0·001). Mean CTX-I values also tended to be lower in contraceptive users (P = 0·1 vs. nonusers). Another study examining the effects of exercise on markers of bone turnover and BMD found that baseline values of serum OC, TRAcP and urinary hydroxyproline were lower in COC users than in nonusers, but were not affected by regular exercise in either group.49 Another cross-sectional study of female elite athletes and sedentary controls demonstrated that the bone turnover markers serum bone specific alkaline phosphatase (BSAP) and CTX-I were significantly higher in athletes not using COC than in nonexercising subjects not using COC.50 However, COC use decreased both markers significantly reaching identical levels in athletes and controls. The authors interpreted this observation as a potentially protective effect of COC in athletes.
| Design | Author | Preparation | Time of use (year) | N | Age | BSAP | OC | PINP | PINCP | uDPD | uCTX | uNTX | uHprol | sCTX | sICTP | sNTX | TRAP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross-sectional | de Papp AE et al. | Nonspecified | 9 | Co: 118COC: 119 | 28–45 | ↓ | ↓ | ↓ | ↓ | ||||||||
| Adami S et al. | Non specified | ? | Co: 584OC: 83 | 20–49 | ↓ | ↓ | (↓) | ||||||||||
| Garnero P et al. | Formulations nonspecified | 7 | Co: 156COC: 44Others: 8 | 35–49 | ↓ | ↓ | ↓ | ↓ | ↓ | ||||||||
| Ott SM et al. | Formulations nonspecified | ? | Co: 82COC: 39 | 18–39 | ↓ | ||||||||||||
| Weaver CM et al. | Formulations nonspecified | ? | Co: 60COC: 78 | 18–31 | ↓ | ↓ | ↓ | ||||||||||
| Zittermann A | Formulations nonspecified | ? | Co: 19OC: 12 | ↓ | ↓ | ||||||||||||
| Longitudinal | Endrikat J et al. | EE 20/LNG 100 EE 30/LNG 150 | 3 | Co: 0COC: 48 | 20–35 | ↑ | ↓ | ||||||||||
| Nappi C | EE 20/GTD 75 EE 15/GTD 60 | 1 | Co: 19COC: 37 | 22–34 | (↓) | ↓ | |||||||||||
| Nappi C | EE 30/DRSP 3000 EE 30/GTD 75 | 1 | Co: 23COC: 48 | 22–34 | (↓) | ↓ | |||||||||||
| Paoletti AM et al. | EE 30/DRSP 3000 | 0.5 | Co: 26COC: 28 | 20–30 | ↓ | ↓ | ↓ | ||||||||||
| Rickenlund | EE 30/LNG 150 | 0.8 | Co: 0COC: 38 | 16–35 | ↓ | ||||||||||||
| Rome E et al. | EE 20/LNG 100 | 1 | Co: 152COC: 165 | 12–18 | ↓ | ↓ | |||||||||||
| Elgan C | Formulations nonspecified | 2 | Co: 44COC: 74 | 18–26 | ↔ | ||||||||||||
| Mais et al. | EE 20/DSG 150 | 1 | Co: 0COC: 19 | 20–30 | ↓ | ||||||||||||
| Polatti et al. | EE 20/DSG 150 | 5 | Controls: 71COC: 76 | 19–23 | ↓ | ||||||||||||
| Vescovi J et al. | EE 25/NGS 180–250 | 0.04 | Controls: 6COC: 6 | 18–35 | ↓ | ↓ | |||||||||||
| Warren MP et al. | EE 25/NGS 180–250 | 0.8 | Co: 0COC: 19 | 18–40 | ↓ | ↓ | ↓ | ↔ | ↓ | ||||||||
| Wreije et al. | EE 30/DSG 150 | 1 | Co: 0COC: 33 | 18–45 | ↓ | ↓ |
- ↓, decrease; (↓), potential decrease; ↑, increase; (↑), potential increase; ↔, no change effect.
- Numbers provide daily doses in μg.
- EE, ethinyl estradiol; LNG, levonorgestrel; DSP, drosperinone; NGS, norgestimate; GTD, gestodene; Co, controls; COC, combined oral contraceptive; OC, oral contraceptive.
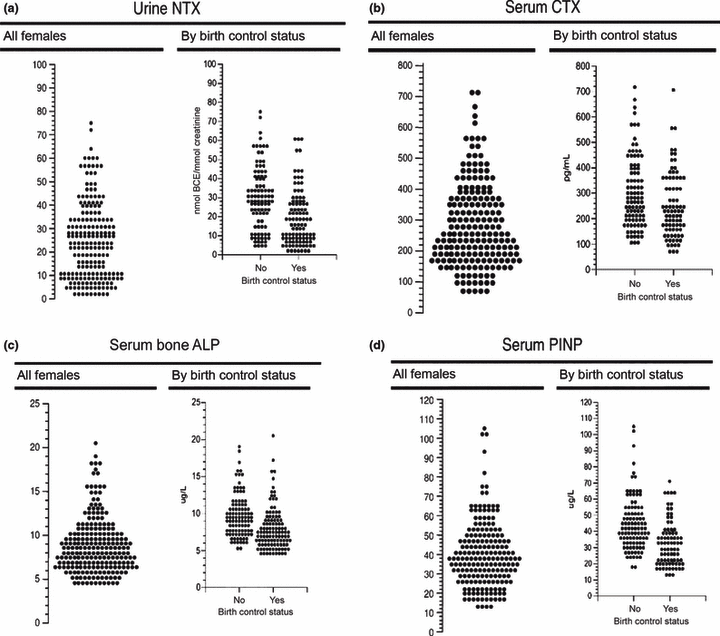
Distribution of bone turnover markers in COC users and non-users. (a) Urine N-telopeptide of type 1 collagen (NTX), (b) serum C-telopeptide of type I collagen (CTX), (c) serum bone-specific alkaline phosphatase (bone ALP), (d) serum N-terminal propeptide of type 1 procollagen (PINP).46
Taken together, most if not all cross-sectional studies indicate that markers of bone turnover are significantly lower in users of oral contraceptives than in nonusers. It should be noted, however, that most of these studies were performed in very young women in whom ‘high’ levels of bone turnover markers are considered to reflect physiological bone modelling and remodelling. This raises the unanswered question whether a ‘suppression’ of normal bone turnover in young (bone building) women has adverse effects on their bone health.
Longitudinal studies on the effects on COC on bone turnover provide less consistent results than their cross-sectional counterparts. The largest study so far included 165 adolescent girls aged 12–18 years who at baseline commenced oral contraception with 20 μg ethinyl estradiol and 100 μg levonorgestrel, and 152 age-matched controls (nonusers).51 After 12 months, serum BSAP (P < 0·05) and urinary DPD (P < 0·08) concentrations were approximately 10% lower in COC users than in nonusers. However, bone markers levels were not available at baseline, making the interpretation of these results somewhat difficult.
Another relatively large study by Polatti et al. compared 76 young (19–23 years) COC users (20 μg ethinyl estradiol and 150 μg desogestrel) with 71 nonuser controls before and after 60 months of treatment.52 At study end, urinary hydroxyproline levels were 90% lower in the user than in the nonusers. This decrease was associated with a reduction in bone accrual: while the controls experienced a significant increase in BMD over the 60 month period, and no change in BMD was observed in the COC users.
In a smaller study, using the same combination of 20 μg ethinyl estradiol and 150 μg desogestrel for 12 months, Mais et al. observed similar effects on the urinary excretion of hydroxyproline.53 However, the main shortcoming of this study is the lack of a control group. Other longitudinal studies measured a larger panel of bone turnover markers, including BSAP, OC and urinary desoxypyridinoline cross-links (uDPD). In a well-performed study from Italy, Paoletti et al. treated 28 women (20–30 years) with a combination of 30 μg of ethinyl estradiol and 3 mg of drospirenone and compared these to untreated age-matched women.54 After 6 months, the bone formation markers BSAP and OC had decreased by 20–30% in COC users but remained constant in controls (Fig. 3). Similarly, the excretion of the bone resorption marker uDPD was reduced by 25% compared with controls.
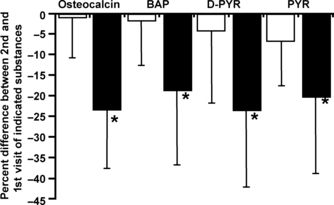
Effect of a 6-month treatment with a COC containing drospirenone (3 mg) and ethinyl estradiol (30 μg) on osteocalcin, bone-specific alkaline phosphatase, pyridinoline and deoxypyridinoline.54□– control group (n = 26) and – COC group (n = 28), *P < 0·001.
The effects of COC on bone turnover markers seem to be more pronounced in women with high bone turnover at baseline. Grinspoon et al. treated osteopenic women with hypothalamic amenorrhea for 3 months with a triphasic COC containing 35 μg ethinyl estradiol and 18–250 μg norgestimate.55 At the end of the third 28-day-cycle, the bone formation markers serum BSAP, OC and pro-collagen type I C-terminal peptide as well as the bone resorption markers uNTX-I and uDPD were all reduced by 20–45% in the COC group when compared with baseline and the changes were significantly greater in COC than in controls. These results are supported by another smaller trial by Vescovi et al.56 In this study, six women with exercise-induced menstrual irregularities were treated with 25 μg ethinyl estradiol and 18–250 μg norgestimate; results were compared with 6 untreated controls. After 2 weeks, the bone formation marker serum PINP and the bone resorption marker serum CTX-I had dropped by 30 and 40% respectively. Rickenlund et al.40 observed similar changes in OC levels in oligomenorrhoeic and regularly menstruating women after 10 month of treatment; however, these changes were observed independently of the level of physical exercise performed by individual subjects.
Although nearly all investigations into the effects of COC on bone turnover found significant reductions in bone turnover markers in COC users, there are two studies that did not. Elgan et al.41 divided 118 healthy young women into four groups: smokers and nonsmokers not using oral contraceptives and smokers and nonsmokers using oral contraceptives. Subjects were seen at baseline and after 2 years of follow-up. Persistence of COC use throughout the study was assessed using a questionnaire. At study end, none of the individual groups showed a significant change in urinary DPD and the use of oral contraceptives was not associated with changes in BMD. However, urinary DPD was the only marker measured in this study and its baseline levels varied between groups. No bone formation markers were assessed. Moreover, the report provides no information on the composition of the oral contraceptives used by the participants.
Contrasting most other reports, Endrikat et al.42 described a significant increase in serum BSAP levels after 3 years of either 20 μg ethinyl estradiol and 100 μg levonorgestrel or 30 μg ethinyl estradiol and 150 μg levonorgestrel in women aged 20–35 years. The bone resoprtion marker urinary NTX decreased by approximately 30% in both groups. The major limitation of this study is the lack of a control group.
Given the reductions in bone turnover markers associated with the use of COC, the question arises whether these changes may be linked to differences in BMD and fracture risk. Generally, the analysis of potential effects of COC use on fracture risk is limited by the fact that fragility fractures are extremely rare among premenopausal women. Potential adverse outcomes of COC in regards to skeletal fragility may only become evident after menopause, i.e. many years following the cessation of the drug and during a time, when other endocrine and health-related problems affecting fracture risk start to occur. The situation, therefore, is rather complex and it may be plainly impossible to determine the risk of fracture conferred by the use of COC during adolescence and younger adulthood.
Probably, the most robust data come from four large epidemiological studies, each of which includes at least 1000 fracture cases. The Women’s Health Initiative (WHI) study of 80,947 individuals revealed a slightly increased risk of fracture in short-term (<5 years) users of COC (hazard ratio = 1·15, 95% CI 1·04–1·27).57 However, when COC were taken for more than 5 years, the increase in fracture risk disappeared. Similar findings were reported by Cooper et al. analysing a total of 482,083 woman-years.58 In the unrestricted analysis, the relative risk for ever-users of COC was 1·2 (95% CI 1·08–1·34). However, when the analysis was limited to women over the age of 50, no difference between never-users and ever-users was observed. Vessey et al. also reported a slight increase in fracture risk in COC users.59 Another very recent population based study from Denmark investigated the relation between COC use and fracture risk in young women.60 In the 64,548 fracture cases and 193,641 controls analysed, COC use was not consistently related to fracture risk. The main advantage of these studies is that they included all fracture sites. Studies focusing on one specific fracture site or a specific sub-group of fracture patients, such as patients with a previous fracture, revealed a decrease or no change of fracture risk.61–64 Given that all of these studies are observational, the currently available data do not allow for a general conclusion in regards to the long-term effects of COC on fracture risk.
Bone mineral density is a powerful predictor of fracture risk in men and women. The effect of COC on BMD therefore deserves special attention, particularly within the context of the aforementioned changes in bone turnover during COC use. There is good evidence that adolescent and young adult women using COC generally exhibit lower BMD than nonusers of comparable age.52,65–68 Moreover, longitudinal studies in adolescents indicate that low dose COC (containing 20–30 μg ethinyl estradiol and 100–150 μg desogestrel or levonorgestrel) may inhibit bone mass accrual.52,65–68 A recent large observational study comparing adolescent girls on oral contraceptives or depot-medroxyprogesterone acetate (DMPA) with nonuser controls revealed that girls on oral contraceptives gained less BMD at the lumbar spine and at the hip than their aged-matched nonusing peers.69 By contrast, data from prospective trials indicate that in postadolescent women COC have no effect on BMD.70–72 Furthermore, a number of smaller cohort studies with a relatively low level of evidence found negative49,73 or positive74 effects of COC on BMD in women between 18 and 33 years of age. In a recent systematic review, Martins et al. showed that apart from adolescents (<23 years) the age of first COC use (4, 5) and the duration of use (Fig. 6) play no significant role for BMD at any site.5 This again supports the notion that adolescent girls and young adult women are particularly prone to the potential adverse effects of COC on bone, while postadolescent women are not affected.
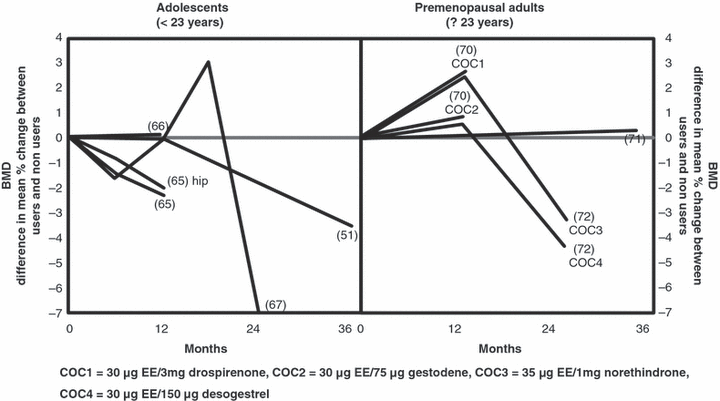
Difference in mean percentage change in bone mineral density at the spine and femoral neck between COC users and nonusers in adolescents/young adults and premenopausal healthy women (modified from).5 For each line, the corresponding reference is provided (#) in the graph.
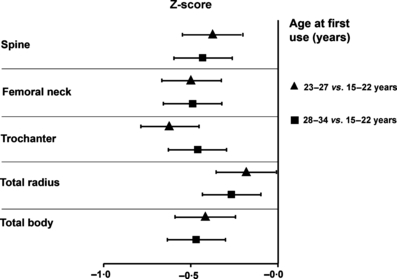
This cross-sectional analysis in early postmenopausal women shows that only for very young women (<23 years) the age of first COC use is related to BMD. The graph displays BMD Z-scores according to age at the first use of COCs at various anatomical sites.88 23–27 vs. 15–22 years, 28–34 vs. 15–22 years.
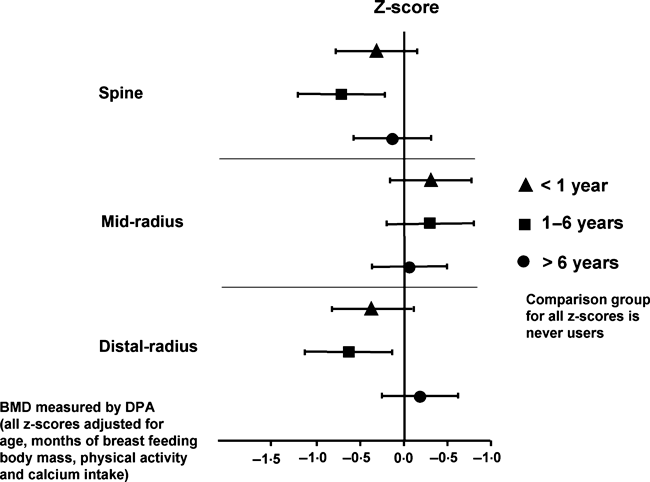
This cross-sectional analysis in early postmenopausal women illustrates that the duration of COC use plays no significant role for BMD at any site. The graph displays BMD Z-scores according to the duration of COC use at various anatomical sites.89 <1 year, 1–6 years, • >6 years.
Taken together the majority of cross-sectional and longitudinal studies suggest that the use of COC induces a significant reduction in most if not all markers of bone turnover. This effect seems to be most pronounced in adolescent girls and women with high baseline bone turnover. With regards to BMD, it appears that COC inhibit adequate bone accrual in adolescents, while the effect in postadolescent and older premenopausal women seems to be negligible. At present, it is unclear whether these metabolic and structural skeletal changes are associated with changes in fracture risk. Some larger and well powered observational studies suggest a slight increase in fracture risk at any skeletal site in COC users. However, most of these studies are limited by their methodology and may not be generalized.
Progestin only hormonal contraceptives (POHC)
While COC are the most widely used method of hormonal contraception, other methods based on progestins only (progestin-only hormonal contraceptives; POHC) have found wide distribution and use.75 While these agents are considered highly efficacious as regards contraception and under certain circumstances may offer advantages, the effects of POHC on bone differ from those of COC. This is particularly true for DMPA, one of the oldest and most widely used POHC (Table 4). Two of the three available cross-sectional studies investigating the effects of POHC on bone health included DMPA users only.
| Design | Author | Preparation | Time of use (year) | N | Age | BAP | OC | PINP | PINCP | uDPD | uCTX | uNTX | uHprol | sCTX | sICTP | sNTX | TRAP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cross sectional | Ott SM et al. | DMPA 150 mg | ? | Co: 82DMPA: 115 | 18–39 | (↑) | ↑ | ||||||||||
| Walsh JS et al. | DMPA 150 mg | Min: 1 year Mean: 3 years | Co: 100DMPA: 100 | 18–345 | ↑ | ↑ | |||||||||||
| Van der Jagt | LNG 30–60 μg | 1–4 | Co: 25LNG: 90 | 25–50 | ↓ | ↑ | |||||||||||
| Shaarawy M et al. | DMPA 150 mg | – | Co: 20DMPA: 40 | 21–44 | ↑ | ↑ | |||||||||||
| Longitudinal | Naessen P | DMPA 150 mg LNG 30–60 μg | 3 | DMPA: 9LNG: 10 | 20–45 | LNG: ↑↑ DMPA: ↑ | LNG: ↑ DMPA: ↑↑ | ||||||||||
| Rome E et al. | DMPA 150 mg | 1 | Co: 152DMPA: 53 | 12–18 | ↓ | (↓) |
- ↓, decrease; (↓), potential decrease; ↑, increase; (↑), potential increase; ↔, no change effect.
- Numbers provide daily doses in μg; DMPA, depot medroxyprogesterone acetate; Co, controls.
The largest of these studies compared both bone turnover markers and BMD in 115 DMPA users and 82 nonusers.47 Urinary levels of the bone resorption marker uNTX-I were 20% higher in DMPA users than in controls, while no differences between these two groups were observed in serum BSAP concentrations. This constellation is indicative of a selective stimulation of bone resorption in DMPA users, a notion supported by the fact that BMD at both the hip and spine was lower in DMPA users when compared with controls. However, as the duration of DMPA use was not specified in this study, the data are somewhat difficult to interpret.
Shaarawy et al. compared 20 controls and 40 DMPA users and categorized them according to the duration of DMPA use.76 Mean serum OC levels were 80–300% higher in DMPA users compared with nonusers, and this increase was positively related to the duration of DMPA use. Thus, subjects who had been on DMPA for more than 5 years had OC levels twice as high as those using DMPA for less than 1 year. A comparable and time-dependent increase was also reported for uDPD as a marker of bone resorption.
In a recent case–control study including 100 DMPA users Walsh et al. observed a 5% deficit in BMD at the femoral neck and lumbar spine, accompanied by significantly higher levels of serum PINP und urinary NTX-I.77 Interestingly, the differences between users and nonusers were more pronounced in younger women (18–25 years) than in older women (35–45 years).
Thus, all cross-sectional studies available to date seem to indicate that DMPA is associated with accelerated bone turnover similar to that seen in postmenopausal women. By contrast, other POHC such as levonorgestrel appear to decrease bone turnover markers. Thus, a cross-sectional study of 90 Nigerian women using levonorgestrel implants up to 4 years showed levels of serum BSAP and serum NTX-I to be 40–60% lower than in nonuser controls.78 The decrease in serum NTX-I levels was significantly correlated with the duration of treatment, with the lowest serum NTX-I concentrations seen in women using the implants for 4 years. By contrast, most of the reduction in serum BALP levels occurred during the first year of treatment and values remained stable thereafter. Of note, no associations were seen with changes in calcaneal ultrasound parameters.
A number of longitudinal studies offer additional information. In a 6-month head-to-head comparison of DMPA (n = 9) and levonorgestrel (n = 10) implants, Naessen et al. confirmed the known effects of DMPA on bone turnover markers: after 6 months of treatment, serum OC and urinary hydroxyproline levels were increased over baseline by 30% and 100% respectively,79 However, in contrast to the results of the cross-sectional studies mentioned earlier (61), treatment with levonorgestrel implants was found to increase, rather than decrease serum OC (by 150%) and urinary hydroxyproline (by 20%) levels.79 Interestingly, BMD at the proximal (group difference at 6 months 3·4%, P = 0·025) and distal forearm (group difference at 6 months 4·1%, P = 0·077) was increased in subjects receiving levonorgestrel implants.79 The results of the latter study are partially incongruent with the results of other investigations, although it should be noted that the numbers of subjects was small, the treatment interval was rather short and there was no adequate control group. Hence, it is unclear whether the results of this study are of sufficient power to permit general conclusions.
Rome et al., investigating 53 adolescent DMPA users and 152 nonusers over a period of 1 year, observed a 10% reduction in serum BSAP levels (P = 0·001) and a trend towards lower uDPD levels (P = 0·08).51 During the treatment period, BMD at the femoral neck decreased by 2·2% in the DMPA users but increased by 2·3% in the control group, resulting in a 4·5% difference after only 12 months.
Taken together, the available data are scant and do not allow for final conclusions in regards to the effects of POHC on bone. However, it seems that POHC may induce an increase in bone resorption markers while their effects on bone formation markers are variable, depending on the progestin used. It further appears that the increase in bone resorption caused by long-term DMPA use is detrimental to bone health as several investigations have demonstrated either bone loss in adult women, or a delay in bone accrual in adolescent girls.73,80–85 However, in their recent systematic review Kaunitz et al. conclude that bone loss occurring with DMPA use is reversible within 24 weeks and is therefore not likely to be an important risk factor for low bone density and fractures in older women, although data on fracture risk in DMPA users are lacking.86 The mechanisms responsible for the effects of DMPA on bone are currently insufficiently understood. DMPA is known to suppress endogenous sex hormone production and hence induces a hypogonadal state, similar to that of postmenopausal women.82 Other authors propose GR related mechanism.13 However, taking into consideration that progestins such as levonorgestrel and medroxyprogesterone acetate bind with different affinities to various steroid receptors, it is probable that the net-effect of an individual progestin on bone is the sum of various partial effects caused by binding to different steroid receptors in bone cells and other tissues.
Conclusion
The existing evidence indicates that combined oral contraceptive are associated with significant reductions in most if not all markers of bone turnover. Potential effects of these metabolic changes on bone health are currently a matter of debate. Although the available studies are not consistent in terms of bone health outcomes, the largest and most powerful investigations suggest a slight increase in fracture risk at any site. With regards to bone mineral density, it seems that combined oral contraceptive inhibit bone accrual in adolescents but not in postadolescent women. Conversely, progestin only hormonal contraceptive increase bone resorption markers and show inconsistent effects on bone formation markers. Especially depot-medroxyprogesterone acetate seems to induce an imbalance between bone resorption and bone formation in favour of bone resorption, which is accompanied by significant bone loss.
Competing interests/financial disclosure
Nothing to declare.




