Urothelial progenitor cells: regional differences in the rat bladder
Abstract
Abstract. Objectives: Because the trigone is a unique region in the caudal bladder with a higher risk of neoplasia, we hypothesized that this area would have a high proportion of progenitor cells. As yet there is no marker nor methodology to specifically isolate urothelial stem cells, and thus demonstrate multi-potential differentiation and self-renewal. Here, our goal was to evaluate the distribution of progenitor cells that carry two general major attributes of stem cells: clonogenicity and proliferative capacity. Materials and methods: The bladders of Fisher rats were divided into caudal and cephalic segments and primary cultures were established from the harvested urothelial cells. Results: We found that colony-forming efficiency was almost 2-fold higher for cells from the caudal bladder compared to the cephalic bladder. Doubling time was significantly faster for cells harvested from the caudal bladder at initial plating. This suggested that the caudal bladder harbours a higher density of urothelial progenitor cells. With passage to p4, the differences between the upper and lower bladder were lost, suggesting selection of proliferative cells with serial passage. Based on Ki-67 staining, there was no geographical difference in cell proliferation under normal homeostatic in vivo conditions. Conclusions: These results demonstrate geographical sequestration of urothelial progenitor cells to the area of the bladder that encompasses the bladder neck and trigone, which may be a factor in pathological disparities between the trigone and remaining bladder.
INTRODUCTION
Stem cells are a small population of cells that possess unique characteristics of high self-renewal, low turnover, long life and the ability to undergo differentiation to produce mature tissue. These attributes allow them to drive development and regeneration of tissues over the lifetime of an organism. In the haematopoietic system, stem cells play a pivotal role in the development of leukaemia (Bonnet & Dick 1997) and in other tissues are thought to play a role in the development of neoplasia as well (Jordan 2004; Miller et al. 2005). Because stem cells have the capacity for self-renewal and generate multiple lineages during their long lifespan, mutagenic transformation in a single stem cell can result in the formation of a clonal growth of neoplastic cells and thus in tumour formation (Reya et al. 2001). The importance of stem cells in maintaining tissue homeostasis and the profound consequences of their malignant transformation have fuelled the quest for identifying them in tissues and in understanding their growth characteristics.
The precise location of urothelial stem cells in the bladder has not yet been identified. In other organs, epithelial stem cells have been found in unique and well-protected geographical niches that may reduce their exposure to trauma. For example, corneal stem cells are found in the basal layer of the limbus, sequestered in the peripheral cornea (Ebato et al. 1988; Pellegrini et al. 1999), while in the epidermis, stem cells are located at the lower ‘bulge area’ of hair follicles (Lavker et al. 2003; Webb et al. 2004). Similarly, intestinal stem cells have a protected niche deep within crypts (Brittan & Wright 2004) and prostatic stem cells appear localized to the proximal region of glandular ducts (Tsujimura et al. 2002). These studies utilized the high proliferative capacity of stem cells for their localization. Bladder urothelial stem cells, although their exact location was unknown, were presumed to reside in the basal layer of the epithelium. Indeed, our preliminary data with nucleotide-label retention support this presumption (Kurzrock et al. 2005a). However, whether they are scattered evenly throughout the bladder or grouped in particular regions as in other organs, was unknown.
The mammalian bladder is divided into several regions: the dome, the lateral, posterior and anterior walls, the trigone and the bladder neck (Fig. 1). The distribution and configuration of the mesenchyme, muscle and nerve supply is different in each geographical area. On the other hand, the epithelium is thought to be homogenous without significant variation (Jost et al. 1989b). Yet, there is a regional disparity in occurrence of inflammatory and neoplastic conditions of the epithelium (Stephenson et al. 1990; Sciarra et al. 2004). For this reason, we have hypothesized that the urothelium is not homogenous and that progenitor cells are not evenly distributed.
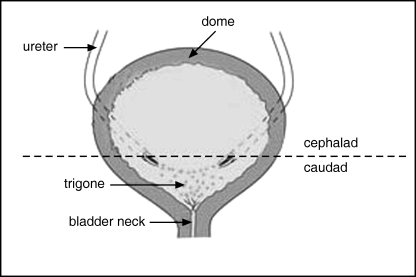
Depiction of posterior aspect of bladder opened in the coronal plane. Dotted line along trigonal ridge illustrates line of division between cephalic and caudal segments.
Unlike pluripotent embryonic stem cells, no definitive biochemical markers are available to identify urothelial or some other epithelial stem cells (Tumbar et al. 2004). Epithelial stem cells can be identified by their unique growth characteristics. In vitro, culture conditions stimulate stem cells to proliferate and regenerate. High in vitro proliferative potential is therefore one attribute that can be used to identify putative stem cells (Lavker & Sun 2000). Without a marker or methodology to isolate epithelial stem cells, and thus demonstrate multi-potential differentiation and self-renewal, our goal was to evaluate the distribution of progenitor cells that carry two major attributes of ‘stemness’, clonogenicity and proliferative capacity. To determine if there are regional differences in the distribution of progenitor cells in the bladder, we evaluated in vivo and in vitro growth characteristics of rat urothelium using protocols similar to those employed for skin and corneal investigation (Ebato et al. 1988; Webb et al. 2004). Our data support the concept that in the bladder, as in other epithelia, progenitor cells are not evenly distributed.
MATERIALS AND METHODS
Cell population growth kinetics were determined using colony-forming efficiency (CFE), a measure of clonogenic capacity, and replicative doubling time, a measure of cell proliferative capacity. Both CFE and doubling time were assessed at initial plating of cells derived from the specific areas of the bladder, with 12 matched cephalic and caudal specimens. These were defined as p0 colonies. Five of the 12 matched specimens were also serially passaged four times to assess any effect of prolonged in vitro culture on proliferative capacity. These were designated as p1, p2, p3 and p4. The Ki-67 staining index of cephalic and caudal bladder segments was also evaluated to assess for differences in in vivo cell proliferative activity between the two areas.
Animals
Animal care and procedures were performed in compliance with University of California Institutional Animal Care and Use Committee guidelines. One-month-old Fischer 344 rats were housed in a climate-controlled facility and were maintained on standard chow and water.
Cell harvest
The cell harvest and culture procedures used have been described previously (Kurzrock et al. 2005b); we employed the following modifications. Rats were euthanized and the bladders were dissected and placed in 1% antibiotic–antimycotic solution, consisting of penicillin (500 U, 1 U/ml), streptomycin (500 µg, 1 µg/ml) and amphotericin (1.25 µg, 2.5 ng/ml) (Invitrogen Corp., Carlsbad, CA, USA). The bladders were divided at the trigonal ridge into caudal and cephalic segments (Fig. 1). Segments were opened and were placed epithelial side down in 0.25% trypsin −1 mm ethylenediaminetetraacetic acid solution (Invitrogen Corp.) for 1 h at 37 °C. A sterile glass slide edge was used to scrape the epithelial surface to release the urothelial cells into solution. This solution was aspirated and the trypsin deactivated by addition of fibroblast media consisting of Dulbecco's modification of Eagle's medium (Invitrogen Corp.) supplemented with 10% foetal bovine serum (Omega Scientific, Tarzana, CA, USA), l-glutamine (2.92 µg/ml) (Invitrogen Corp.), and 1% antibiotic–antimycotic solution. The cell suspension was then centrifuged at 150 g for 5 min, the supernatant was aspirated, cells were re-suspended in supplemented keratinocyte growth medium (KGM+) consisting of KGM (Cambrex Corp., East Rutherford, NJ, USA) supplemented with 2% foetal bovine serum and cholera toxin (8.3 ng/ml) (Calbiochem, San Diego, CA, USA), and the cells were counted using a haemocytometer.
Cell culture
Cells were plated onto 60-mm cell culture plates (Corning, Corning, NY, USA) seeded with a mitomycin C-treated murine 3T3 (Swiss albino) feeder layer (Kurzrock et al. 2005b) at a density of 2000 cells for determination of CFE and 30 000 cells for determination of proliferation. Cells were cultivated in KGM+ and the medium was changed every 2–3 days. Each sample was plated in triplicate. The plates were incubated in a 37 °C humidified atmosphere of 5% carbon dioxide. Between 7 and 9 days, prior to confluency, CFE plates were fixed in Streck tissue fixative (Streck Laboratories, La Vista, NE, USA) and were stained with rhodamine blue (Sigma, St. Louis, MO, USA) to count colonies (greater than 50 cells). The cobblestone urothelial colonies were easily distinguished from the loosely associated or unassociated 3T3 fibroblasts.
At the same time point as preparation of CFE plates, proliferation plates were treated with 0.25% trypsin −1 mm ethylenediaminetetraacetic acid to release the cells for counting. Cell doubling time was calculated using the following formula: DT = (t − t0) log2/(logN − logN0) where t, to indicate time points at counting and initial plating, respectively; and N, N0 indicate number of cells at respective time points. N0 was determined by multiplying total cells plated by the respective CFE. For cell culture experiments, results are depicted as the average of triplicate samples. Comparisons between groups were made using paired Student's t-test.
Immunohistochemistry
Caudal and cephalic bladder segments were immunostained for expression of Ki-67 protein to determine in vivo proliferative activity in the normal homeostatic state. Five bladders were evaluated. Slides of upper and lower bladder segments were heated in citrate buffer at a pH of 6.0 for 20 min. Blocking was performed with avidin for 10 min and with biotin for 10 min. Slides were incubated with rabbit anti-Ki-67 monoclonal antibody (Abcam, Cambridge, MA, USA) diluted 1 : 200 in 10% horse serum overnight at 4 °C. Secondary antibody, donkey anti-rabbit-biotinylated IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) diluted 1 : 200 in 1% horse serum was applied for 30 min at room temperature. Samples were then incubated with Vectastain ABC kits (Vector Laboratories, Burlingame, CA, USA) for 30 min. The antibody staining final reaction product was then visualized using DAB (Vector Laboratories). A methyl green (DAKO, Carpinteria, CA, USA) counterstain was then applied. The Ki-67 staining index was determined by counting 1000 urothelial cells from each sample. The percentage of cells that were positively stained in for expression of the Ki-67 protein was defined as the staining index. For specimens with less than a total of 1000 cells, all cells were counted.
RESULTS
Primary harvest cell culture (p0)
Colony-forming efficiency was significantly higher for cells harvested from caudal bladder segments than from cephalic bladder segments at the initial plating (4.8 versus 2.6, P < 0.01) (2, 3). Doubling time was significantly shorter for cells harvested from caudal bladder segments at the initial plating (20.3 versus 22.6, P < 0.03) (Fig. 4). This suggests that there are substantially more clonogenic cells in the lower bladder and they are minimally more proliferative.
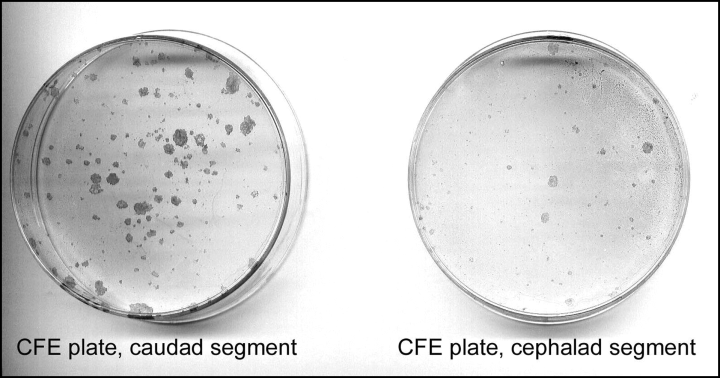
Representative colony forming efficiency plates for caudal and cephalic bladder segments at primary harvest.
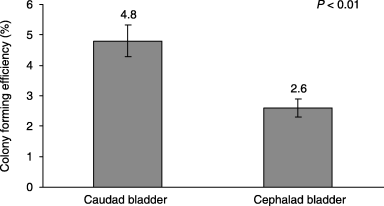
Colony forming efficiency of primary cell cultures from harvested urothelial cells (p0). Error bars ± SEM.
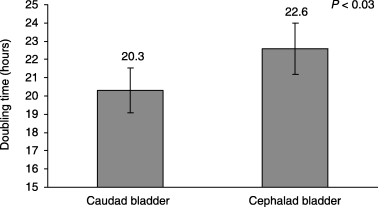
Doubling time of primary cell cultures from harvested urothelial cells (p0). Error bars ± SEM.
Serial passage cell culture (p1–p4)
For the subset of specimens that underwent serial passage, p1, p3 and p4 cells demonstrated no significant differences in CFE between caudal and cephalic segments. P2 cells from the caudal bladder had significantly higher CFE (Fig. 5). Doubling time of p1 cells from caudal bladder segments was significantly shorter. The difference in doubling time between cephalic and caudal cells became progressively smaller with each passage (Fig. 5).

Colony forming efficiency and doubling time for serial passages of urothelial cells from caudal and cephalic bladder sections. Error bars ± SEM (*denotes P < 0.05).
In vivo proliferative index
Ki-67 staining index was not significantly different for the caudal and cephalic bladder segments (2.55 versus 2.72, P = 0.89) (6, 7).
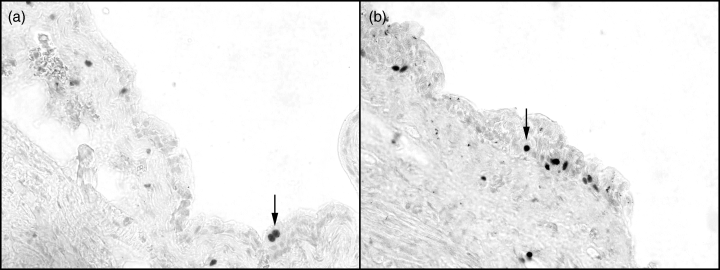
Representative sections from cephalic (a) and caudal (b) rat bladder stained for Ki-67. Arrows point to one of several positively stained nuclei within the urothelium (×40 magnification).
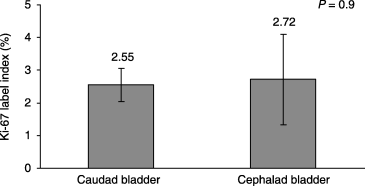
Comparison of Ki-67 staining index in caudal and cephalic bladder. Error bars ± SEM.
DISCUSSION
Our initial work has indicated that nucleotide-label retaining cells, which are putative epithelial stem cells, are localized to the basal layer of the bladder epithelium (Kurzrock et al. 2005a). The present investigation focused on determining if there is a geographically preferred locale for progenitor cells. Understanding the distribution of urothelial progenitor cells within the bladder will hopefully facilitate further work on characterization and isolation of stem cells and improve our knowledge of urothelial neoplasia and cell biology.
Cell culture experiments indicated that cells from the caudal segment of rat bladders have a substantially higher clonogenic capacity at initial plating (p0). This suggests that there is a sequestration of progenitor cells within the bladder neck and trigone. Prior to this study, bladder urothelium was considered to be homogenous. This piece of data is the first to demonstrate regional differences within bladder anatomy.
By serial passaging, differences in clonogenic and proliferative capacity were diminished. This observation is consistent with the finding that proliferating keratinocytes become terminally differentiated in culture and manifest changes in morphology (Lavker & Sun 2000). It is controversial whether or not growth characteristics of serially passaged cells reflect the qualities of the primary harvested cells in vivo. In this experiment, the convergence of CFE values of the different bladder regions over time suggests a selection of clonogenic cells with serial passage.
Although epithelial stem cells are relatively more proliferative in vitro, in normal, non-wounded tissue, they are slow growing (Lavker & Sun 2000). Thus, stem cells in vivo would be expected to have a lower level of Ki-67 expression (Jensen et al. 1999). In this study, we were not able to demonstrate a difference between Ki-67 staining for the cephalic and caudal bladder segments (label index about 3%). This implies that proliferation under normal homeostatic conditions is balanced equally between the caudal and cephalic portions of the bladder.
In the epidermis, there is a presumed hierarchy of cells defined by cell kinetic analysis and experiments on cultured keratinocytes. There is the undifferentiated compartment of stem cells with unlimited regenerative capacity, an intermediate layer of transit amplifying cells (stem cell daughters) with limited proliferative activity that are responsible for most of the cellular division, and a terminally differentiated, committed, compartment with no proliferative capacity (Jensen et al. 1999). Although our data demonstrate regional differences in the distribution of progenitor cells, this does not necessarily reflect a stem cell niche. The clonogenic capacity demonstrated in vitro may possibly be secondary to transit amplifying population, not yet identified in urothelium.
The trigone has long been considered to be a unique area of the bladder with different pathologies and embryologic origin than the remaining bladder. Trigonal epithelium is postulated to derive from mesodermal ingrowth, whereas the remaining bladder is of endodermal origin (Park 2002). This was thought to explain the clinical disparities. However, recent research using stromal–epithelial recombination experiments (Baskin et al. 1996) suggests that the trigonal urothelium is also derived from endodermal tissue (De Marco et al. 2004).
Stem cells are thought to be a target for carcinogenesis (Reya et al. 2001). Our finding of a higher concentration of progenitor cells within the caudal rat bladder may be a factor in the clinical observation that a number of pre-malignant conditions are predominantly found in the caudally located trigone and bladder neck of the human bladder. Squamous metaplasia primarily affects the female trigone (Wiener et al. 1979; Jost et al. 1989a). Inverted papillomas, which represent benign urothelial neoplasms with a low potential for malignant transformation, are found predominantly in the bladder neck and trigone (Asano et al. 2003).
Transitional cell carcinoma is found more commonly on the lateral walls. The bladder neck is the second most common site (29%) and the trigone is the third (22%) (Sciarra et al. 2004). Considering the relatively small surface area of the bladder neck and trigone compared to the lateral walls, the incidence of carcinoma per area is much higher in the caudal bladder. Bladder neck tumours have a higher likelihood of multiplicity (Sciarra et al. 2004). Trigonal tumours occur at younger ages (Stephenson et al. 1990). These variations in disease presentation may be a reflection of a higher concentration of urothelial progenitor cells in the caudal bladder.
In summary, cells harvested from the caudal rat bladder demonstrate substantially higher clonogenic capacity than cells from the cephalic bladder. The results of this study support our hypothesis that bladder urothelium is not homogenous and there are regional disparities within the bladder. We postulate that there is a sequestration of progenitor cells to the area of the bladder that encompasses the bladder neck and trigone. This may be a factor in the pathological disparities between the upper and lower human bladder.
ACKNOWLEDGEMENTS
This work was supported by grants from Shriners Hospitals for Children and Children's Miracle Network. We appreciate the assistance of Ralph deVere White, MD, (Director, UC Davis Cancer Center) for his thoughtful review and assistance with the preparation of this manuscript.