Targeted phototherapy of plaque-type psoriasis using ultraviolet B–light-emitting diodes
Conflicts of interestL.K., Z.C., E.B. and L.K.: none declared. A.H.B. receives royalty income from licensing of FOXP3 monoclonal antibodies. A.K. is employed by Allux Medical Inc.
Summary
Background One of the major technological breakthroughs in the last decade is represented by the diversified medical applications of light-emitting diodes (LEDs). LEDs emitting in the ultraviolet (UV) B spectrum might serve as a more convenient alternative for targeted delivery of phototherapy in inflammatory skin diseases such as psoriasis.
Objectives We investigated the efficacy and safety of a new UVB-LED phototherapeutic device in chronic plaque-type psoriasis.
Methods Twenty patients with stable plaque-type psoriasis were enrolled into a prospective, right-left comparative, open study. Symmetrical lesions located on extremities or trunk were chosen; one lesion was treated with the study device, whereas the other lesion served as an untreated control. Two treatment regimens were used in the study, one with an aggressive dose escalation similar to those used for outpatient treatment and one with slow increase in dose, similar to those used for treatment at home.
Results Patients in both groups responded rapidly to the UVB-LED therapy. Early disease resolution was observed in 11 patients (seven in the first group and four in the second group). Overall improvement at end of therapy was 93% in the high-dose group and 84% in the low-dose group. Four patients from the high-dose group and five from the low-dose group were still in remission at the 6-month follow-up visit.
Conclusions These results suggest that this innovative UVB-LED device is effective in the treatment of localized psoriasis and may be useful in other UV-responsive skin diseases.
Ultraviolet (UV) B radiation (280–320 nm) is a highly effective treatment in psoriasis. Initially, broadband UVB sources were applied that emit wavelengths throughout the whole UVB spectrum. Action spectrum studies have established that wavelengths between 304 and 313 nm are the most effective to clear psoriatic lesions, while wavelengths from 290 to 300 nm produce sunburn reaction, but have no therapeutic effect.1 These findings led to the introduction of narrowband UVB sources (peak emission at 311 ± 2 nm), which have been successfully used to treat psoriasis for more than 20 years. The efficacy of UVB phototherapy has been demonstrated in several studies and therefore is recommended for the treatment of psoriasis in the guidelines of dermatological societies all over the world.2–5
Conventional phototherapy is characterized by simultaneous exposure of both diseased and normal skin. Healthy skin has a lower tolerance to UV radiation, evidenced by the development of skin erythema, and therefore is a limiting factor for a proper dose escalation which is typically required for rapid clearance of psoriatic plaques. We previously introduced targeted phototherapy using the 308-nm excimer laser for the treatment of mild to moderate psoriasis, which represented a significant advance in treatment options.6 This type of therapy allows the delivery of high-intensity UVB radiation directly to the psoriatic plaque and spares the healthy skin from unnecessary treatment, potentially reducing the risk for long-term side-effects such as carcinogenesis and photoageing.7 Clinical outcome and remission periods following therapeutic regimens with targeted phototherapy are similar to those following conventional phototherapy.8
The therapeutic effect of UVB radiation is mostly attributed to its immunosuppressive and immunomodulatory action.9 The most important mechanism that explains the immunosuppressive effect of UV radiation is apoptosis induction.10,11 Ozawa et al.12 observed that UVB treatment of psoriatic skin lesions resulted in a consistent and profound depletion of T lymphocytes from the epidermis and dermis. Other effects that result from the application of UVB radiation are decreased antigen presentation, and modulation of the synthesis, release and activity of inflammatory mediators and cytokines. In combination with apoptosis induction, these mechanisms are believed to be the main contributors to the therapeutic effect of UVB irradiation.9
One of the major technological breakthroughs in the last decade is represented by the diversified medical applications of light-emitting diodes (LEDs). LEDs emitting in the UVB spectrum may serve as a more convenient alternative for targeted delivery of phototherapy in inflammatory skin diseases such as psoriasis.
We investigated the efficacy of a new, UVB-LED phototherapeutic device in chronic plaque-type psoriasis.
Patients and methods
This was a prospective, left-right comparative, open-label pilot study. The aim of this proof of concept study was to address the efficacy and safety of UVB-LEDs in plaque-type psoriasis. Twenty subjects recruited at the Department of Dermatology and Allergology, University of Szeged, Hungary were enrolled in the study. The study was approved by the Central Ethics Committee of Hungary. All subjects gave written informed consent before participating in any study-related procedure. Subjects with stable mild to moderate plaque-type psoriasis involving < 20% body surface area were recruited. Stable plaques were defined as those that had been present and unchanged for a minimum of 2 months. Patients who had received systemic treatments (e.g. ciclosporin, methotrexate, retinoids and biologic agents) and/or phototherapy for treatment of psoriasis within the last 6 months were excluded. Also excluded were patients who had received any photosensitizing medication within the past 4 weeks or had used topical treatments (other than emollients) within the past 2 weeks. Two symmetrically localized plaques with approximately the same dimensions and same disease severity were selected on the trunk or extremities. One plaque received phototherapy and the other one served as a control.
The ultraviolet B phototherapy system
The Resolve™ UVB Phototherapy System (Allux Medical Inc., Menlo Park, CA, U.S.A.) uses LED technology to deliver localized UV radiation directly to the psoriatic plaques.
The phototherapy device is composed of two distinct components: the patient attached unit (PAU) which delivers the therapeutic radiation and a medical-grade DC power supply plus associated power cords. The dimensions of the PAU are 128 × 90 × 38 mm. The therapeutic radiation source in the UVB phototherapy system is an array of 72 UV-LEDs in a hexagonal 9 × 8 structure contained within the PAU. The typical emission spectrum from an individual UV-LED is Gaussian-like and has a peak emission wavelength of approximately 310 nm and a full width at half maximum of approximately 15 nm (Fig. 1). An ensemble or array of 72 virtually identical LEDs will accordingly produce a similar emission spectrum from the device.
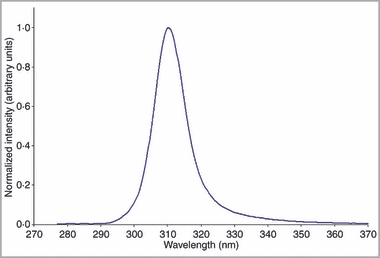
Ultraviolet (UV) emission spectrum of the UVB phototherapy system light-emitting diodes.
In order to achieve a uniform illumination density from the UV-LED array at the fused silica exit window, each LED is driven with a unique electric current value to compensate for any individual LED variations. The position and configuration of the LED array were designed so that a uniform distribution of radiation was generated across the entire output periphery to within ± 10%.
The device needed no warm-up time before reaching a constant output. A randomly selected representative UV-LED device was tested for any measurable degradation over time at a constant forward current of 30 mA; there was less than a ± 10% variation in output power during the course of this test, which entailed over 600 h of continuous testing.
The intensity of the delivered radiation in this clinical study was 1 mW cm−2, enabling the delivery of a dose of 350 mJ cm−2 in 5 min and 50 s.
The intended effective use of the Resolve UVB Phototherapy System does not require sterility; therefore, the product is not sterilized nor is it compatible with typical terminal sterilization processes such as steam, ethylene oxide or irradiation (gamma or electron beam). The PAU is compatible with cleaning and disinfecting agents commonly used in clinical settings. The device received 510(k) clearance from the U.S. Food and Drug Administration in 2007. The device used in the current research was manufactured exclusively for performing the research reported here, and is not currently commercially available.
For masking during delivery of the UVB treatment a clear, UV-blocking aromatic polyester polyurethane film (American Polyfilm Inc., Branford, CT, U.S.A.) was used. The mask is flexible to conform to a patient’s body surface, and clear to aid the physician in defining particular areas to expose to UV radiation during treatment. The masking material was placed on the target lesion and the treatment area outline was traced on to the masking material using a permanent marker. The intended treatment area was then cut out of the mask using scissors. The custom-shaped mask was returned to the treatment location, positioned around the treatment area, and secured to the patient using tape. The PAU was then fixed to the patient using attachment straps (Fig. 2).
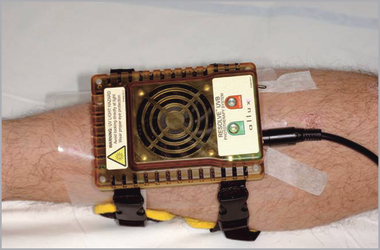
The device was temporarily affixed to the patient via custom attachment straps for comfort during treatment.
Treatment protocol
Before the first treatment each patient’s minimal erythema dose (MED) was determined on unexposed, uninvolved skin using increasing doses of UVB radiation. MED testing was performed in each patient with the same UV-LED device used during the clinical study. Six spots (each 1 cm2 in size) were exposed to increasing doses of UVB radiation. The doses used for MED determination were based on the subject’s skin type: skin type I and II: 100, 150, 200, 250, 300, 350 mJ cm−2; skin type III and IV: 250, 300, 350, 400, 450, 500 mJ cm−2.
The evaluation of patients was scheduled 24 h after UV exposure. The MED was determined as the lowest dose that caused a detectable pinkness of the skin.
An ointment of 10% salicylic acid was used as a keratolytic agent for 2–3 days before the first phototherapy session. Treatments were administered four times weekly for up to 8 weeks or until complete clearance, whichever occurred first. Treatments were scheduled for Monday, Tuesday, Thursday and Friday but the protocol provided some flexibility for patients who preferred one of the treatments to be conducted during the weekend. During the treatment regimen patients did not receive concomitant medications (except emollients). Control plaques were treated only with emollients.
Two treatment regimens were used in the study, one with an aggressive dose escalation similar to those currently used in outpatient treatment (first regimen) and one with a slow increase in dose, similar to those currently recommended for home use (second regimen). To increase penetrance of UV radiation into the skin with less scatter, mineral oil (Paraffinum liquidum; Hungaropharma, Budapest, Hungary) was applied to the surface of the plaques before each treatment.
First regimen
Ten subjects were enrolled in this group. The starting dose was 1 MED. The dose was increased every visit by 20–50% of the previous dose up to 5 MED and kept at this dose for the remaining treatments. The dose increase was decided based on therapeutic response and presence of side-effects. Usually the first few treatments were delivered with a 50% increase and thereafter lower increments were used. If blisters occurred the plaques were not treated on the next scheduled treatment. A physical mask was used to shield healthy tissue. The outer treatment area was defined as the edge of the erythema of the psoriatic plaque.
Second regimen
Ten subjects were enrolled in this group. The starting dose was 0·7 MED. The dose was increased every visit by 0·1 MED for the whole treatment period. The treatments were delivered in a semitargeted way, by using a physical mask, which was cut larger than the psoriatic plaque (the treatment area outline was traced approximately 1 cm outside the plaque margins) allowing exposure of some healthy skin next to the plaque. The highest delivered dose was 3·8 MED.
Assessment
The local Psoriasis Severity Index (PSI) was used to evaluate clinical improvement. The local PSI was derived from the standard Psoriasis Area and Severity Index score by omitting the area, thus assigning a score of 0–4 (0, none; 1, mild; 2, moderate; 3, severe; 4, very severe) for erythema, induration and desquamation. The PSI was calculated for both treated and control plaques before starting the treatment regimen, once weekly until the end of the treatment period, on clearing if clearing occurred and at the follow-up visits. In addition, standardized photographs were taken at the same visit when PSI was calculated. The treated areas were evaluated for side-effects at each visit.
Follow-up investigations were performed at 1, 3 and 6 months after end of treatment. Relapse during the follow-up period was defined as reduction of > 50% in PSI from the achieved maximal improvement in PSI score.13 Treated plaques did not receive any therapy during the follow-up period.
Skin biopsies and immunohistochemistry
In a subset of 10 patients (seven from the first regimen and three from the second regimen) a 3-mm punch biopsy was taken before starting the treatment regimen from a psoriatic plaque with similar localization and severity index as the treated and control plaques. Another punch biopsy was taken from the treated plaque after the last treatment was delivered. The tissue was formalin fixed and paraffin embedded. Routine haematoxylin and eosin as well as immunohistochemical stains for CD3, CD4, CD8, CD25, FOXP3, CD83, Ki-67 and CK16 were performed. See also Data S1 (Supporting information).
Statistical analysis
The two-tailed paired t-test was used for statistical analysis: P < 0·05 was considered significant.
Results
Twenty subjects (three women and 17 men; age range 29–71 years, mean 51·7) were enrolled in the study. Duration of psoriasis ranged from 1 to 35 years. All patients enrolled in the study were white, with skin types II (five patients), III (13 patients) and IV (two patients). MED levels ranged from 150 to 450 mJ cm−2.
First regimen
Ten subjects were enrolled in this group; nine of them completed treatment and all follow-up visits. One subject dropped out after one treatment because of acute prostatitis which was presumed to be unrelated to the UVB phototherapy. The number of treatments delivered per patient ranged from 9 to 32 (mean 20). Maximal dose delivered at end of treatment varied between 0·9 and 1·75 J cm−2.
At baseline the mean ± SD local PSI score was 8·6 ± 1·4 for the treated plaques and 8·4 ± 1·4 for the control plaques.
At 2 weeks the mean PSI score for the whole treated group had already decreased from 8·6 to 5·5 (P < 0·001). Improvement remained significant compared with baseline throughout the course of treatment. Early resolution (clearance) occurred in seven patients (Fig. 3). Eight of nine patients reached 75% improvement in local PSI score. All subjects achieved 50% improvement in the PSI score. Overall improvement in the PSI score at the end of the study was 93%.
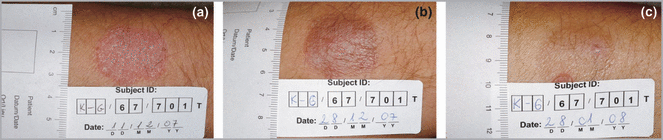
Example of a subject treated with the first regimen. Baseline Psoriasis Severity Index of the treated lesion was 9 (a). The subject had early resolution after 11 treatments (b) and was in remission at the 1-month follow-up visit (c).
No improvement occurred during and at the end of the treatment regimen in PSI scores of the control plaques.
Two subjects experienced small blister formation (both after 50% increase of the previous dose). Blisters resolved rapidly, and only one treatment session was skipped. As reported also by others,14,15 blister formation was followed by rapid therapeutic response: both subjects had early resolution (after nine and 11 treatments, respectively).
At the 1-month follow-up visit all patients who completed the study were in remission (Fig. 3). At the 3-month follow-up visit six patients were in remission while at the 6-month visit four of these patients were still in remission.
Second regimen
Ten subjects were enrolled in this group; all of them completed treatment and attended the 1-month follow-up visit. One patient who had a relapse at 1 month follow up was lost to further follow up. The number of treatments delivered per patient ranged from 12 to 32 (mean 27). Maximal dose delivered at end of treatment varied between 0·63 and 1·5 J cm−2.
At baseline the mean ± SD local PSI score was 9·5 ± 1·1 for the treated plaques and 9·4 ± 1·2 for the control plaques. No significant difference was detected between PSI scores of patients enrolled in the first regimen and those enrolled in the second regimen.
As in the first group, the mean PSI score for the whole treated group had already decreased at 2 weeks, from 9·5 to 5·4 (P < 0·001). Improvement remained significant compared with baseline throughout the course of treatment. Early resolution (clearance) occurred in four patients (Fig. 4). Seven patients reached 75% improvement in the local PSI score. All subjects achieved 50% improvement in the PSI score. Overall improvement in the PSI score at the end of the study was 84%.
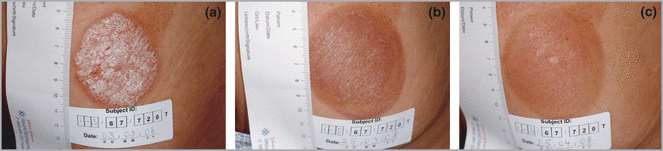
Example of a subject treated with the second regimen. Baseline Psoriasis Severity Index of the treated lesion was 11 (a). The subject had early resolution after 12 treatments (b) and was in remission at the 1-month follow-up visit (c).
No improvement occurred during and at the end of the treatment regimen in PSI scores of the control plaques. No adverse events were reported.
Of the 10 subjects, eight were in remission at the 1-month follow-up visit and five of these patients were still in remission at the 3- and 6-month follow-up visits.
Histology
A significant reduction in inflammatory cells was seen in the biopsy specimens collected at the end of the treatment period compared with baseline. This was accompanied by significant reduction in the expression of proliferation marker Ki-67 and of cytokeratin 16. See also Figure S1 (Supporting information).
Discussion
Psoriasis is a chronic relapsing inflammatory disease of the skin which affects at least 2–4% of the population. Psoriasis has a profound effect on the quality of life of affected patients, its greatest impact being in adults aged 18–45 years, a period when individuals are usually the most productive both occupationally and socially.16
There is no cure for psoriasis and the goal of applied therapies is to achieve remission and to keep the patients disease free for as long as possible. Unfortunately, this objective is not always reached. According to a recent survey of the U.S. National Psoriasis Foundation, less than one-third (29%) of patients are satisfied with current treatment and this may affect their adherence to prescribed regimens.17
Phototherapy is one of the most effective treatments available for psoriasis. The clearance rates following narrowband UVB phototherapy are between 63% and 86%, and between 70% and 90% following psoralen + UVA (PUVA). Remission following phototherapy lasts between 4 and 12 months, which is considered to be among the longest of periods that can be achieved with the currently available therapies. Full-body phototherapy (such as given by narrowband UVB cabins and PUVA) has the disadvantage of concomitant exposure of both psoriatic plaques and healthy skin, increasing the short- and long-term risks of phototherapy. Therefore these treatments are reserved only for severe psoriasis and are not used in mild to moderate psoriasis which represents 75% of all patients with psoriasis. In the last decade new phototherapy devices have emerged such as the XtracTM 308 nm excimer laser and lamp (Photomedex, Montgomeryville, PA, U.S.A.) and the BClearTM Targeted PhotoClearing System (Lumenis Inc., Santa Clara, CA, U.S.A.) which allow a targeted delivery of UVB radiation to the plaques and spare healthy skin. These devices deliver high doses of UV radiation only to the psoriatic plaques and their use is accompanied by similar clinical response as conventional full-body phototherapy.6,8,18,19 The use of high doses (several MEDs) is accompanied by a high incidence of short-term side-effects such as blister formation. Two independent studies reported that during treatment with the 308 nm excimer UVB laser, approximately 40% of patients developed blisters, some needing 7–14 days until complete healing.14,20 In the current study, we have demonstrated that the use of LED technology results in similar clinical outcome as that of the 308 nm laser,21 and has a better side-effect profile. Eight weeks of targeted phototherapy using UVB-LEDs resulted in similar decreases in PSI scores and improvement rates as targeted delivery with the BClear system (a UVB lamp which has the peak emission of 305–313 nm).22 The maximal dose delivered with the BClear system in the study of Lapidoth et al.22 was 8 MED, which was much higher than the maximal dose delivered during either the first (5 MED) or the second (3·8 MED) regimen of the present study. In most patients PSI improvement appeared sooner with the UVB-LED device than with the BClear device (at 2 weeks 36% in group 1 and 43% in group 2 compared with 12% with BClear). Remission times in both groups were similar to those of standard phototherapy and to other targeted phototherapies.
The main mechanism of action of UVB radiation is induction of apoptosis in inflammatory cells, mirrored by dramatic reduction of these cells at the end of treatment. UVB-LED phototherapy resulted in significant reduction of inflammatory cells and was followed also by reduction in keratinocyte proliferation as judged by expression of the Ki-67 nuclear protein. These results are in concordance with reports published by other groups.12,23,24
In our study, mineral oil was used to moisten the plaques before each treatment session. Mineral oil has been frequently used in targeted phototherapy as it does not absorb radiation, and by decreasing the reflectance it increases the therapeutic effect of UV radiation.21,25
The disadvantage of both the 308 nm laser and the BClear system is that they are limited to outpatient use. A fundamental requirement of successful phototherapy is application of several treatments over a short time period, resulting in frequent clinic visits which leads to low compliance. Home-based phototherapy may represent an alternative for clinic-based phototherapy. To be widely acceptable by the medical community and patients, home-based phototherapy should fulfil basic requirements such as good efficacy, minimal and controlled exposure of healthy skin, be easy to use, be convenient and give the physician a level of control on how the treatment is delivered. UVB-LEDs allow the design of a wearable and portable small phototherapy device which can be used at home, thus allowing light physical work (e.g. computer work). The second treatment regimen in our study used a conservative escalation of doses, similar to those currently recommended in the literature for home use,26 and was accompanied by clinical results comparable with those obtained in treatment regimens characteristic of clinic-based use.21,22 Future development of the device, such as adding the capability of the physician to preprogram the treatment regimen, will fulfil the physician’s requirement to be able to control and track the patient’s phototherapy at home and would ultimately result in a decrease of uncontrolled self-administered treatments. Therefore UVB-LEDs may represent an attractive alternative therapy for mild and moderate psoriasis. In addition, targeted phototherapy may be used as an add-on therapy in patients receiving systemic drugs.
In the present study, we have demonstrated that UVB-LEDs represent a promising technology for targeted phototherapy of psoriasis. They are highly effective in treating localized psoriatic lesions and their safety profile is outstanding. As the technology allows the design of small, wearable, portable devices coupled with the ability to achieve uniform optical distribution to the skin and treatment regimen programmability by the physicians they may represent the next generation of home-based phototherapy devices. Besides psoriasis, this technology may be successfully applied to treat other localized photoresponsive skin diseases such as vitiligo. The device may also be used for photosensitivity testing and MED testing in routine clinical practice.
What’s already known about this topic?
- •
Phototherapy is an efficient treatment of psoriasis recommended by guidelines round the world.
- •
Based on action spectrum studies narrowband ultraviolet B phototherapy is the treatment of choice in psoriasis.
- •
Full-body phototherapy is applied in patients with severe psoriasis, whereas targeted phototherapy has recently been introduced to treat mild and moderate psoriasis.
- •
Targeted phototherapy is characterized by limited exposure of healthy skin, therefore reducing the short- and long-term side-effects.
What does this study add?
- •
This is the first study reporting the efficacy and safety of a new phototherapy device based on light-emitting diode technology
- •
Targeted phototherapy delivered with this device is effective in chronic plaque-type psoriasis.
- •
The device has an excellent safety profile, significantly reducing short-term side-effects such as blister formation.
- •
This technology may represent a platform for development of clinic- and home-based phototherapy devices for psoriasis and other inflammatory skin diseases.
Acknowledgments
The research was sponsored by Allux Medical Inc., Menlo Park, CA, U.S.A., OTKA NK77434, OTKA-K68680 and TAMOP 4.2.2-08/1 grants. A.H.B. was supported by Leukaemia Research, U.K.




