Dietary effect of squid and fish meals on growth and survival of Pacific white shrimp Litopenaeus vannamei in the presence or absence of phytoplankton in an indoor tank system
Abstract
This study was conducted in 36 indoor 40-L fibreglass aquaria to determine the weight gain and survival of Litopenaeus vannamei fed different dietary levels of fish (FM) and squid meal (SM) and to evaluate the potential of phytoplankton to reduce FM and SM levels in shrimp feeds. Six experimental isonitrogenous (35% protein) and isocaloric (17.5 kJ g−1) diets were formulated to contain either 5%, 10% or 20% SM combined with either 6.5% or 12% FM. Dietary effects on growth and survival were compared in two systems: a ‘CLEAR water system’ (CWS) without the presence of microalgae and a ‘GREEN water system’ (GWS) with microalgae in the culture water. Shrimp cultured in the GWS had 28–57% greater weight gain than those cultured in the CWS, regardless of dietary treatment. However, survival was not different. Shrimp cultured in the CWS or the GWS, and fed diets containing combinations of FM and SM did not show differences in weight gain and feed conversion ratio. These results suggest that under the conditions existing during this research, 6.5% FM and 5% SM can be used as a cost-effective combination in commercial feeds for shrimp production and that growth can be enhanced in the presence of primary productivity.
Introduction
Global shrimp culture commonly experiences variations in price of feed ingredients (Coutteau, Ceulemans, Meeuws, Van Halteren, Robles & Nur 2008), largely due to demand and availability. The physical and nutritional quality of prepared feeds and efficacy of feed management are important because supplemental feeds can represent 20–50% of variable production costs, depending on the intensity of the culture system (Akiyama, Dominy & Lawrence 1992; Lawrence & Houston 1993; Tacon & Barg 1998). Thus, development of more cost-effective feeds with improved management is required for further development of the shrimp farming industry.
Natural productivity plays an important role in shrimp nutrition and needs to be considered when formulating shrimp feeds (Leber & Pruder 1988; Moss & Pruder 1995; Otoshi, Montgomery, Look & Moss 2001; Michele, Melony, Stuart, Sandy, Peter & Nigel 2004). Juvenile shrimp reared in organically rich, hyper-eutrophic water and fed two commercial diets, grew 50–73% faster than shrimp fed an identical diet, but maintained in well water devoid of natural productivity (Leber & Pruder 1988). Growth enhancement has been attributed to the assimilation of microalgae and microbial-detrital aggregates present in pond water by shrimp (Moss & Pruder 1995). Moss, Pruder, Leber and Wyban (1992) showed that water from traditional shrimp production ponds containing high levels of organic matter (i.e. microalgae) increased the growth of cultured shrimp by as much as 53%. The greater white shrimp growth in the presence of biofloc including microalgae versus clear water has also been previously evaluated (Tacon, Cody, Conquest, Divakaran & Forster 2002; Burford, Thompson, McIntosh, Bauman & Pearson 2004; Cuzon, Lawrence, Gaxiola, Rosas & Guillaume 2004; Moss, Forster & Tacon 2006; Wasieleski, Atwood, Stokes & Browdy 2006). However, no one has previously reported that the level of marine animal meals can be reduced in shrimp diets in the presence of microalgae.
The most common marine animal meals in shrimp feeds are fish meal, squid meal and krill meal (Lim & Dominy 1990; Akiyama & Dominy 1991; Tacon 1993; Tacon & Barg 1998). However, all three of these ingredients are natural, supply limited aquatic resources and thus relatively high priced. Efforts should be made to determine the best relative levels of these critical sources of protein in shrimp diets using feed performance and cost, especially considering availability of intrinsic sources of nutrition such as phytoplankton. Forster, Dominy, Lawrence, Castille and Patnaik (2010) evaluated growth and survival of Litopenaeus vannamei in a 35-day growth trial with 25 different combinations of squid, krill and fish meal in an indoor recirculating system in the relative absence of natural productivity. Growth of shrimp was greater when fed diets containing 11.6% fish and 22.9% squid meals when compared with a combination of 5.8%, 9.7%, 11.6%, 14.5%, 17.4%, 13.5% of fish meal with 7.6%, 11.4%, 15.2%, 19.1% and 22.9% of squid meal. These combinations, in the presence of and absence of krill meal resulted in similar growth, indicating that krill meal was not required with those levels of fish and squid in a CLEAR water system. The growth response to different dietary levels of fish and squid meal in the presence of phytoplankton was not evaluated. To optimize diets for shrimp in GREEN water systems, it is critical to evaluate the potential of microalgae to reduce fish and squid meal inclusion levels in commercial feeds for L. vannamei. The objectives of the present study were (1) to determine the effect of fish and squid feed levels in the presence and absence of microalgae on growth performance and survival of juvenile L. vannamei; and (2) to evaluate whether the presence of microalgae in the shrimp culture environment has the potential to reduce inclusion levels of squid and fish meals in commercial feeds.
Materials and methods
This study was conducted in a greenhouse at the Alicorp Aquarium System, located at a shrimp farm in Tumbes, on the north Pacific coast of Peru. Specific-pathogen-resistant L. vannamei postlarvae in their 14th day of development after metamorphosis (PL14), were obtained from the Lobo Marino N°1 Laboratory (Salinas, Ecuador) and maintained in a greenhouse nursery system for 24 days prior to use. The nursery system was a high-density polyethylene-lined, sediment-free wooden tank system (28 m3 volume). Aeration was provided by regenerative blower. The PL14 (0.02 g, 2% CV) were stocked at a density of 65 per m2, and manually fed 20 g of a commercial 40% crude protein (CP) crumble of 0.3–0.8 mm diameter (Nicovita® PC-1 40% CP; Alicorp, Lima, Peru) three times daily at 6:00, 12:00 and 18:00 hours. Newly hatched live Artemia sp. nauplii were also fed daily (50 nauplii per PL day−1) for 2 weeks. For the next 10 days, only a commercial 40% CP crumble of 0.8–1.5 mm diameter (Nicovita® KR-1 40% CP; Alicorp) was fed at a rate of 45 g, three times (6:00, 12:00 and 18:00 hours) daily until harvest. This conditioning period allowed for acclimation to laboratory conditions (temperature 30.8°C ± 1.07 SD and salinity 18.1 g L−1 ± 0.4 SD) and achievement of sufficient body weight for initiation of the experimental trial. Juveniles used in the trial were netted and transferred to the experimental units and allowed to acclimate for 1 week to a commercial 35% CP diet of 2 × 2 mm (length × diameter) (Nicovita®; Alicorp – Bag Tag: min 35% protein, min 5% fat, max 12% moisture, max 4% fibre and max 12% ash) prior to starting the trial. The shrimp were netted and individually weighed to determine the initial weight before the 8-week trial.
The experimental system consisted of 36 indoor rectangular fibreglass aquaria (40 L volume; 0.1-m2 bottom surface area) connected to either of the following two water supply systems : (1) a 2680-L semi-closed recirculating system, consisting of a sump tank, three mechanical 200-, 75- and 5-μ filters, a biosphere biological filter and UV sterilizer and (2) an open system consisting of four, 28-m3 wooden PVC-lined reservoir tanks. The water supply, which was also used for the shrimp farm ponds, was pumped from the ‘El Venado’ estuary, through a 280-μ filter bag into four 28-m3 wooden PVC-lined reservoir tanks. The stock water was chlorinated to minimize the introduction of pathogens from wild vectors, and kill plankton and benthos. A dose previously proven effective of 10 mg L−1 of calcium hypochlorite solution (65% active ingredient) was used to provide a free chlorine residual concentration of 4 mg L−1, 30 min post application with a targeted residual chlorine level of 1 mg L−1 after 24 h (analysis HACH-DR 2800, Loveland, CO, USA). After 72 h of aeration, the chlorine concentration of the water was reduced to <0.05 mg L−1 and was used to fill 18 aquaria of the semi-closed recirculating system, hereafter referred to as the CLEAR water system . Two of the four chlorinated reservoir tanks were maintained in bloom with microalgae through a continuous fertilization programme. The water was not seeded with microalgae. It bloomed from natural air spores in the reservoir water, as typically occurs in commercial lined ponds. Natural light source was used to maintain the algae. The water was fertilized with N : P and N : Si ratios of 15:1 and 1:1.25 respectively. Inorganic fertilization was applied at mid-day (12:00 ± 2 hours) with NUTRILAKE® (15% nitrogen), Triple Super Phosphate (20% phosphorus) and NUTRICIL® (23% silicate). A total of 30 g of 35% ground CP commercial feed (5.6% nitrogen, 0.85% phosphorus) and 30 g of commercial organic fertilizer (2.1% nitrogen, 0.65% phosphorus) were also added daily, as sources of carbon, nitrogen and phosphorus. After day 7, no NUTRILAKE® and Triple Super Phosphate were added. The source of metasilicate, NUTRICIL®, was maintained at a rate of 140 g day−1 (the equivalent of 50 kg ha−1 day−1) throughout the trial. Ground commercial diet (Nicovita® 35% CP; Alicorp) and a commercial organic fertilizer (Nicovita® FB 12% CP; Alicorp) were maintained at the same rate of 30 g day−1 throughout the trial. This water was used to fill another 18 aquaria and was labelled as the GREEN water system. The CLEAR water system had a 21% new water exchange rate (1.1-L shrimp−1 day−1 – incoming chlorinated water that passed through 20- and 5-μ filters) and a recirculating rate of 0.95 L min−1 tank−1 (3409% exchange tank−1 day−1). The GREEN water system was exchanged by 20% (1.0-L shrimp−1 day−1) daily for 6 days per week and by 50% (2.5-L shrimp−1 day−1) on the 7th day of the week.
Eight (equivalent to 80 shrimp m−2) L. vannamei, very similar in size, were stocked in each aquarium. The mean weight per aquarium, weighed as a group, varied from 1.56 to 2.66 g, with no significant differences among treatments (P = 0.964). Aeration was provided to each aquarium by one 2.5 × 2.5 × 5 cm air stone connected to a 0.5-hp air blower to maintain oxygen at a minimum of 6 mg L−1. A light : dark photoperiod of 12 : 12 h was provided using supplemental compact fluorescent lighting. The distance between the white fluorescent lights (36 W) and the water in the aquarium system was 1.60 m.
Six experimental isonitrogenous (35% CP) and isocaloric (17.5 kJ g−1) diets were formulated to contain one of three levels of squid meal (S – 5%, 10% or 20%) combined with one of two levels of fish meal (F – 6.5% or 12%) (Table 1 – The diets will be referred to hereafter as 5S6.5F; 5S12F; 10S6.5F; 10S12F; 20S6.5F; 20S12F with S and F representing squid and fish meals respectively). De-hulled and de-fatted soybean meal was added to maintain equal protein and energy levels in experimental diets. At the Nicovita® pilot feed mill plant (NPP, Lima, Peru), all ingredients were finely ground at 250 ± 100 μ, mixed, steam conditioned, pelletized (compacted and cut), dried and cooled to obtain a fast sinking and water stable 2 × 2 mm pellet. Proximate analyses and pepsin digestibility (AOAC 971.09, 2005) were determined on each experimental diet. Diets were placed in labelled plastic containers and placed into a refrigerator at 4°C prior to feeding.
| Feed ingredient | Diet designation | |||||
|---|---|---|---|---|---|---|
| 5S6.5F | 5S12F | 10S6.5F | 10S12F | 20S6.5F | 20S12F | |
| Squid meala | 5 | 5 | 10 | 10 | 20 | 20 |
| Fish meala | 6.5 | 12 | 6.5 | 12 | 6.5 | 12 |
| Soybean meala | 39.77 | 34.31 | 34.78 | 29.54 | 20.04 | 12.48 |
| Wheata | 33.7 | 33.7 | 33.7 | 33.5 | 38.5 | 40.6 |
| Lecithina | 4 | 4 | 4 | 4 | 4 | 4 |
| Fish oila | 2 | 2 | 2 | 2 | 2 | 2 |
| Crustacean mealb | 2 | 2 | 2 | 2 | 2 | 2 |
| Brewers yeasti | 2.7 | 2.7 | 2.7 | 2.7 | 2.7 | 2.7 |
| Marine salth | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Calcium carbonateh | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Potassium phosphate dbh | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Potassium chlorideh | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 |
| Magnesium oxideh | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| DL-methioninec | 0.18 | 0.14 | 0.17 | 0.11 | 0.11 | 0.07 |
| Cholesterold | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Vitamin premixe | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Mineral premixf | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Stay C 35%g | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
- a Alicorp, Lima, Peru: Peruvian giant squid muscle meal (Dosidiscus gigas), Peruvian fish meal (Engraulis ringens) and fish oil, Dehulled and defatted soybean meal, liquid lecithin and hard red winter wheat.
- b Cervimunida johni – BAHIA SpA, Santiago, Chile.
- c Sigma Chemical Company, Cleveland, OH, USA.
- d Dishman, Utrecht, The Netherlands.
- e DSM Vitamin premix: Vit. A 10 000 IU g−1; B1 30 mg kg−1; B2 15 mg kg−1; DL Ca panthoten 50 mg kg−1; B6 35 mg kg−1; B12 40 μg kg−1; Ascorbic acid 150 mg kg−1; K3 3 mg kg−1; D 33 500 IU g−1; E 150 IU g−1; niacin 100 mg kg−1; folic acid 4 mg kg−1; biotin 1000 μg kg−1.
- f DSM Mineral premix: Mn 40 mg kg−1; Zn 40 mg kg−1; Cu 25 mg kg−1; Fe 100 mg kg−1; Se 0.3 mg kg−1; I 0.35 mg kg−1.
- g DSM, Lima, Peru.
- h ICN Biomedicals, Aurora, OH, USA.
- i Inactive Saccharomyces cerevisiae – ICC, Sao Paulo, Brazil.
The biochemical composition of the diets was determined as follows: CP (DUMAS method; ISO 16634-1:2008; protein combustion analyser LECO TruSpec®TRSCNC, LECO Corp., St. Joseph, MI, USA; Mugford 2000); crude fat (AOAC 920.39 C, 2005 Ether Extract); crude fibre (AOCS Ba 6-84, 1998); ash (AOAC 942.05, 2005); moisture (NTP-ISO 6469, 2002); nitrogen-free extract [100 − (CP + crude fibre + ether extract + ash)]; gross energy (Parr 6200 Oxygen Bomb Calorimeter; Parr, Moline, IL, USA); amino acid concentrations (AOAC 2007, No. 988.15); and phospholipids (AOCS 1998, Ca 12 – 55 – phosphorus). Prior to high pressure liquid chromatography analysis of amino acid concentration, duplicate feed samples (100 mg) were hydrolysed in vacuum with 5 mL of HCl 6N for 22 h at 112°C.
The feeding trial was conducted by feeding each experimental diet in triplicate over a period of 56 days using a randomized block design. Automated feeders were used to feed shrimp five times daily (08:00, 11:00, 14:00, 17:00 and 20:00 hours) with equal rations at each feeding. The feed from the automatic feeder fell only in a specially designed 10-cm diameter plastic feed tray, which allowed daily removal and weighing of uneaten feed. Faeces and moults were also removed daily. Feeding rates were based on a feed table beginning at 0.13 g feed shrimp−1 day−1 and gradually increased to a maximum of 0.42 g shrimp−1 day−1. Feeding rate was always above satiation for all treatments. Feed rations were adjusted on a weekly basis, according to a projected weight gain of 2 g shrimp−1 week−1 and survival.
With the exception of salinity (g L−1) that was measured during the afternoon, water temperature (°C), dissolved oxygen (mg L−1) and pH were monitored twice daily (6:00 and 18:00 ± 0.5 hours) in the experimental aquaria and sump tank. Total ammonia nitrogen (TAN), nitrite nitrogen (NO2-N) and alkalinity were monitored weekly in 24 experimental aquaria and the sump tank. The 24 aquaria were randomly chosen every week. Species identification of microalgae and cell density (cell mL−1) were determined twice weekly, and chlorophyll (mg m−3) analysis was performed weekly in the GREEN water aquaria. Samples were collected at 12:00 hours using 500-mL plastic bottles. Subsamples (~150-mL) were preserved by adding 2 mL of Lugol's solution (Throndsen 1978). Cells were allowed to settle for 10 h and subsequently processed for taxonomic and cell count analysis using an improved Neubauer haemocytometer at 600× magnification. Methodology for cell counts followed that of Venrick (1978). Malca's (1997) manual was used to separate microalgae into taxonomic divisions. Total chlorophyll was measured according to Strickland and Parsons (1972).
Crude protein (nitrogen × 6.25), essential amino acids, crude fat, ash, gross energy and moisture of phytoplankton samples were also determined. These samples were collected once weekly, filtering approximately 180 L of the system incoming water containing primary productivity, using a set of 100-, 75- and 5-μ mesh filters for 2 min, and then storing samples in a freezer (−12 to −18°C). The frozen samples were freeze-dried at −50°C for 48 h for reduction in moisture to less than 10%. Eight freeze-dried samples were combined and analysed as two composite samples to verify the accuracy of results.
Nutritional responses of the shrimp to the experimental diets were evaluated using the following indicators: (1) total weight gain (final mean wet weight − initial mean wet weight); (2) survival (final number of animals/initial number of animals) × 100; and (3) FCR – feed conversion ratio (total feed intake in dry weight basis/total gained biomass). The microalgae per cent contribution to shrimp growth for each treatment also was estimated by comparing the shrimp weight gain in the GREEN versus CLEAR water systems [(GREEN mean wet weight gain − CLEAR mean wet weight gain)/CLEAR mean wet weight gain] × 100.
A 2 × 3 × 2 factorial analysis of variance (anova) was used to determine significant differences and their interaction among treatments (two fish meal levels, three squid meal levels and two microalgae levels) on weight gain and survival of shrimp. Survival was transformed using arcsine square root before being submitted to statistical analyses, to comply with the assumption of data being normally distributed. The normality was tested using ‘Kolmogorov–Smirnov’. When significant (α = 0.05) F values were obtained, differences among treatments were determined using Fisher's least significant difference multiple range test. The data were analysed using the spss statistical software version 16 for Windows (SPSS, Chicago, IL, USA).
Results
Proximate composition of the experimental diets (Table 2) varied slightly from the formulated values for CP and gross energy, although they were constant in all diets. The desired levels of lipid, ash and fibre were achieved. Amino acid composition of the diets (Table 3) was also relatively constant.
| Component | Diet designation | |||||
|---|---|---|---|---|---|---|
| 5S12F | 5S6.5F | 10S12F | 10S6.5F | 20S12F | 20S6.5F | |
| Crude protein (%) | 39.7 | 40.0 | 40.8 | 39.6 | 42.3 | 43.0 |
| Crude fat (%) | 9.4 | 9.4 | 9.7 | 9.2 | 9.8 | 9.4 |
| Ash (%) | 10.1 | 7.8 | 10.5 | 10.1 | 10.0 | 8.0 |
| Crude fibre (%) | 2.0 | 2.3 | 1.9 | 2.0 | 2.1 | 2.2 |
| NFE (%)b | 38.8 | 40.5 | 37.1 | 39.1 | 35.8 | 37.4 |
| Gross energy (kJ g−1) | 18.2 | 18.5 | 18.2 | 18.3 | 18.1 | 18.7 |
| Phospholipids (%) | 3.7 | 3.4 | 3.4 | 3.8 | 3.9 | 3.8 |
| Digestibility (%)c | 94.3 | 93.1 | 93.2 | 94.0 | 93.7 | 93.8 |
- a Values represent averages of duplicate samples.
- b Nitrogen-free extract.
- c Pepsin.
| Amino acids | Diet designation | |||||
|---|---|---|---|---|---|---|
| 5S12F | 5S6.5F | 10S12F | 10S6.5F | 20S12F | 20S6.5F | |
| Non essential amino acids | ||||||
| Aspartate+asparagine | 4.1 | 4.3 | 4.2 | 4.1 | 4.1 | 4.2 |
| Serine | 1.8 | 1.9 | 1.9 | 1.8 | 1.8 | 1.9 |
| Glutamate+glutamine | 6.9 | 7.3 | 7.1 | 6.9 | 6.9 | 7.1 |
| Glycine | 2.1 | 2.0 | 2.2 | 2.1 | 2.5 | 2.5 |
| Alanine | 1.9 | 1.9 | 2.1 | 1.9 | 2.2 | 2.2 |
| Proline | 2.2 | 2.3 | 2.3 | 2.2 | 2.3 | 2.4 |
| Tyrosine | 1.2 | 1.2 | 1.1 | 1.3 | 1.2 | 1.2 |
| Total NEAA | 20.1 | 20.9 | 20.8 | 20.3 | 21.0 | 21.6 |
| Essential amino acids | ||||||
| Histidine | 1.2 | 1.3 | 1.3 | 1.2 | 1.3 | 1.3 |
| Arginine | 3.0 | 3.0 | 3.1 | 3.0 | 3.3 | 3.4 |
| Threonine | 1.6 | 1.7 | 1.7 | 1.6 | 1.8 | 1.9 |
| Valine | 1.9 | 2.0 | 2.0 | 1.9 | 2.1 | 2.1 |
| Methionine | 0.8 | 0.9 | 0.9 | 0.9 | 1.1 | 1.1 |
| Methionine+cysteine | 1.4 | 1.5 | 1.7 | 1.6 | 1.9 | 2.0 |
| Lysine | 2.5 | 2.5 | 2.6 | 2.5 | 2.7 | 2.7 |
| Iso-leucine | 1.8 | 1.9 | 1.9 | 1.8 | 2.1 | 2.1 |
| Leucine | 2.8 | 3.0 | 3.0 | 2.9 | 3.2 | 3.3 |
| Phenylalanine | 1.8 | 1.9 | 1.9 | 1.8 | 1.9 | 2.0 |
| Total EAA | 18.0 | 18.8 | 19.2 | 18.2 | 20.1 | 20.6 |
| Total AA | 38.1 | 39.7 | 40.0 | 38.6 | 41.1 | 42.2 |
| EAA/Total AA (%) | 52.8 | 52.7 | 52.2 | 52.7 | 51.1 | 51.1 |
- a Values represent averages of duplicate samples.
There were no significant differences in treatments with respect to water quality indicators within each water system, with the exception of nitrite and alkalinity (P < 0.001; Table 4).
| Parameter | GREEN water system | CLEAR water system | P |
|---|---|---|---|
| Temperature (°C) | 30.4 ± 1.07a | 30.4 ± 0.98a | 0.11 |
| Salinity (g L−1) | 25.5 ± 0.94a | 25.5 ± 0.98a | 0.827 |
| pH | 7.77 ± 0.13a | 7.75 ± 0.14a | 0.43 |
| Dissolved oxygen (mg L−1) | 6.0 ± 0.22a | 6.0 ± 0.21a | 0.228 |
| Chlorophyll (ug L−1) | 12.1 ± 6.8 | ||
| Total ammonia nitrogen (mg L−1) | 0.017 ± 0.01a | 0.016 ± 0.01a | 0.69 |
| NO2-N (mg L−1) | 9.35 ± 5.96a | 0.52 ± 0.23b | <0.001 |
| Alkalinity (mg L−1) | 100.43 ± 13.45a | 92.96 ± 11.95b | 0.001 |
- Values are means ± standard deviation of daily and weekly determinations over the 8-week trial.
- n = 2016 for temperature, pH and DO; 1008 for salinity; 96 for ammonia, nitrite and alkalinity; 126 for chlorophyll.
- Means within rows with the same letter are not significantly different (least significant difference, α = 0.05).
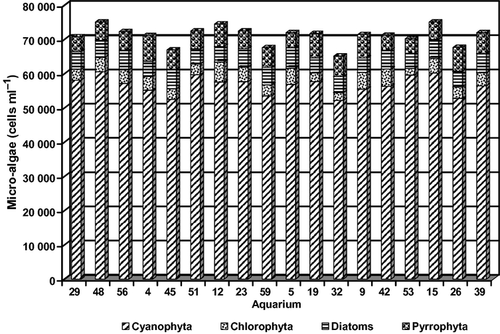
The level of phytoplankton in the CLEAR water system was negligible throughout the feeding trial and contrasted with that of the GREEN water system. Mean cell counts of microalgae among aquaria were not significantly different (anova: P > 0.05) in the GREEN water system (Fig. 1). Cyanobacteria were the predominant microalgae division, followed by Pyrrophytes, Diatoms and Chlorophytes (Table 5). Phytoplankton cell counts among dietary treatments were not significantly different (anova: P > 0.05), and had similar distribution patterns based on the average values of each aquaria in the GREEN water system (Fig. 2). The proximate and amino acid composition of composite microalgae samples were rather similar (Table 6).

| Division | Genus | Frequency (%)GREEN |
|---|---|---|
| Diatoms | Chaetocheros | 27.3 |
| Navicula | 20.9 | |
| Nitzchia | 18.8 | |
| Cymbella | 21.9 | |
| Thalassiosira | 6.0 | |
| Gyrosigma | 3.6 | |
| Amphiprora | 1.0 | |
| Amphipleura | 0.5 | |
| % Total division | 7.0 | |
| Cyanobacteria | Oscillatoria | 100.0 |
| % Total division | 79.6 | |
| Pyrrophytes | Phacus | 70.9 |
| Glenodinium | 29.1 | |
| % Total division | 8.1 | |
| Chlorophyts | Chlamydomonas | 96.2 |
| Oocysts | 3.8 | |
| % Total division | 5.4 |
| Analyte | Composite | |
|---|---|---|
| 1 | 2 | |
| Dry matter (%) | 5.8 | 6.2 |
| Crude protein (%) | 15.3 | 14.9 |
| Crude fat (%) | 0.3 | 0.4 |
| Ash (%) | 45.6 | 45.9 |
| Gross energy (kJ g−1) | 7.5 | 7.7 |
| Essential amino acids (% of dry matter) | ||
| Histidine | 0.18 | 0.18 |
| Arginine | 0.72 | 0.71 |
| Threonine | 0.67 | 0.68 |
| Valine | 0.78 | 0.77 |
| Methionine | 0.26 | 0.25 |
| Methionine+cysteine | 0.39 | 0.37 |
| Lysine | 0.62 | 0.61 |
| Isoleucine | 0.61 | 0.60 |
| Leucine | 0.93 | 0.92 |
| Phenylalanine | 0.64 | 0.63 |
Factorial anova indicated that weight gain of shrimp in the GREEN water system was significantly higher, and that FCR was significantly lower (Table 7). However, survival was not significantly different among dietary treatments and water systems. In all cases, the interaction between the three factors (fish meal level, squid meal level and productivity) was not statistically significant (Table 7). No statistical differences in weight gain and FCR were evidenced in shrimp fed diets containing the various combinations of fish meal and squid meal, cultured in the CLEAR (with no phytoplankton) and GREEN water system (Table 7). Survival of shrimp among all water systems was not significantly different. Shrimp FCR values for those in the GREEN water system was significantly lower (anova: P < 0.001) among dietary treatments. The per cent contribution of phytoplankton to weight gain of shrimp were 35% (5S6.5F), 47% (5S12F), 52% (10S6.5F), 28% (10S12F), 57% (20S6.5F) and 36% (20S12F).
| SM level | FM (%) | Water system | Initial weight (g) | Weight gain (g) | Survival (%) | FCR |
|---|---|---|---|---|---|---|
| 5 | 6.5 | GREEN | 2.2 | 12.3a | 96 | 1.74a |
| 5 | 12 | GREEN | 2.0 | 12.5a | 83 | 1.98a |
| 10 | 6.5 | GREEN | 1.8 | 12.2a | 96 | 1.82a |
| 10 | 12 | GREEN | 1.9 | 11.9a | 92 | 1.96a |
| 20 | 6.5 | GREEN | 2.0 | 12.9a | 96 | 1.68a |
| 20 | 12 | GREEN | 1.9 | 12.7a | 100 | 1.66a |
| 5 | 6.5 | CLEAR | 1.8 | 8.2b | 100 | 2.32b |
| 5 | 12 | CLEAR | 1.8 | 8.5b | 96 | 2.54b |
| 10 | 6.5 | CLEAR | 1.8 | 8.0b | 83 | 2.77b |
| 10 | 12 | CLEAR | 1.9 | 9.3b | 92 | 2.45b |
| 20 | 6.5 | CLEAR | 1.8 | 8.3b | 92 | 2.59b |
| 20 | 12 | CLEAR | 1.8 | 9.3b | 84 | 2.92b |
| PSEa | 0.063 | 0.75 | 0.21 | 0.43 | ||
| anova: P-values | ||||||
| Fish meal | 0.254 | 0.265 | 0.369 | |||
| Squid meal | 0.473 | 0.908 | 0.695 | |||
| Water system | <0.001 | 0.463 | <0.001 | |||
| Fish meal × squid meal × water system | 0.632 | 0.224 | 0.307 | |||
- Water system: level of microalgae in aquaria.
- Means within columns with the same letter are not significantly different (least significant difference, α = 0.05).
- a Pooled standard error of treatment means = square root (MSE/n).
Discussion
Mean NO2-N for aquaria in the CLEAR water system were lower than those in the GREEN water system, probably due to the nitrifying bacteria present in the bio-filter of the clear recirculation water system . Alkalinity was slightly lower in the CLEAR water system, probably due to a reduction in calcium carbonate (CaCO3) levels, caused by a relatively low new water exchange rate in the recirculation system. Despite the differences in nitrite and alkalinity levels among the water systems, all water quality factors were within acceptable ranges for survival and growth of L. vannamei (Wickins 1976; Van Wyk, Davis-Hodgkins, Laramore, Main, Mountain & Scarpa 1999; Lin & Chen 2003; Handy, Samocha, Patnaik, Gandy & McKee 2004; Sowers, Young, Shawn, Isely, Jeffery, Browdy & Tomasso 2004; Cohen, Samocha, Fox, Gandy & Lawrence 2005; Mishra, Samocha, Patnaik, Speed, Gandy & Abdul-Mehdi 2008) throughout the duration of the 8-week experiment.
In the present study, no diet-related differences in weight gain and FCR were observed in the CLEAR and in the GREEN water system. Forster et al. (2010) observed that the optimum dietary combination of marine animal meals in diets for L. vannamei in indoor, clear water tanks was 11.6% fish meal and 22.9% squid meal. Even though results from this study and the present one are not comparable due to differences in shrimp strain, initial weight, growth rate, water source, diet ingredients and conditions of the trial, trends concerning the levels of fish and squid meal inclusion in diets for L. vannamei were observed. Taking into consideration the assemblage of phytoplankton characterized under the experimental conditions of the present study, the most cost-effective combination of ingredients was the diet containing 6.5% fish meal and 5% squid meal, suggesting that fish and squid meal levels in commercial feeds for L. vannamei can possibly be reduced in the presence of microalgae in the culture water.
The estimation of per cent contribution of phytoplankton to weight gain of shrimp in this study ranged from 28% to 57% for L. vannamei stocked at 80 m−2. Lawrence and Houston (1993) estimated 77% to 83% contribution of natural productivity versus prepared diets to weight gain of shrimp in pens and in earthen ponds stocked at 15 and 20 m−2 respectively. Anderson, Parker and Lawrence (1987) and Parker, Anderson and Lawrence (1989) using a stable carbon isotope tracer technique obtained similar estimates of per cent contribution of natural productivity for assimilation of organic carbon by L. vannamei at similar stocking densities (20 m−2) with values ranging from 53% to 77% and 44% to 86% compared with prepared feed. Even though there were not significant differences in the reported percentage contribution between the two studies, the variation in ranges could have been due to the different tracers used and variations in the characteristics of the pond biota, as they were conducted in different years. Moss et al. (1992) evaluated weight gain of L. vannamei in 40 m2 tanks provided with selected solid fractions of pond water with biofloc. Their study estimated contributions of 53% for particles between 0.5 and 5 μ and an additional 36% for particles >5 μ, resulting in a total contribution of 89%. Wasieleski et al. (2006) detected significant differences in growth and survival of juvenile L. vannamei fed two levels of protein (35% and 25%) and reared for 20 days (stocking weight of 1.82 ± 0.71 g) in 50-L plastic bins at a density of 300 m−2 within a raceway with and without natural productivity (zero-exchange system with suspended microbial floc). Conceptually, as the stocking density and harvest biomass increase in an earthen pond, the per cent contribution of natural productivity to the nutritional requirements of shrimp would decrease if all other factors did not change (Lawrence & Houston 1993). Furthermore, using higher stocking densities in an aquarium with no soil, as in the present study, the contribution of the four divisions of microalgae for L. vannamei growth, would probably be relatively less. Thus, the relative contribution of natural productivity obtained in the present study indicates that the method can be used for estimating the contribution of microalgae to the weight gain of shrimp in aquaria systems.
As none of the analysed nutrients were limiting in any of the diets, differences in growth within diets cannot be attributed to any of the analysed microalgae nutrients. The greater weight gain in the presence of primary productivity was possibly due to either (1) the phytoplankton contribution to a specific, but unknown nutrient(s) in the shrimp diet, (2) unknown growth factors or (3) affecting of some water quality or other system factor. Further studies are recommended to better understand the specific nutrient(s) that may contribute to the better growth of shrimp in the presence of microalgae.
The significant weight gain exhibited by shrimp fed the different dietary treatments in the GREEN water system could have also been related to the greater digestion of unknown microalgae nutrients through the stimulation of the digestive enzymatic system of the shrimp when exposed to a microalgal environment. Moss, Divakaran and Kim (2001) compared the digestive enzyme activity of L. vannamei (mean weight = 0.07 g, SD = 0.004 g) reared at 40 m−2 in well and pond water plastic tanks for 35 days and fed a commercial 45%-protein feed. These authors observed that specific activities of serine protease, collagenase, amylase, cellulase, lipase and acid phosphatase in the shrimp digestive gland were more than two times higher in pond water than in well water, and that shrimp reared in pond water had significantly greater growth (4.9 g) compared with well water (0.97 g). Divakaran and Moss (2004) investigated whether there was any difference in the specific activity of laminarinase between shrimp grown in an indoor CLEAR water system and an outdoor zero-water exchange shrimp culture system. Specific activity of laminarinase was nearly seven times higher in shrimp grown in the outdoor zero-water exchange system, compared with shrimp grown in the indoor CLEAR water system, thereby indicating the possibility of substrate specificity and enhanced enzyme production. If, as this study suggests, laminarinase is present in the digestive gland of shrimp reared in the presence of microalgae, this enzyme may serve an important role in the digestion of microalgae nutrient(s) and other sources of beta-1,3-linked glucans present in shrimp pond water.
The data obtained in the present study suggest that the squid and fish meal levels in commercial feeds for L. vannamei can be reduced. Furthermore, microalgae present in the culture system significantly improved weight gain and FCR of shrimp, thus potentially reducing the feed cost associated with shrimp production.
Acknowledgments
The authors acknowledge Alicorp for the financial support in the execution of this work and their technicians for assistance in conducting the experiment.




