Triploidization in rohu × mrigal hybrid and comparison of growth performance of triploid hybrid
Abstract
The present study was aimed at the identification of treatment optima to induce triploidy in ‘Labeo rohita (rohu) × Cirrhinus cirrhosus (mrigal)’ hybrid using heat shock treatment. The eggs were exposed at four different temperature regimes viz., 38, 39, 40 and 41°C for 1–3 min, applied 3–5 min after fertilization. After 4 min of fertilization, heat shock treatments for 1 and 1.5 min durations were found the best inducing triploidy up to 100% and 96% respectively. Survival rates upto yolk sac absorption were found to be 73% and 71% in rohu and mrigal, 68% and 67% in the reciprocal diploid hybrids and 61% and 60% in the reciprocal triploid hybrids (RTH). Triploidy was confirmed by chromosome counting that revealed the diploid chromosome number of rohu and mrigal at 2n = 50 and in their triploid hybrid chromosome number was found to be 3n = 75. Growth rate of the RTH showed a significant difference (P < 0.05) from the single species and the diploid hybrids. Triploids also showed higher survival rate over the diploids.
Introduction
Bangladesh waters are rich in biological diversity with 260 fresh water fish, 475 marine fish, 24 freshwater prawn and 36 marine shrimp species (FSY 2008). Inland aquaculture in Bangladesh mainly consists of the Indian major carps viz., rohu (Labeo rohita Hamilton), mrigal (Cirrhinus cirrhosus Block), catla (Catla catla Hamilton) and some Chinese carp species. Until the mid-1980s, fish culturists depended on wild seed, but because of damage to the natural breeding grounds and over-exploitation, the hatchery productions have been the mainstay of seed supply. There are about 77 governmental and 860 private hatcheries that meet the fish demand for culture fishery in Bangladesh (FSY 2009). However, in an attempt to produce more seeds to meet the increasing demand, the hatcheries did not pay attention to the genetic quality and the seeds produced are of poorer quality (Hussain & Mazid 2005; Biswas, Shah, Takii & Humai 2008). Hence, it has become essential to carry out research into genetic improvement of the species through various approaches. The different approaches for fish improvement are: (1) fish breeding (selection and inbreeding), (2) hybridization, (3) chromosome set manipulation (gynogenesis, androgenesis, triploidy, tetraploidy), (4) sex reversion and (5) transgenesis. Thus, a suitable approach can be applied to the targeted species for achieving the desired goal of improvement. In the present study, the two approaches, hybridization and chromosome set manipulation, were adopted for genetic improvement in two carp species of Bangladesh.
In hybridization, the common desired goal is to produce offspring that outperform both the parental species. It is also used to transfer desirable characteristics from one species to another, manipulate sex ratios, produce sterile animals, improve flesh quality, increase disease resistance, improve environmental tolerance and exploit degraded aquatic environments (Bartley, Rana & Immink 2004; David & Pandian 2006). The Indian major carp hybrids are fertile and can be backcrossed to the parental species (Padhi & Mandal 1977). Triploidization can be used to improve the results of interspecific hybridization when the cross produces sub-viable offspring (Scheerer & Thorgaard 1983). Triploids are created by shocking newly fertilized eggs shortly after fertilization that disrupts the spindle formation and suppresses the extrusion of second polar body from the egg (Padhi & Mandal 2000; Yoshikawa, Morishima, Fusimoto, Yamaha & Arai 2007) or by crossing the tetraploids and diploids. By whatever means the ploidy of a species is manipulated, the produced triploid, tetraploid, gynogen and androgen by chromosome set manipulation, all have different implications in fish culture, fisheries management or in fish genetics studies. Triploids are important for two reasons: increased growth and sterility. Triploid fish usually grow faster than their diploid counterparts because of larger cell size (Ferguson 1992; Shah, Thorgaard & Wheeler 1999); the nucleus of triploid fish contains 33% more genetic materials (Tave 1992). Hybrid triploids do not produce functional gametes due to imbalanced chromosomal distribution during meiosis (Garner, Madison, Bernier & Neff 2008) and thus they do not have to spend extra energy to gamete production (Tave 1992; Johnson, Shrimpton, Cho & Heath 2007). Currently, the most suitable method for producing sterile fish, in large numbers, is through triploidy induction (Jhingan, Devlin & Iwama 2003). Hybrid viability, which is often poor, could be improved through artificial triploidization. Diploid hybrids generally survive less, while triploid hybrids survive better (Chevassus, Guyomard, Chourrout & Quillet 1983; Shah et al. 1999). Early attempts in chromosome manipulation of rohu and mrigal were initiated by Reddy, Kowtal and Tantia (1990), Islam, Shah, Rahaman and Chowdhury (1994), Hussain (1996) and Mia, Islam and Shah (2001). But triploidization of the hybrids of Indian major carp is a new attempt in Bangladesh as well as in the Indian sub-continent.
With the growing feed demand by world population and the increased pressure on our natural resources, sustainable aquaculture has to produce more, yet have minimal environmental perturbations. Thus, triploidy can be induced in some commercially important fish species for increasing the growth potential, disease resistance and reproductive capacity to boost aquaculture production. As aquaculture in Bangladesh is facing several problems, the application of genetic knowledge for a candidate species will help tide over the problematic situations. In the present study, attempts were made to induce triploidy in rohu × mrigal hybrid using heat shock treatment. A shock at 40°C temperature for 1–1.5 min, 4 min after fertilization produced 100% triploidy. Growth and survival of the hybrid triploids were compared with reciprocal diploid hybrids (RDH) and single species of rohu and mrigal and growth performance of triploids was found higher than the diploids. Thus, hybrid triploidization can effectively be applied to improve the genetic quality of fish, which will enhance aquaculture production and culture efficiency as well.
Materials and methods
Experimental design
The gametic materials of the experiment were obtained from the brood fish of Fish Seed Multiplication Farm, Gallamari, Khulna of the Department of Fisheries, Bangladesh. The chromosome manipulations were performed in the same hatchery premise. In total, 24 trials (each with four replications) were given to find out the optimum treatment combinations for triploidy induction in the reciprocal hybrids of rohu and mrigal. After getting the optimum treatment combination, another trial with four replications was given to compare the fertilization, hatching, growth and survival rates of different fish groups produced. As a result, six different crosses were produced viz., single species of rohu and mrigal, reciprocal diploid hybrid1 (RDH1) (Hybrid1: ♂ rohu × ♀ mrigal), reciprocal diploid hybrid2 (RDH2) (Hybrid2: ♀ rohu × ♂ mrigal), reciprocal triploid hybrid1 (RTH1) (Triploid1: triploid of hybrid1) and reciprocal triploid hybrid2 (RTH2) (Triploid2: triploid of hybrid2). The karyological analyses were performed in the Fish Biology Laboratory, FMRT Discipline and the growth performance study was carried out in the mini pond complex of Khulna University.
Hypophysation and hybridization
Induced breeding was performed by using carp pituitary intramuscular hormone injection. The doses were 2 and 6 mg kg−1 body weight for the females administered at 6 h duration, whereas the males were given only single injection at 2 mg kg−1 body weight at the time of second dose to the female. Eggs and milt were collected by hand stripping and were mixed well with physiological saline solution (5% dextrose and 0.9% NaCl); temperature of the saline solution was 30°C.
Heat shock treatment
Fertilized eggs were immediately heat shocked by dipping in scoop nets in batches in a thermostatically controlled water bath (HHS 4: WS2-133-75, Anjue Equipment Co. Ltd, Anjue, China). During each trial, about 1000 eggs were taken in the scoop nets for heat shock treatment. Various combinations of temperatures ranging from 38 to 41°C and duration of time ranging from 1 to 3 min were applied, 3–5 min after fertilization (Table 1).
| Temperature (°C) | Time A. F. (min) heat shock applied | Duration of heat shock (min) | Fertilization rate (%) | Hatching rate (%) | Triploidy induced (%) | |||
|---|---|---|---|---|---|---|---|---|
| Hybrid1 | Hybrid2 | Hybrid1 | Hybrid2 | Hybrid1 | Hybrid2 | |||
| 38 | 3 | 1 | 97 | 92 | 47 | 44 | 0 | 0 |
| 1.5 | 86 | 83 | 43 | 46 | 2 | 0 | ||
| 2 | 80 | 84 | 38 | 38 | 3 | 5 | ||
| 3 | 95 | 90 | 28 | 26 | 0 | 0 | ||
| 4 | 1 | 88 | 87 | 45 | 43 | 0 | 0 | |
| 1.5 | 85 | 82 | 48 | 49 | 0 | 0 | ||
| 2 | 94 | 91 | 42 | 45 | 0 | 7 | ||
| 3 | 84 | 73 | 30 | 32 | 4 | 3 | ||
| 5 | 1 | 92 | 78 | 43 | 49 | 0 | 0 | |
| 1.5 | 90 | 91 | 41 | 42 | 0 | 0 | ||
| 2 | 80 | 81 | 35 | 38 | 0 | 0 | ||
| 3 | 73 | 72 | 32 | 33 | 0 | 0 | ||
| 39 | 3 | 1 | 94 | 93 | 30 | 38 | 13 | 12 |
| 1.5 | 95 | 89 | 33 | 36 | 38 | 35 | ||
| 2 | 90 | 90 | 27 | 27 | 24 | 27 | ||
| 3 | 83 | 81 | 26 | 28 | 32 | 34 | ||
| 4 | 1 | 93 | 90 | 24 | 26 | 80 | 96 | |
| 1.5 | 87 | 85 | 23 | 21 | 100 | 90 | ||
| 2 | 91 | 79 | 21 | 22 | 90 | 100 | ||
| 3 | 86 | 87 | 17 | 20 | 100 | 100 | ||
| 5 | 1 | 89 | 89 | 29 | 28 | 17 | 13 | |
| 1.5 | 83 | 78 | 22 | 27 | 11 | 18 | ||
| 2 | 77 | 74 | 21 | 31 | 62.5 | 10 | ||
| 3 | 75 | 70 | 18 | 21 | 14 | 26 | ||
| 40 | 3 | 1 | 93 | 91 | 20 | 23 | 64.5 | 95 |
| 1.5 | 90 | 88 | 18 | 21 | 94 | 94 | ||
| 2 | 82 | 78 | 16 | 17 | 93 | 100 | ||
| 3 | 71 | 73 | 13 | 19 | 100 | 90 | ||
| 4 | 1 | 89 | 86 | 19 | 20 | 92 | 96 | |
| 1.5 | 94 | 95 | 17 | 18 | 84 | 88 | ||
| 2 | 90 | 91 | 15 | 19 | 75 | 70 | ||
| 3 | 69 | 65 | 12 | 16 | 100 | 100 | ||
| 5 | 1 | 92 | 88 | 23 | 21 | 0 | 0 | |
| 1.5 | 91 | 90 | 23 | 17 | 40 | 0 | ||
| 2 | 86 | 87 | 13 | 15 | 15 | 60 | ||
| 3 | 65 | 72 | 11 | 14 | 19 | 40 | ||
| 41 | 3 | 1 | 81 | 85 | 17 | 16 | 43 | 40 |
| 1.5 | 53 | 59 | 14 | 15 | 84 | 80 | ||
| 2 | 43 | 52 | 10 | 11 | 70 | 80 | ||
| 3 | 41 | 49 | 8 | 7 | 100 | 90 | ||
| 4 | 1 | 60 | 68 | 12 | 14 | 50 | 100 | |
| 1.5 | 38 | 43 | 9 | 11 | 40 | 60 | ||
| 2 | 30 | 38 | 7 | 10 | 80 | 75 | ||
| 3 | 28 | 36 | 4 | 5 | 100 | 90 | ||
| 5 | 1 | 55 | 64 | 13 | 11 | 25 | 5 | |
| 1.5 | 40 | 51 | 6 | 9 | 20 | 0 | ||
| 2 | 25 | 29 | 5 | 6 | 0 | 0 | ||
| 3 | 20 | 27 | 3 | 4 | 0 | 10 | ||
Incubation of eggs
The treated eggs were incubated together with controls in some hatching jars of 6-L holding capacity with continuous flow of water (30°C) for continuous churning movement of the developing eggs. After complete hatching, the larvae were reared in the jars for 3 days. Blended egg yolk was given twice daily as supplementary feed.
Assessment of fertilization and hatching rates
The eggs were taken in some Petri-dishes and looked under a magnifying glass. The fertilized eggs were easily separated from the unfertilized eggs by the presence of transparent shell with grey spot within the egg shell, while the unfertilized eggs were opaque. Then the number of fertilized eggs was counted and rate was determined by counting the exact number of fertilized eggs from the total number of eggs taken. To determine the hatching rate exactly, 250 fertilized eggs were transferred in some small hatching jars with three replications. Hatching was completed within 24 h. The hatching rate was ascertained by counting the numbers of ultimate recovery of spawn from each hatching jar. Then the hatchlings were transferred to other round jars to check survivability up to third day.
Survival rate up to yolk sac absorption
Yolk sac was attached with the abdomen of the newly hatched larvae up to third day. To calculate the survival rate of the larvae up to the stage of absorption of the yolk sac, the number of such larvae was counted after 3 days.
Chromosome karyotyping
The karyotypes were prepared from newly hatched, 1-day-old larvae. Embryos were placed to swim in Petri dish containing 0.005–0.008% colchicine solution for 4–6 h at room temperature followed by fixing in 3:1 methyl alcohol and acetic acid solution for 3 min (Reddy et al. 1990; Hussain, Chatterji, McAndrew & Johnstone 1991). The larvae were minced well on depression slide by using a glass rod with 25 μL 50% acetic acid. The cell suspension was withdrawn into a micro-haematocrit tube fitted with a rubber bulb and expelled onto a glass slide on a warm plate (45–50°C) and the excess was quickly withdrawn back into the capillary tube leaving a fine and clean ring of cells. Slides were then stained with freshly prepared 4% Giemsa's stain for 20 min. The stained slides were washed, air-dried and mounted in DPX to have permanent slides. The haploid and diploid chromosome numbers in the two species were reported previously as 25 and 50 (Reddy et al. 1990; Islam et al. 1994 and Mia et al. 2001). Thus, their triploid number was ascertained with the presence of 75 chromosomes.
Growth performance
The 3-day-old larvae were stocked in the prepared earthen nursery rearing ponds. Thirty different nursery ponds (with five replications) were used to check the growth of hybrid triploids with their reciprocals, diploid hybrids and the single species of rohu and mrigal. In each pond, 2000 larvae were stocked. Larvae were reared in the ponds for a period of 16 weeks. Samples were collected randomly once in a week to assess the growth performance and body weight was taken for the growth measurement. The water quality and pond characteristics were maintained properly to keep the larvae free from all sorts of diseases, parasitic attack and from predation.
Survival rate
The survival rate was measured by counting the number of larvae stocked at the beginning of culture and the number of fish obtained by the end of the experiment.
Statistical analysis
The results of the various growth trials were statistically compared using one-way anova followed by Duncan's multiple range test. These statistical analyses were performed with the aid of the computer software spss. Test of significance was done at 5% level of significance.
Results
Heat shock treatment
Table 1 depicts the details of the experiments conducted and the results obtained. In the present experiment, triploidy was induced successfully in reciprocal hybrids of ‘rohu × mrigal’ by applying heat shock treatment. Heat shock at 39°C temperature for 1.5 min, 4 min after fertilization was the best combination for hybrid1 and 39°C temperature for 1 min, 4 min after fertilization for hybrid2. In several trials, highest rate (100%) of triploidy was induced; however, the rates of hatching were very low.
Fertilization rate
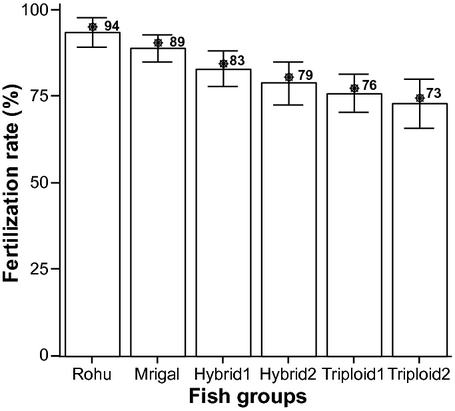
The average fertilization rate of rohu, mrigal, RDH1, RDH2, RTH1 and RTH2 are shown in Fig. 1. Rohu showed the highest fertilization rate (94%) and RTH2 showed the lowest rate (73%).
Hatching rate
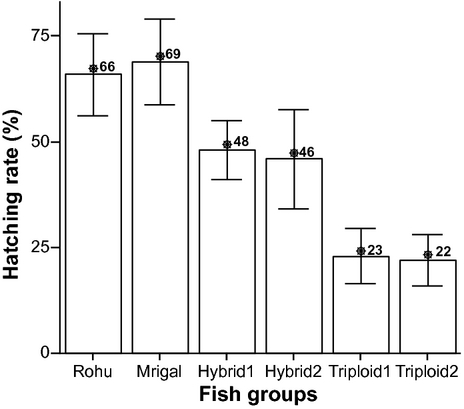
The average hatching rates of the two single species and their reciprocal diploid and triploid hybrids are shown in Fig. 2. During the experiment, mrigal showed the highest hatching rate (69%), whereas triploid2 showed the lowest rate (22%).
Survival rate up to yolk sac absorption
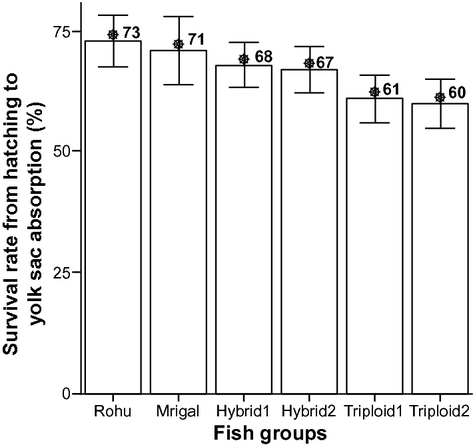
Figure 3 shows the comparative survival rate of six different fish groups during the present study where rohu showed the highest survival rate (73%) and RTH2 showed the lowest (60%).
Chromosome karyotyping and ploidy determination
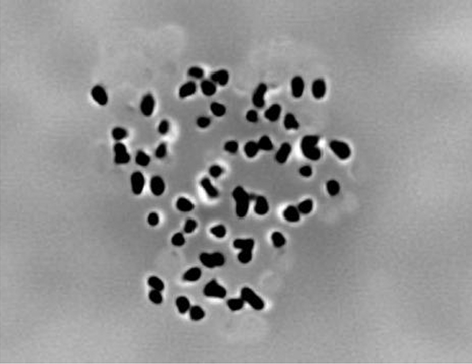
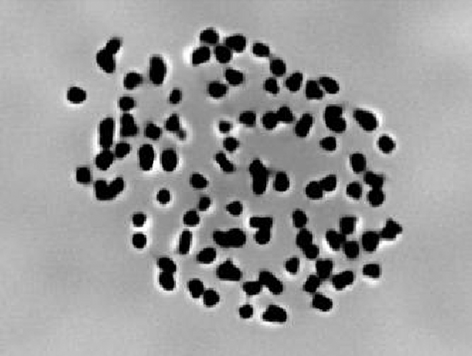
After complete hatching, the larvae were checked for triploidy and were recognized by counting the number of chromosomes. In diploid hybrids, 50 (2n) chromosomes were recorded in every trial and in triploid hybrids, the chromosome number was 75 (3n). The number of chromosomes for both the diploid and triploid hybrids and their metaphase preparations are presented in Figs 4 and 5.
Growth performance
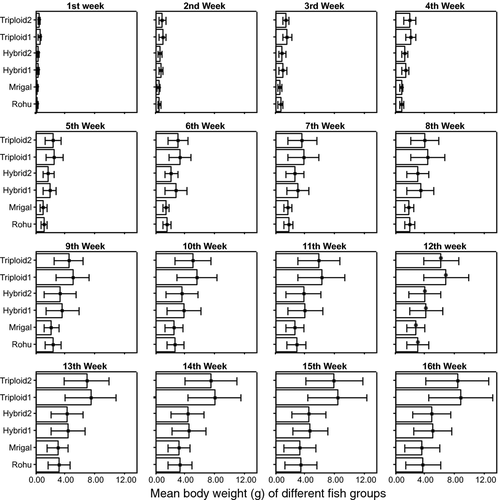
The growth performances for six different fish groups are presented in the Fig. 6. Sampling once in a week, the comparison of growth performance showed significant differences (P = 0.000 < 0.05) in the mean body weight among the different fish groups in each week. By the end of the culture period, it was observed that the body weight of RTH1 and RTH2 was 8.76 and 8.37 g, whereas for rohu, mrigal, RDH1 and RDH2 were 3.72, 3.58, 5.01 and 4.88 g respectively.
Survival rate
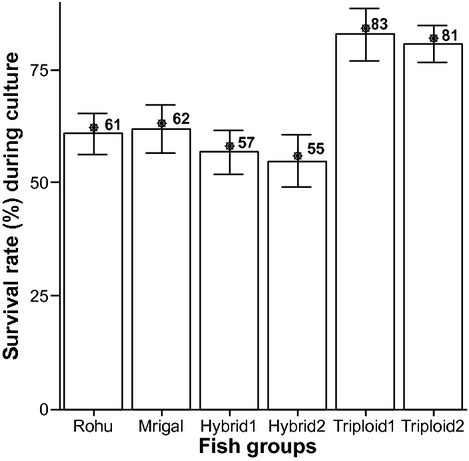
The results for the survival rate of different fish groups are given in Fig. 7. RTH1 showed the highest (83%) survival rate, whereas RDH2 showed the lowest (55%) rate.
Discussions
Heat shock treatment
The present experiment was successful in the induction of triploidy in ‘rohu × mrigal’ hybrid achieving about 100% triploidy using heat shock. Four minutes after fertilization at a temperature of 39°C with a duration of 1.5 min for reciprocal hybrid1 (male rohu × female mrigal) and 4 min after fertilization at a temperature of 39°C with a duration of 1 min for reciprocal hybrid2 (male mrigal × female rohu), were the best combinations for triploidy induction (Table 1). At 38°C temperature with different durations of heat shock and time after fertilization, the heat shocks applied were ineffective in inducing triploidy in both the reciprocal hybrids although these combinations showed higher fertilization and hatching rates. At 39°C, 4 min after fertilization at 2 and 3 min duration of shock, 90% and 100% triploidy were induced in reciprocal hybrid1 but although the rate of fertilization was reasonably higher (91% and 86%), the hatching rates were comparatively low (21% and 17%). When 100% triploidy was induced at 39°C temperature, 4 min after fertilization at 1.5 min duration of shock, it was considered as the best combination for triploidy induction in reciprocal hybrid1 because of its comparatively higher fertilization (87%) and hatching rate (23%). At 39°C, 4 min after fertilization at 2 and 3 min duration of heat shock, 100% triploidy was also induced in reciprocal hybrid2 having the almost similar fertilization (79% and 87%) and hatching (22% and 20%) rates. Although 96% triploidy was induced at 39°C temperature 4 min after fertilization at 1 min duration of heat shock, it was considered the best combination for triploidy induction in hybrid2 because of its higher fertilization (90%) and hatching rate (26%). At 40 and 41°C temperatures, both the hatching and fertilization rates were very low compared with those at 38 and 39°C in both the RTH.
Felip, Piferrer, Zanuy and Carrillo (2001) describe that correct handlings and treatment optima may induce 100% triploid and gynogenetic fish. Hussain (1996) reports that the physical shock treatments had species-specific effectiveness; a treatment may be less efficient in cold-water fish, whereas the same treatment may be more efficient in warm-water fish and vice-versa. It is generally thought that warm water species are more susceptible to cold shock (Padhi & Mandal 1977; Wolters, Libey & Chrisman 1981) than to heat shock, whereas heat shock is more effective for cold water species (Chourrout 1980; Nagy 1987). The present experiment differed from this contention as rohu and mrigal are the warm water species and are supposed to be highly susceptible to heat shock treatment for triploidy. The present study also suggests that triploidy rate declined sharply with delayed (≥5 min after fertilization) application of heat shock.
Fopp-Bayat, Jankum, Woznicki and Kolman (2007) induced triploidy successfully in Siberian sturgeon and bester hybrids by applying heat shock at 37°C temperature in 18th minute after fertilization for 2 min duration. Hussain et al. (1991) induced 100% triploidy in Oreochromis niloticus optimizing pressure (8000 psi, 2 min duration applied 9 min after fertilization), heat shock (41°C, 3.5 min duration applied 5 min after fertilization), and cold shock (9°C 30 min duration applied 7 min after fertilization) and found that heat shock treatment was not the best means of inducing triploidy because of variable survival rates of the triploids induced. Reddy et al. (1990) induced 12% triploidy in rohu, by administering heat shock to zygotes, 7 min after fertilization at 42 ± 0.5°C for a duration of 1–2 min. Islam et al. (1994) induced 60% triploidy in Labeo rohita (rohu) by heat shock at 40°C for 2 min starting 4 min after fertilization. Mia et al. (2001) arrested the second polar body of mrigal (Cirrhinus cirrhosus) by heat shocking at 39°C temperature for 1 min duration and applied 3 min after fertilization. The best combinations for heat shock treatment in the present study support the idea and findings of the foregoing studies, but differed slightly due to species and strain differences. Thus, heat shock could be an effective means of triploidy induction, but the shock at more than 5 min after fertilization may not be effective for the triploidization in ‘rohu × mrigal’ hybrid.
Fertilization and hatching rates
In the present study, the single species of rohu and mrigal showed the highest fertilization hatching rates being recorded at 94%, 89% and 66%, 69% (Figs 1 and 2). The hatching rates of RDH1, RDH2, RTH1 and RTH2 were obtained at 48%, 46%, 23% and 22% respectively. The diploid hybrids showed lower fertilization and hatching rates, the reason for which could most probably be ascribed to general problem of survivability of offspring from mixing of heterospecific gametes. The fertilization and hatching rates were even lower in case of the triploid hybrids, which might similarly be ascribed to the general difficulty of retaining the second polar body set chromosome in the heterospecific combination of genomes by heat shock. Due to the application of heat shock, a considerable amount of eggs were deformed.
The highest values reported for hatching success of hybrid triploids range from 58% (brook vs. brown trout, Galbreath & Thorgaard 1994) to 83% (rainbow vs. coho salmon, Quillet, Chevassus & Devausx 1988). Lower values, however, were also reported, which ranged between 1% (masu salmon Oncorhynchus keta vs. rainbow trout, Oshiro, Deng, Higachi & Takashima 1991) and 14% (chum salmon Oncorhynchus keta vs. brook trout, Gray, Evans & Thorgaard 1993). Gheyas, Mollah and Hussain (2001) observed lower fertilization and hatching percentage in cold shock treated triploids (91% and 37%) than those of diploid controls (95% and 46%). Such lower hatchability of triploid individuals compared with control has been reported by other authors as well (Chrisman, Wolters & Libey 1983; Krasznai, Marian & Kovscs 1984; Solar, Donaldson & Hunter 1984). After the heat shock treatment, the fertilization rates of bester and Siberian sturgeon hybrids were observed as 90.5% and 95% by Fopp-Bayat, Jankum, Woznicki et al. (2007). Heat shock treatment (at 41°C temperature for 2 min) provides 51% hatching rate, whereas cold shock treatment (at 6°C temperature for 2 min) provides 45% in triploid hybrid tetrads observed by David and Pandian (2006). The present study also confirms that rohu × mrigal hybrid zygotes are highly susceptible to heat shock.
Survival rate up to yolk sac absorption
The survival rate of hatchlings up to yolk sac absorption was found to be 73%, 71%, 68%, 67%, 61% and 60% for rohu, mrigal, RDH1, RDH2, RTH1 and RTH2 (Fig. 3). In this stage, the survival rate was lower in the diploid and triploid hybrids than the single species.
At earlier stage (up to 7 days after hatching), the survival rates of diploid Siberian sturgeon, diploid hybrids of ‘Siberian sturgeon × bester’ and triploid hybrids of ‘Siberian sturgeon × bester’ were 58%, 53% and 43% (Fopp-Bayat, Jankum, Woznicki et al. 2007). The survival rate of hatchlings of triploid stinging catfish (Heteropneustes fossilis) up to yolk sac absorption ranged between 4.26% and 71.14% (Gheyas et al. 2001). Survival of triploids at hatching was <25% as observed by David and Pandian (2006). McKay, Ihssen and Mcmillan (1992) reported that at hatching stage triploids show 58–61% survival rate in brown trout (Salmo trutta) and brook trout (Salvelinus fontinalis) and survival of triploids up to the feeding stage is significantly lower. Yoshikawa et al. (2007) obtained 37.5–91.5% survival rate for the larvae of triploid mosaic loaches after 7 days of hatching at different shocking conditions. The survival rate of hatchlings up to 3 days were found to be 9.8–94.9% in triploid Nile tilapia at different temperature ranges observed by Hussain et al. (1991).
The present study supports that the survival rate of triploids at earlier stage (from hatching to several days) is comparatively lower than the later stage. It is expected that higher ploidy level makes the embryonic development stage faster due to increased genetic materials in them (Gray et al. 1993; Hussain 1996). This may cause the lower survival of triploid hybrids at earlier life stage as the embryos get less time for proper development.
Chromosome karyotyping and ploidy determination
The diploid (2n) and triploid (3n) chromosome numbers of rohu × mrigal hybrid were found to be 50 and 75 (Figs 4 and 5). There may have chromosome numbers as 75 ± 3-5 because of having overlapping chromosomes and/or some chromosomes may be torn during maceration. Kibria, Sufi, Khatun and Alam (2001) reported that the 2n chromosome numbers of H. fossilis, Mystus tengara and Pangasius sutchi were found to be 56, 54 and 60 respectively. The diploid and triploid chromosome numbers were found to be 54 and 81 in Clarius gariepinus (Marx & Sukumaran 2007) and 44 and 66 in O. niloticus (Hussain et al. 1991). Mia et al. (2001) reported haploid (n), diploid (2n) and triploid (3n) chromosome numbers of mrigal as 25, 50 and 75. The diploid chromosome numbers of Acipenser baeri and ‘Huso huso × Acipenser ruthenus’ hybrids were described as 120 and 120 by Fontana, Tagliavini and Congui (2001) and Fontana (2002) respectively. The chromosome numbers of all the studied diploid and triploid hybrids of ‘A. baeri × (H. huso × A. ruthenus)’ were 180 and 300 (Fopp-Bayat, Jankum, Woznicki et al. 2007). Gheyas et al. (2001) reported the 2n and 3n number of chromosome in H. fosillis as 56 and 84.
Growth performance
In the present study, the growth rate between triploid (RTH1 and RTH2) and diploid (rohu, mrigal, RDH1 and RDH2) groups of fish differed significantly (P < 0.05). During the culture period, the same aged (3 days) larvae of each group were stocked in separate ponds. By the end of the culture period, it was observed that the mean body weights of triploids were much higher than the rest of the fish groups. After the 16-week culture period, the mean body weights were found to be 8.76 and 8.37 g for RTH1 and RTH2, 5.01 and 4.88 g for RDH1 and RTH2, 3.72 and 3.58 g for single species of rohu and mrigal (Fig. 6). The maximum body weights for six different fish groups were obtained as 6.10, 5.93, 7.63, 7.45, 13.09 and 12.63 g for rohu, mrigal, RDH1, RDH2, RTH1 and RTH2. The higher growth rate in triploid hybrids was explained to be due to the presence of an extra chromosome set (extra 33% genetic material in each nucleus), which might lead to increased capacity of metabolism. The diploid hybrids also showed a considerably higher growth rate over the single species of rohu and mrigal during the study period, which might also be ascribed to increase heterozygosity in the hybrids causing vigour for performance.
Indeed, some studies have identified a positive relationship between growth and heterozygosity (Shah 1984; Ferguson 1992; Pogson & Fevolden 1998; Shah et al. 1999), although some others have found no relationship (Borell, Pineda, McCarthy, Vazquez, Sanchez & Lizana 2004; Pujolar, Maes, Vancoillie & Volckaert 2006; Garner et al. 2008). Some other studies showed that higher stress on triploid larvae due to shock treatment may lead to differences in growth rate through reduced appetite and lower efficiency in converting food into body mass (De Boeck, Alsop & Wood 2001; Bernier, Bedard & Peter 2004). Felip et al. (2001) observed similar growth in diploid and triploid fish for the first 2 years and then slower in triploids. Lincoln (1981) observed faster growth in triploid female of ‘plaice × flounder’ hybrids than the diploids over one spawning season. Uneo, Ikenaga and Kariya (1986) described that the edible portions of female and male triploids were about 30% and 15% larger than those of diploids. Johnson et al. (2007) observed excessive growth rate (almost two times higher) of triploid Chinok salmon (Oncorhynchus tshawytscha) over diploids during 7 months’ culture period. Li, Hu, Wang, Zhu and Fu (2009) observed reduced swimming abilities and slower movement in fast growing triploid and transgenic common carp (Cyprinus carpio) that may also be the potential reasons of higher growth in triploids. Growth rate and food conversion rate of triploid fish were better as compared with diploids because of sterility in triploids (Wasow, Kuzminski, Woznicki & Ziomek 2004). Thus, hybrid triploidization in the present study resulted in increased overall phenotypic variances (notably on growth and survivability). Triploids exhibited significantly higher growth and survival rate during culture period than diploids except survival during incubation period.
Survivability
The survival rates were obtained at 61% for rohu, 62% for mrigal, 57% for RDH1, 55% for RDH2, 83% for RTH1 and 81% for RTH2 (Fig. 7). Triploids showed the highest survival rate, probably due to the application of heat shock treatment that made the triploids better fit to adopt with culture conditions. Another important probable reason for higher survivability in triploids was due to their extra genetic materials, which increase their immune functions and hormonal secretion for adaptability (Benfey 1991; Bernier et al. 2004). Thus, survivability in triploids during embryonic development was significantly less than during the post-hatching period in the present study.
The survival rate of hybrid triploid tetrads was found to be 5% after a 180-day culture period and the triploids produced by heat shock showed higher survivability than the triploids produced by cold shock (David & Pandian 2006). Altimiras, Axelsson, Claireaux, Lefrancois, Mercier and Farrell (2002) observed 38% survivability in triploid rainbow trout and 87.7% survivability in triploid brown trout during 16 consecutive weeks’ culture period in laboratory. Fopp-Bayat, Jankum, Woznicki et al. (2007) observed 71% survivability in diploid Siberian sturgeon, 62.2% and 54.4% in diploid and triploid hybrids of Siberian sturgeon and bester. McKay et al. (1992) observed both reduced survival rate in un-eyed egg stage and higher survival in older triploid brook and tiger trout. Triploid salmonids possess cells with increased volume, but maintain normal organ sizes by decreasing cell numbers (Benfey 1991). Triploidy does not always appear to impair the health of fish (Yamamoto & Iida 1995). Fopp-Bayat, Jankum and Kuzminski (2007) reported higher survival in triploids of brook trout (S. fontinalis) over their diploid and gynogenetic counterparts. The present study supported several studies that hybrid triploidization can increase the survival rate and adaptability in culture condition and that could be explicated to their extra genetic materials in them. The results obtained in the present study further suggested that artificial triploidization in ‘rohu × mrigal’ hybrid could be resorted to aquaculture production increase over diploid culture.
Conclusion
In the present experiment, the triploidy could be successfully induced in ‘rohu × mrigal’ hybrid through heat shock. There has been an explosive increase in aquaculture production in the last few decades through several traditional and modern means, especially through the application of several genetic technologies. However, still the enterprise is being confronted with severe challenges from pollution, inbreeding and spread of diseases and loss of biodiversity. For creation of appropriate manpower in aquaculture genetics, many crucial issues of technological, biological, ecological and economics are waiting to be resolved. The introduction of triploid hybrid fish culture to the inland aquaculture can serve as a basis for increase of production over the culture with diploids that suffer from growth inefficiency. The results of the present study could be further tested for continued better growth performance of the triploid hybrids. Other than the better growth potential of the triploid hybrids, their sterility aspect can be even more wanting in production and management goals of aquaculture and fisheries. This work can serve as the basis for further work to test other shock protocols and to scale up the most practical method for a commercial production of triploid hybrid seed.




