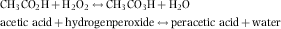Reduction of in vitro growth in Flavobacterium columnare and Saprolegnia parasitica by products containing peracetic acid
Abstract
Commercial products containing peracetic acid (PAA) are strong disinfectants with a wide spectrum of antimicrobial activity and have been suggested as potential therapeutic agents in aquaculture. The aim of this study was to compare the in vitro reduction of growth on two fish pathogens, Flavobacterium columnare and Saprolegnia parasitica, by seven commercial PAA-containing products. Flavobacterium columnare was exposed to 1, 2, 4, 6, 8 and 10 mg L−1 PAA and S. parasitica was exposed to 0.5, 1, 2, 4, 6, 8 and 10 mg L−1 PAA in petri dishes for 24 h incubation. The reduction of growth was measured in comparison to a PAA-free control. A reduction of the growth was observed for both pathogens with increasing PAA concentration. Hydrogen peroxide (H2O2) possibly has a role in the effectiveness of the products, since products with lower PAA concentrations had a higher concentration of H2O2. The commercial products with a low concentration of PAA and a low PAA:H2O2-ratio were generally more effective against pathogens. The practical application of the products with low PAA concentration should be prioritized.
Introduction
Flavobacterium columnare and Saprolegnia parasitica are two important fish pathogens in freshwater aquaculture. Flavobacterium columnare is the etiological cause of columnaris disease; it is a Gram-negative, filamentous, yellow-pigmented bacillus (Wakabayashi 1993) that infects a large number of fish species worldwide in water temperatures above 15°C (Anderson & Conroy 1969; Wakabayashi 1993). Infected fish are characterized by whitish areas on the fins, on the head or on the body surface as spots or saddleback-shaped lesions (Decostere, Haesebrouck & Devriese 1998).
Saprolegnia parasitica is a pathogenic oomycete; this fungus is ubiquitous and can affect several life stages of all freshwater fish species (Southgate 1993). The fungus invades eggs where mycelial growth results in death of the embryo and spreads to the adjacent eggs (van West 2006). On the body of fish, the infection appears as raised white plaques on epidermal tissue (Southgate 1993). Mycelial growth causes tissue damage and cellular necrosis, and hyphae can invade muscle and blood vessels (van West 2006).
In intensive aquaculture, high density and animal handling can increase the transmission of pathogens and the susceptibility of fish to diseases. Therapeutic agents must be used in order to keep these infections under control. Malachite green has been used extensively in aquaculture to control fungal and protozoan infections for a wide variety of fish (Srivastava, Sinha & Roy 2004). However, since 2002, malachite green has been banned on food-fish worldwide due to its carcinogenic and toxicological effects (van West 2006). Acriflavine was traditionally used to control F. columnare in fish; however, use of this substance is no longer permitted (Schlotfeldt 1998). Only a few therapeutic agents are allowed in Europe (Schlotfeldt 1998), and there is currently a crisis for treatment of diseases in aquaculture. Therefore, alternative therapeutic agents must be investigated in order to treat diseases without harmful effects to the fish or the user and without leaving dangerous residues in the environment.
Peracetic acid kills microorganisms by oxidation and subsequent disruption of the cell membrane; it is a strong disinfectant with a wide spectrum of antimicrobial activity. Kitis (2004) reported the bactericidal, fungicidal and sporicidal activity at very low concentrations. Reactions of PAA with organic matter produce little to no mutagenic or toxic by-products (Monarca, Richardson, Feretti, Grottolo, Thruston, Zani, Navazio, Ragazzo, Zerbini & Alberti 2002). Peracetic acid demonstrates antimicrobial activity in a wide temperature range (Stampi, De Luca & Zanetti 2001), but it is affected by pH with decreasing activity in alkaline conditions (Colgan & Gehr 2001). Peracetic acid is used for the disinfection of wastewater, in food industries and in hospital facilities.
The successful use of PAA as a treatment against fish pathogens has recently been investigated (Rintamäki-Kinnunen, Rahkonen, Mannermaa-Keränen, Suomalainen, Mykrä & Valtonen 2005; Meinelt, Richert, Stüber & Bräunig 2007a; Meinelt, Staaks, Staaks, Stüber & Bräunig 2007b; Meinelt, Matzke, Stüber, Pietrock, Wienke, Mitchell & Straus 2009; Pedersen, Pedersen, Nielsen & Nielsen 2009; Pedersen, Pedersen, Nielsen & Nielsen 2010; Straus & Meinelt 2009; Sudová, Straus, Wienke & Meinelt 2010), and the results are promising for applications in freshwater aquaculture. The aim of this study was to compare the in vitro reduction of growth on two fish pathogens, F. columnare and S. parasitica, by seven commercial PAA products.
Materials and methods
Seven commercial PAA products (Table 1) having specific PAA and H2O2 concentrations were obtained from the manufacturers for use in these experiments. The molar PAA:H2O2-ratio was calculated to explain effects of H2O2. The experiments were designed to evaluate the growth reduction potential of the products on F. columnare and S. parasitica.
| ID | Product | Supplier | PAA (%) | H2O2 (%) | PAA:H2O2 |
|---|---|---|---|---|---|
| Lspez | Wofasteril L. Spez | KESLA PHARMA WOLFEN GmbHa | 3 | 40 | 0.034 |
| E35 | Wofasteril 035 | KESLA PHARMA WOLFEN GmbHa | 3.5 | 10 | 0.156 |
| SC50 | Wofasteril SC50 | KESLA PHARMA WOLFEN GmbHa | 5 | 8 | 0.28 |
| AC150 | Peressigsäure 15% reinst | AppliChem GmbHb | 15 | 24 | 0.28 |
| E250 | Wofasteril E250 | KESLA PHARMA WOLFEN GmbHa | 25 | 30 | 0.37 |
| SI400 | Sigma-Aldrich Peracetic Acid Solution | Sigma-Aldrich Coc | 39 | 6 | 2.91 |
| E400 | Wofasteril E400 | KESLA PHARMA WOLFEN GmbHa | 40 | 12 | 1.49 |
- a KESLA PHARMA WOLFEN GmbH (Greppin, Germany).
- b AppliChem GmbH (Darmstadt, Germany).
- c Sigma-Aldrich Co. (St. Louis, MO, USA).
A pure strain of F. columnare [CVUA (2009100)8608] was obtained from Chemisches und Veterinäruntersuchungsamt Stuttgart (Stuttgart, Germany). The bacteria were cultured on supplemented plate count agar (Atlas 2004). The culture media was chosen as a result of preliminary tests on various media and was composed of 15.0 g agar, 5.0 g peptone, 2.5 g yeast extract, 1.0 g glucose, 5.5 mg KCl, 294 mg CaCl2.2H2O, 123.3 mg MgSO4.7H2O and 63 mg NaHCO3 dissolved in 1 L of deionized water. The pH of the media was 7.7 and the buffering capacity was 0.75 mmol L−1. The bacteria culture was sub-cultured every week.
To determine the sensitivity of F. columnare to each of the PAA products, petri dishes (52 mm) were filled with 10 mL supplemented plate count agar and inoculated with 2 mL of 4-day old bacterial broth. The broth was removed from the petri dishes after 2 min, and a 6 mm diameter hole was made in the centre of each dish with a sterile punch; a 100 μL aliquot (100, 200, 400, 600, 800 or 1000 mg L−1 PAA) was pipetted in the hole to give final concentrations, according to manufactures data and assuming no decay of PAA, of 1, 2, 4, 6, 8 and 10 mg L−1 PAA for the 10 mL agar. A PAA-free control and a negative control without the inoculation of bacteria were included; the tests were performed in triplicate at 20°C. Two thresholds were chosen for the measurement of PAA effects: the bacteriostatic and the bactericidal thresholds. The inhibition zone was measured to the nearest 1 mm from the edge of the hole to the bacterial layer after 24 h of incubation at 22°C using analySIS image analysis software (Olympus Life Science Europa, Hamburg, Germany). The reduction of growth of F. columnare was calculated as the radius of the inhibition zone divided by the radius of the petri dish.
The effect of the PAA products on S. parasitica was evaluated by obtaining a strain of S. parasitica (ATCC 22284) from the American Type Culture Collection (Manassas, VA, USA). The fungus was cultured on glucose-peptone-yeast agar (Meinelt, Paul, Phan, Zwirnmann, Krüger, Wienke & Steinberg 2007c) and was sub-cultured every 2 weeks. Briefly, 1 mL of diluted PAA solution (5, 10, 20, 40, 60, 80 and 100 mg L−1 PAA) was mixed with 9 mL of 55°C liquid GY-agar and was immediately poured in sterile 90 mm petri dishes. Final nominal PAA concentrations according to manufactures data and considering no decay of PAA, were 0.5, 1, 2, 4, 6, 8 and 10 mg L−1 in the agar. Next, the middle of the plate was inoculated with a 6 mm plug of S. parasitica from a 3–5 day old culture. A PAA-free control and a negative control without inoculation material were prepared; the test was performed in duplicate at 20°C. Maximum growth diameters were measured after 24 h of incubation. The difference of growth diameter between samples of S. parasitica in the presence of PAA and the PAA-free control were calculated; the growth was calculated as the percent reduction compared to the growth of the PAA-free control.
The effect of two variables (concentration of PAA and the PAA-products) on growth or growth inhibition was investigated by a two-way ANOVA for the whole range of concentrations (>0 to 10 mg L−1) or for a potential therapeutic range (>0 to 2 mg L−1; Pedersen et al. 2009). The mean toxicity of the products were compared post hoc by Student's t-test. Spearman's rank correlation was used to describe the relationship between three variables (molar PAA:H2O2 ratio, the concentration of PAA in agar and the concentration of H2O2 in agar) and the growth reduction of the pathogens. Correlations close to 1 indicate an excellent relationship between the variables. The level of significance was determined at P < 0.025.
Results and discussion
For the experiments with F. columnare, the reduction of bacterial growth significantly increased with increasing PAA concentrations (Fig. 1). Growth reduction was significant at 1 mg L−1 for Lspez and E35, at 2 mg L−1 for SC50, AC150 and E250, and at 4 mg L−1 for SI400 and E400. The inhibition of growth for the products with a lower molar PAA:H2O2 ratio such as Lspez and E35, required a lower concentration of PAA (Table 1). The molar PAA:H2O2 ratios are given in Table 1 for each PAA product. The ratio was lowest for Lspez and highest for SI400. For F. columnare, the best correlation (−0.7423) was observed at the lower range of PAA concentrations. Products with higher H2O2 concentrations were particularly more toxic at lower PAA concentrations in the present study.
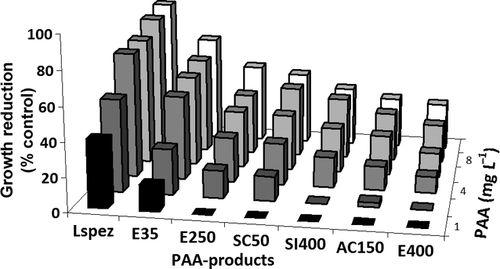
Inhibition of S. parasitica was achieved with all PAA products dependent on concentration (Fig. 2). At lower concentrations, all PAA products affected the growth of S. parasitica to some extent. Minimum inhibitory concentrations at 24 h ranged from 4 mg L−1 for Lspez and E35, to 6 mg L−1 for SC50, 8 mg L−1 for AC150, SI400 and E400, and 10 mg L−1 for E250. Above these concentrations, no visible growth was observed in the petri dishes. Below these concentrations, the growth of S. parasitica was decreased in comparison to the control. The correlation was higher for lower PAA concentrations (Table 3.) The fit of the correlation decreased due to 100% inhibition reached at several higher concentrations. Mean growth reductions (Table 2) indicated the products with lower concentrations of PAA and a lower molar PAA:H2O2 ratio were more toxic. The substances with the lowest ratio, Lspez and E35, were most effective against F. columnare. In this experiment, the product with nearly equivalent, moderately high concentrations of PAA and H2O2 (E250) had the lowest inhibitory effect. There was a significant inhibition of the growth of the S. parasitica mycelium after PAA exposure; however, sporicidal concentrations were not evaluated. Additionally, zoospores of S. parasitica are encysted and are resistant to intense environmental changes; a higher PAA concentration would likely be required for a sporicidal effect. Greenspan and MacKellar (1951) indicated sporicidal concentrations 100 times higher than fungicidal concentrations for PAA.
| Pathogen (PAA concentration range) | Student's mean comparison | ||||||
|---|---|---|---|---|---|---|---|
| Lspez | E35 | SC50 | AC150 | E250 | SI400 | E400 | |
| Flavobacterium columnare (1–10 mg L−1) | 68.4a | 44.4b | 26.1c | 16.5d | 26.5c | 19.4d | 12.4e |
| F. columnare (1–2 mg L−1) | 47.2a | 21.3b | 7.1c | 1.3d | 7.9c | 0.0d | 0.0d |
| Saprolegnia parasitica (0.5–10 mg L−1) | 74.8a | 62.5b | 55.3bc | 45.6cd | 38.3d | 39.4d | 40.9d |
| S. parasitica (0.5–2 mg L−1) | 41.2a | 12.6b | 13.1b | 14.3b | 10.6b | 4.3c | 3.5c |
| Pathogen (PAA concentration range) | Growth reduction vs. molar PAA:H2O2 | Growth reduction vs. PAA in agar | Growth reduction vs. H2O2 in agar | |||
|---|---|---|---|---|---|---|
| P | Spearman's ρ b | P | Spearman's ρ b | P | Spearman's ρ b | |
| Flavobacterium columnare (1–10 mg L−1) | <0.0001a | −0.5700 | <0.0001a | 0.6406 | <0.0001a | 0.8470 |
| F. columnare (1–2 mg L−1) | <0.0001a | −0.7423 | 0.0547 | 0.2987 | <0.0001a | 0.8452 |
| Saprolegnia parasitica (0.5–10 mg L−1) | <0.0001a | −0.2842 | <0.0001a | 0.8815 | <0.0001a | 0.7797 |
| S. parasitica (0.5–2 mg L−1) | <0.0001a | −0.5136 | 0.0005a | 0.5142 | <0.0001a | 0.8480 |
- a indicates a significant correlation between pathogen inhibition and the PAA concentration of the products at P < 0.025.
- b rho measures the statistical dependence between pathogen inhibition and the PAA concentration of the products. If rho = ±1, the variables are perfectly correlated.
As investigated by Straus and Meinelt (2009) with Ichthyophthirius multifiliis theronts, the present experiments confirm that different PAA products have different toxicity to fish pathogens. In both ranges of PAA concentrations, the experiments indicated that products with the lowest PAA concentration and a low PAA:H2O2 ratio tend to be more effective with regard to the reduction of fish pathogens in vitro. One outlier (E250) showed less inhibition to S. parasitica, although the ratio of 0.37 is relatively low. Indeed, results indicate the two products with the lowest PAA concentrations and very low PAA:H2O2 ratios (Lspez = 0.034 and E35 = 0.156) were significantly more effective on both pathogens. The additional amount of H2O2 in these products may have caused additive toxic effects against the two fish pathogens.
The data suggest a dose-dependent decrease of pathogen toxicity relative to the PAA concentration and PAA:H2O2 ratio of the products (Table 3). The nominal concentrations of PAA in the experiments were equal for all products, but the amount of H2O2 varied resulting in different molecular ratios. For example, a solution with a nominal concentration of 10 mg L−1 PAA resulted in 133, 28.5, 16, 16, 12, 1.5 and 3 mg L−1 H2O2 and 0.034, 0.156, 0.28, 0.28, 0.37, 2.91, 1.49 (PAA:H2O2 ratios) for Lspez, E35, SC50, AC150, E250, SI400 and E400, respectively. Therefore, H2O2 had a dose-dependent additive effect on pathogen growth. Significant positive correlations were observed in all cases. The Spearman's ρ indicated a strong correlation for F. columnare (between 0.8452 in the therapeutical range and 0.8473 in the tested range of PAA concentrations). The correlation remained strong for S. parasitica in the tested range (0.7818) and in the therapeutic range (0.8480). The mechanism behind this effect is unknown. Future studies should analytically verify concentrations of PAA and H2O2 to document actual concentration and decay.
Larger doses of H2O2 are required when compared to PAA for similar disinfection (Wagner, Brumelis & Gehr 2002). In the F. columnare experiment, a 100 μL aliquot containing 100, 200, 400, 600, 800 or 1000 mg L−1 PAA was applied in the centre of each petri dish. As a result, the H2O2 concentration was high enough to induce major bacterial growth reduction at the inner gradient. Straus and Meinelt (2009) suggested additional increased effects of H2O2; the mortality of theronts was higher with a product containing 4.5% PAA compared to a 40% product with H2O2 concentrations of 22% and 12%, respectively. Thus, the higher efficiency of the 4.5% PAA product may have been due to higher H2O2 concentration (Straus & Meinelt 2009). These findings are supported by the present study.
In conclusion, all products reduced growth of F. columnare and S. parasitica. Products with a higher concentration of PAA (vs. H2O2) did not inhibit growth as well as products with lower PAA/higher H2O2 concentrations. Additive effects of H2O2 play an important role in the reduction of pathogen growth by PAA in vitro. The practical application of the products with low PAA concentration should be prioritized. Future research should investigate in vitro toxicity to pathogens in aquatic systems, the safety of PAA products to fish, correlate in vitro to in vivo efficacy and investigate the mechanistic activity of PAA containing products.
Acknowledgments
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the Leibniz-Institute of Freshwater Ecology and Inland Fisheries or the U.S. Department of Agriculture.