The use of probiotics during the nursery rearing of the pink shrimp Farfantepenaeus brasiliensis (Latreille, 1817) in a zero exchange system
Abstract
The present work evaluated the use of probiotics during the nursery rearing of the pink shrimp Farfantepenaeus brasiliensis, in a zero exchange aerobic heterotrophic culture system during 30 days. Three replicate tanks were randomly assigned to the following treatments: (1) Bacillus spp. mixture (Sanolife Pro--W®), (2) Bacillus sp., Enterococcus sp., Lactobacillus spp. mixture (Biomin Start-grow®), (3) Bacillus cereus var. toyoi and (4) control treatment (without probiotic addition). Bacteriological analysis monitored the abundance of presumptive Vibrio spp. in the water of experimental tanks. For the immunological analysis, shrimp haemolymph was collected to determine the granular haemocyte count and total protein concentration. Results showed that mean final weight and specific growth rate of shrimp in the probiotic treatments were significantly higher. Furthermore, shrimp reared in the probiotic treatments showed higher levels of total protein and granular haemocyte. The bacteriological analysis showed that the concentration of Vibrio spp. measured in probiotic treatment tanks was lower than that recorded in the control tanks.
Introduction
Aquaculture is currently the fastest growing activity among all sectors of animal production, and marine shrimp culture is the main economic activity developed in the field of aquaculture (FAO – Fisheries and Aquaculture Department 2007). The fast development of shrimp culture increasingly requires strategies to improve production systems, enhance bio-security and reduce environmental impacts (Avella, Gioacchini, Decamp, Makridis, Bracciatelli & Carnevali 2010; Qi et al. 2009).
The rapid expansion of penaeid shrimp culture is threatened by diseases caused by bacteria of the genus Vibrio, which affect shrimp survival and growth (Aguirre-Guzmán, Vazquez-Juarez & Ascencio 2001). These opportunistic microorganisms are part of the flora of penaeid shrimp and may cause illnesses under unfavourable environmental conditions. Specific effects such as mortality, tissue damage or necrosis and growth retardation are reported. Vibriosis has been also implicated as the cause of high mortalities in juvenile penaeid shrimp worldwide (Lightner & Redman 1998; Castex, Lemaire, Wabete & Chim 2010).
The abuse of antimicrobial drugs, pesticides and disinfectants in aquaculture has caused the evolution of resistant strains of bacteria and brought concern to the society (Boyd & Massaaut 1999; Esiobu, Armenta & Ike 2002). Thus, defining alternative strategies to support aquaculture productivity are extremely necessary (Avella et al. 2010).
Among the alternatives proposed, the use of probiotics has shown promising results and is now widely accepted as a complementary tool for the management of disease and for improving nutrition of aquatic animals (Wang, Li & Lin 2008). Probiotics are also cited as an alternative to antimicrobial drugs, enhancing the growth and disease-resistance of cultured shrimp (Cutting 2011), improving the immunosystem response (Erickson & Hubbard 2000; Picchietti, Mazzini, Taddei, Renna, Fausto, Mulero, Carnevali, Cresci & Abelli 2007), and general welfare (Balcázar, de Blas, Ruiz-Zarzuela, Cunningham, Vendrell & Múzquiz 2006; Silvi, Nardi, Sulpizio, Orpianesi, Caggiano, Carnevali & Cresci 2008).
In addition, there are several studies reporting the development of intensive shrimp culture systems without water exchange, as a way to improve biosecurity and reduce environmental impacts (Burford, Thompson, McIntosh, Bauman & Pearson 2003; Wasielesky, Atwood, Atokes & Browdy 2006; Ballester, Abreu, Cavalli, Emerenciano, Abreu & Wasielesky 2010). However, little information is available regarding the use of probiotics in this type of systems. Therefore, the proposal of the present work was to evaluate the use of two commercial probiotics and one potential probiotic bacteria Bacillus cereus var. toyoi, during the nursery rearing of the pink shrimp Farfantepenaeus brasiliensis in a zero exchange aerobic heterotrophic culture system.
Materials and methods
The experiment was conducted at the Marine Aquaculture Station (EMA/FURG) from 19/08 to 18/09, 2009. The experimental system consisted of 12 rectangular plastic tanks (40 L) with a bottom area of 0.20 m2. Farfantepenaeus brasiliensis early juveniles (0.46 ± 0.13 g) were stocked in the tanks at a density equivalent to 150 shrimp m−2 (30 shrimp/tank). Three replicate tanks were randomly assigned to the following probiotic treatments: (1) Bacillus subtilis, Bacillus licheniformis and Bacillus pumilus mixture (Sanolife Pro-W®, INVE Technologies, Dendermonde, Belgium), (2) Bacillus sp., Enterococcus sp., Lactobacillus spp. mixture (Biomin Start-grow®, Herzogenburg, Austria), (3) Bacillus cereus var. toyoi (Bacteriology Laboratory – Biotechnology Center, Federal University of Pelotas, UFPel) and (4) control treatment (without probiotic addition).
To promote the development of the microbial flocs, all experimental tanks received an inoculum of (500 mL) from a heterotrophic shrimp culture system. Organic fertilization in molasses form was calculated based on Ebeling, Timmons & Bisogni (2006) and Avnimelech (1999) assuming that 6 g of carbon is needed to convert 1 g of TAN (total ammonia nitrogen), generated from feed, into bacterial biomass.
Commercial probiotics were added daily to the treatment tanks to maintain a concentration of 5.104 cfu mL−1 (Sanolife Pro-W®) and 6.103 cfu mL−1 (Biomin Start-grow®), following manufacturers’ recommendation. Previous analyses assure that the dose applied achieved the recommended concentration. For treatment 3, (B. cereus var. toyoi) the probiotic dose used was equivalent to the Bacillus spp. commercial mixture (Sanolife Pro-W®).

Feed conversion ratio was determined based on weight gain, survival, total feed consumed and initial feed moisture content. No water exchange was carried out during the experimental period; only dechlorinated freshwater was added to compensate for evaporation losses.
Throughout the experimental period, water temperature (mercury thermometer, precision ± 0.5°C), salinity (optical refractometer model RTS – 101; Atago® US, Bellevue, WA, USA, ±1 g L−1), pH (digital pH meter model Handylab 2 BNC, ±0.01 precision; Schott®, Hattenbergstr, Germany) and dissolved oxygen (dissolved oxygen meter model Handylab/OXI/set ±0.01 mg L−1 precision; Schott® Cambridge, UK) were measured every day.
Water samples were collected every2 days to determine concentrations of total ammonia nitrogen (NH3 + NH4+ -N; UNESCO 1983) and nitrite (NO2; Bendschneider & Robinson 1952). Chlorophyll α concentration was measured twice weekly according to (Strickland & Parsons 1972); reactive phosphorus was determined three times during the experimental period (PO4; Aminot & Chaussepied 1983) and alkalinity and total suspended solids (TSS) once a week (Eaton, Cleserci & Greenberg 1995).
The concentration of presumptive Vibrio spp. in water was monitored at day 0, 10, 15, 22 and 30 according to the spread plate technique using thiosulfate citrate bile salt sucrose (TCBS) Difco® (Difco Laboratories, Detroit, MI, USA) (Lennette, Spaulding & Truant 1974). At the beginning of the experiment, the water was chlorinated and verified the absence of Vibrio spp.
For the immunological analysis, the haemolymph was collected in the beginning, in the middle and in the end of the experimental period by inserting a 21G needle coupled to a 1-mL syringe, into the shrimp ventral sinus, transferred to a tube and left to coagulate for 24 h at 4°C. The clot was then repeatedly centrifuged at 2000 g for 10 min to obtain the serum, which was either immediately used, or aliquoted and stored at −20°C (Maggioni, Andreatta, Hermes & Barracco 2004).
Granular haemocyte counts (GHC) was determined using a Neubauer chamber, after collecting the haemolymph (six animals per treatment) directly into an anticoagulant solution (1:4) (modified Alsever solution or MAS: 27 mM sodium citrate, 336 mM sodium chloride, 115 mM glucose, 9 mM EDTA, pH 7.0) (Maggioni et al. 2004).
Total protein concentration (TPC) in shrimp serum (six animals per treatment) was determined according to the Bradford method (1976) using bovine serum albumin (BSA) as standard (Maggioni et al. 2004).
Statistical analysis
One-way anova was used to determine significant differences (P < 0.05) on shrimp performance. A two-way analysis of variance (anova, α=0.05) (time × treatment) was used to detect differences in bacteriological and immunological parameters between treatments and the control point. Tukey test was applied when significant differences were detected. All tests were conducted after the confirmation of homogeneity of variances (Cochran test) and normality distribution of data (Kolmogorov–Smirnov's test).
Results
Mean final weight and specific growth rate of shrimp were significantly higher in the probiotic treatments (Table 1). The water quality parameters monitored during the experimental period remained at concentrations suitable for shrimp culture and no significant differences were observed among treatments (P > 0.05) (Table 2).
| Treatment | Survival (%) | Final weight (g) | Specific growth rate (%day) |
|---|---|---|---|
| Sanolife Pro-W | 91.65 ± 11.02a | 1.42 ± 0.40a | 0.036 ± 0.007ª |
| Biomin Start-grow | 81.92 ± 2.40a | 1.39 ± 0.8a | 0.035 ± 0.009a |
| Bacillus toyoi | 81.90 ± 13.4a | 1.34 ± 0.36a | 0.034 ± 0.004a |
| Control | 88.86 ± 6.36a | 1.22 ± 0.38b | 0.030 ± 0.003b |
- Different superscript letters indicate significant differences (P < 0.05).
| Treatments | ||||
|---|---|---|---|---|
| Parameter | Sanolife Pro-W | Biomin Start-grow | Bacillus toyoi | Control |
| Temperature (0C) | 26.4 ± 0.25 | 26.7 ± 0.15 | 26.5 ± 0.1 | 26.7 ± 0.25 |
| pH | 8.1 ± 0.02 | 8.1 ± 0.01 | 8,08 ± 0.03 | 8.1 ± 0.006 |
| Salinity (g L−1) | 31.58 ± 0.12 | 32.32 ± 0.47 | 32.34 ± 0.4 | 31.47 ± 0.23 |
| DO (mg L−1) | 6.19 ± 0.07 | 6.13 ± 0.04 | 6.14 ± 0.02 | 6.11 ± 0.01 |
| TSS (mg L−1) | 618.56 ± 453.4 | 592.31 ± 2.61 | 635.66 ± 485.29 | 538.09 ± 444.7 |
| Alkalinity (mg L−1) | 172.08 ± 15.73 | 186.25 ± 15.53 | 183.33 ± 28.15 | 184.16 ± 12.4 |
| TAN (mg L−1) | 0.91 ± 1.55 | 1.02 ± 1.47 | 1.25 ± 1.89 | 0.92 ± 1.48 |
| Nitrite (mg L−1) | 4.31 ± 3.54 | 4.73 ± 3.88 | 4.11 ± 3.21 | 3.65 ± 3.11 |
| Phosphate (mg L−1) | 3.6 ± 1.8 | 4.29 ± 1.79 | 4.78 ± 2.19 | 3.27 ± 1.32 |
| Chlorophyll α (μg L−1) | 61.41 ± 60.75 | 26.9 ± 19.16 | 48.56 ± 39.78 | 49.78 ± 36.51 |
- TSS, total suspended solids; DO, dissolved oxygen; TAN, total ammonia nitrogen.
Significant differences were observed in granular haemocyte count among treatments (Table 3). All the probiotic treatments presented higher levels of granular haemocyte count compared with control group (Table 3). Biomin® (Herzogenburg, Austria) showed the highest levels of granular haemocyte count and total protein (Tables 3 and 4).
| Granular haemocytes (%HG) days | |||
|---|---|---|---|
| 0 | 15 | 30 | |
| Sanolife Pro-W | 72.16 ± 2.92ª | 72.83 ± 3.65ª | 72.33 ± 4.13ª |
| Biomin Start-grow | 72.16 ± 2.92ª | 76.66 ± 1.86b | 75.5 ± 2.58ª |
| Bacillus toyoi | 72.16 ± 2.92ª | 74 ± 2.19ª | 73.66 ± 3.98ª |
| Control | 72.16 ± 2.92ª | 69 ± 2.36ª | 70.83 ± 2.31ª |
- Different superscript letters indicate significant differences (P < 0.05).
| Total protein (mg mL−1) days | |||
|---|---|---|---|
| 0 | 15 | 30 | |
| Sanolife Pro-W | 121.16 ± 1.72 | 120.83 ± 2.31 | 120.33 ± 2.33 |
| Biomin Start-grow | 121.16 ± 1.72 | 124.5 ± 2.42 | 124.5 ± 1.87 |
| Bacillus toyoi | 121.16 ± 1.72 | 120.66 ± 2.42 | 120.5 ± 1.87 |
| Control | 121.16 ± 1.72 | 121.66 ± 1.96 | 120.66 ± 3.07 |
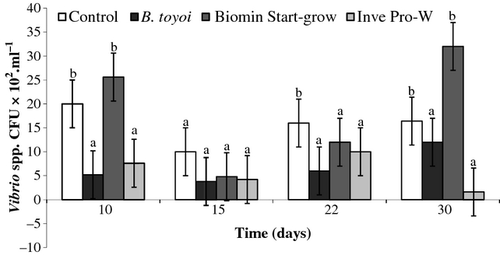
The purpose of the probiotic use was not only to evaluate the performance, but also to control the proliferation of Vibrio in the water. Although we did not assess the presence of Vibrio in the inoculum and in the gastrointestinal tract of the shrimp, the results show that probiotics have contributed to the maintenance of a low concentration of Vibrio in the water, as they are present in seawater and intestinal flora of penaeid shrimp. The bacteriological analysis showed that probiotic treatments maintained the concentration of Vibrio spp. lower than the control group, except for treatment Biomin® which presented the highest concentration of this microorganism (Fig. 1).
Discussion
Probiotic application to marine organisms aims to increase seafood supply and safety, to control the proliferation of harmful microorganisms, and to develop new drugs. Managing microflora in aquaculture to enhance animal welfare, using beneficial bacterial strains, can be considered a biotechnological tool for the development of sustainable and environmental friendly aquaculture (Avella et al. 2010).
Bacillus species have been used as probiotics for at least 50 years. The scientific interest in Bacillus species as probiotics though, has only occurred in the last 15 years (Mazza 1994; Sanders, Morelli & Tompkins 2003; Hong, Duc & Cutting 2005). The safety of Bacillus species has been extensively reviewed elsewhere (Scan 2000a; Ishibashi & Yamazaki 2001; Sanders et al. 2003; Logan 2004).
The manipulation of the gut microbiota through dietary supplementation of beneficial microbe(s) is a novel approach not only from a nutritional point of view, but also as an alternate viable therapeutic modality to overcome the adverse effects of antibiotics and drugs. Those beneficial microorganisms are usually referred as probiotic which after administration are able to colonize and multiply in the gut of host animals, and execute numerous beneficial effects by modulating biological systems in host (Cross 2002; Nayak 2010).
The results of growth and survival of the present study were similar with those reported by Vita (2008), who found that the final weight and growth rate of white shrimp Litopenaeus vannamei were significantly higher in the tanks treated with probiotic than in the control tanks. Wang, Xu and Xia (2005) determined that shrimp reared in ponds treated with probiotics showed significantly higher survival rate, feed conversion ratio and final production compared with control tanks. This indicates that the addition of the commercial probiotics had a noticeable influence on shrimp production and survival rate.
In addition, Wang et al. (2008) observed that the probiotics application significantly decreased the amount of TN (total nitrogen) and TOC (total organic carbon) in pond sediment. The TP (total phosphorus), and TIP (total inorganic phosphorus) in sediment were also reduced during the culture. Zhou, Wang and Li (2009) working with shrimp larvae (Penaeus vannamei) obtained significantly increased survival rates in all treatments over the controls with the application of the probiotic, B. coagulans SC8168.
A potential alternative to intensive shrimp production are zero water exchange systems with microbial flocs (BFT), which has the benefits of bacterial uptake of nitrogen, including ammonia (Burford et al. 2003), and conversion of ammonia into cellular protein, which also provides a supplemental source of nutrition (McIntosh 2000a; McIntosh 2000b; Burford, Thompson, McIntosh, Bauman & Pearson 2004b; Wasielesky et al. 2006).
The microbial community formed in this system is able to rapidly utilize dissolved nitrogen and convert it into microbial protein (McIntosh 2000a; Burford & Williams 2001). This system offers the possibility to simultaneously maintain a good water quality within aquaculture systems and produce additional food for the aquaculture organisms (de Schryver, Crab, Defoirdt, Boon & Verstraete 2008). Juvenile L. vannamei grown in a microbial floc-based system have demonstrated higher growth rates and survival compared with juveniles grown in clear water systems (Wasielesky et al. 2006).
Several studies have demonstrated that shrimp achieve better growth when cultured in biofloc-based ponds. This enhanced growth has been attributed to the nutrients contributed from floc materials present in pond water (Moss & Pruder 1995; Otoshi, Montgomery, Look & Moss 2001; Tacon, Cody, Conquest, Divakaran, Forster & Decamp 2002; Burford, Sellars, Arnold, Keys, Crocos & Preston 2004a; Burford, Sellars, Arnold, Keys, Crocos & Preston 2004b). The floc is composed of aggregated, suspended particles formed in shrimp culture water, and contains phytoplankton, bacteria, zooplankton and detrital materials. It is possible, however, that some components of the floc biomass positively influence shrimp growth through activity not associated with specific nutrients (Ju, Forster, Conquest & Dominy 2008).
The floc biomass could provide a complete source of cellular nutrition as well as various bioactive compounds (Akiyama, Dominy & Lawrence 1992; Fast & Menasveta 2000; Tacon et al. 2002; Truus, Vaher, Koel, Mahar & Taure 2004; Singh, Kate & Banerjee 2005) and may contain an, as yet, undiscovered growth factor (Ju et al. 2008). Floc carotenoids have been reported to provide essential nutritional and many bioactive physiological functions in animal tissues, including stimulating animal immune systems. Ju et al. (2008) concluded that these results suggest that floc materials that develop in low-water exchange shrimp culture systems could be added to diets to obtain better shrimp growth in clear water systems.
These naturally occurring organisms contribute nutritionally and serve as a pre-/probiotic and/or unknown growth promoter. For these reasons, it was hypothesized that production of microbial flocs could produce a viable alternative ingredient for shrimp feed (Kuhn, Boardman, Lawrence, Marsh & Flick 2009). In the present study, even in the presence of a heterotrophic microbial community, probiotics have shown promising results improving the performance of F. brasiliensis juveniles.
A wide range of probiotics, containing either monospecies or multispecies of microorganisms are commercially available. Recently, a number of studies have confirmed the beneficial effects of both forms of probiotics under in vitro and in vivo conditions. However, it is postulated that multispecies/multistrain probiotics are more effective and consistent than their monospecific counter parts as mixed cultures may exert synergistic probiotic properties (Nayak 2010; Timmerman, Koning, Mulder, Rombout & Beynen 2004).
The probiotic Sanolife Pro-W® is a mix of B. subtilis, B. licheniformis and B. pumilus and is known to produce proteases and other enzymes that enable it to contribute to the natural digestion activity of the host (Ziaei-Nejad, Rezaeib, Takamic, Lovettd, Mirvaghefia & Shakourie 2006), and B. licheniformis has been shown to act as an antiviral (Arena, Maugeri, Pavone, Iannello, Gugliandolo & Bisignano 2006). For these reasons, it is plausible to expect positive results from the application of a Bacillus mix to larviculture of commercially relevant species.
Bacteria belonging to both spore former and non-spore formers are used as probiotics. Several spore-forming bacteria, which produce a wide range of antagonistic compounds, can be valuable as probiotics (Moriarty 2003). Among spore formers, Bacillus spores like B.toyoi are routinely being used as probiotics in human and animal practices due to their immunostimulatory properties (Casula & Cutting 2002; Hong et al. 2005).
Spores being heat-stable have a number of advantages over other non-spore formers such as Lactobacillus spp., namely, that the product can be stored at room temperature in a desiccated form without any deleterious effect on viability. In addition, survive extreme environmental conditions enabling long-term survival in conditions that could otherwise kill vegetative bacteria (Nicholson, Munakata, Horneck, Melosh & Setlow 2000; Cutting 2011). Another advantage is the ability of surviving the low pH of the gastric barrier (Spinosa, Braccini, Ricca, De Felice, Morelli, Pozzi & Oggioni 2000; Barbosa, Serra, La Ragione, Woodward & Henriques 2005), which is not the case for most species of non-spore formers (Tuohy, Pinart-Gilberga, Jones, Hoyles, McCartney & Gibson 2007). There is sufficient evidence of the benefits associated with the use of spore-forming bacteria, such as Bacillus spp., as biological agents for improving water quality and reducing disease in aquaculture (Gomez-Gil, Roque & Turnbull 2000; Rengipat, Rukpratanporn, Piyatiratitivorakul & Menasaveta 2000; Irianto & Austin 2002; Sanders et al. 2003; Vaseeharan & Ramamsamy 2003; Wolken, Tramper & van der Werf 2003; Hong et al. 2005; Lalloo, Ramchuran, Ramduth, Gorgens & Gardiner 2007).
The B.cereus var. toyoi is a spore-forming bacterium that was tested for the potential use as probiotic for aquaculture. Spores of Bacillus toyoi, isolated from soil, reduced the mortality of Japanese eel which were infected by Edwardsiella sp., (Kozasa 1986). The same feed additive increased the growth rate of yellowtail (Kozasa 1986).Gatesoupe (1991, 1994) observed that spores of B. toyoi and other Bacillus species when used as feed additive increased the growth of S. maximus and common snook (Centropomus undecimalis) (Irianto & Austin 2002). In the present study, B.cereus var. toyoi showed similar results in commercial multispecies probiotics.
Performance results of shrimp reared in the probiotic treatments during this study was significantly higher compared with the control group. The probiotic treatments did not differ significantly among each other, on the parameters analysed, showing that probiotic based on B. cereus was effective to improve F. brasiliensis culture, during the nursery phase, in a zero water exchange culture system. Moreover, shrimp reared in the probiotic treatments showed higher levels of total protein and granular haemocyte. Also, it was observed that despite the lower concentration of probiotic bacteria in the Biomin® treatment (6.103 cfu mL−1 vs. 5.104 cfu mL−1 in other probiotic treatments) results between probiotic application and Biomin® treatment were similar.
The probiotic effect is more evident when the environmental conditions are unfavourable. Biomin® treatment showed the highest concentration of Vibrio spp., probably because of the lower concentration of probiotic bacteria applied to the tanks in this treatment, due to the recommended dose. However, this treatment presented a more pronounced probiotic effect with the increase of granular haemocytes that seem to be involved in phagocytosis of microorganisms, formation of capsules and nodules and also in the production of toxic molecules and microbicides, extremely important to the immune response of the animals, increasing the capacity to resist unfavourable conditions (Hose, Martin & Gerard 1990; Gargioni & Barracco 1998; van de Braak, Botterblom, Taverne, Van Muiswinkel, Rombout & van der Knaap 2002b).
These results confirm the findings of Krummenauer, Abreu, Lara, Poersch, Encarnação and Wilson (2009) in which, the application of the Biomin® probiotic in Vibrio parahemolitycus contaminated shrimp under bio-floc conditions resulted in significant higher survival when compared with the control where no probiotics were added. This factor probably reflects the characteristics of some of the strains present in the Biomin® treatment like Enterococcus sp. which is a typical intestinal probiotic, known to be able to colonize shrimp gut, hepatopancreas and induce a positive impact on bacterial ecology of the gut by inhibiting Vibrio spp., throughout competitive exclusion and enhancement of the immune response (Supamattaya, Bundit, Boonyarapatlin & Schatzmayr 2006; Swain, Singh & Arul 2009).
The bacteriological analysis showed that the concentration of Vibrio spp. measured in probiotic treatment tanks was lower than that recorded in the control tanks, confirming the successful use of the probiotic as an alternative to the use of an antibiotic. The results showed that the probiotics are effective in the nursery stage of pink shrimp F. brasiliensis, increasing its performance even in a heterotrophic environment. Results also demonstrated that B. cereus var. toyoi is a potentially probiotic microorganism for aquaculture use. Future prospects include tests during other stages of pink shrimp farming as well as their use for the pacific white shrimp L. vannamei.
Acknowledgments
The authors acknowledge the financial support provided by CNPq and Capes- Brazil. Wilson Wasielesky Jr is research fellow of CNPq agency.




