Experimental challenges of Atlantic salmon Salmo salar with incremental levels of copepodids of sea louse Caligus rogercresseyi: effects on infestation and early development
Over the last decade, the native parasite which has worst affected salmonids reared in Chile (Atlantic salmon and Rainbow trout) is Caligus rogercresseyi (González & Carvajal 1994; González, Carvajal & Medina 1995; Boxshall & Bravo 2000; Rozas & Asencio 2007). The financial costs associated with treating Caligus have been estimated at US$0.30 kg−1 or US$160 million annually, principally from intensive use of antiparasitic drugs (Carvajal, Gonzalez & Nascimento 1998; Costello 2009). Consequently with the economic impact caused by this parasite to the Chilean salmon aquaculture, several studies have described significant biological aspects of Caligus species along with their interaction with wild and cultured hosts (González, Carvajal & George-Nascimento 2000; González & Carvajal 2003; Pino-Marambio, Mordue, Birkett, Carvajal, Asencio, Mellado & Quiroz 2007). González and Carvajal (2003) described the life cycle of C. rogercresseyi under laboratory conditions and cultured hosts, which comprised eight developmental stages lasting between 30 and 45 days, and which are strongly dependent on temperature and salinity. Furthermore, field evaluations have shown that rainbow trout is the most susceptible species, followed by Atlantic salmon and Coho salmon (Zagmutt-Vergara, Carpenter, Farver & Hedrick 2005). Coinciding with such evaluations, González et al. (2000) showed through experimental infestations that Atlantic salmon is less suitable to be colonized by infective copepodids, while the Coho salmon is heavily colonized by copepodids although only a low proportion of the parasites reaches the adult stage.
The interaction between salmonid fish and sea lice has been widely studied with Lepeophtheirus salmonis (Krøyer) under laboratory conditions (Bron, Sommerville & Jones 1991; Johnson & Albright 1992; Dawson, Pike, Houlihan & McVicar 1997; Finstad, Bjørn, Grimnes & Hvidsten 2000; Treasurer & Wadsworth 2004; Kolstad, Heuch, Gjerde, Gjedrem & Salte 2005; Gjerde & Saltkjelvik 2009). Patterns that emerged from this parasite–host system related to settlement and early development may be applied to other sea lice parasites and its salmon host. A positive relation between infection pressure (number of copepodids per fish) and settlement may be construed from these studies despite high variability observed, where low infection pressure (28–36 copepodids per fish) produce a low abundance of parasites on fish during settlement (1.66 ± 1.15 and 29.8 parasites per fish) (Bron et al. 1991; Johnson & Albright 1992; Gjerde & Saltkjelvik 2009); intermediate infection pressure (74–145 copepodids per fish) cause mean abundance of parasites on fish (25.1 ± 16.4 and 74.6 ± 42) (Finstad et al. 2000; Treasurer & Wadsworth 2004; Kolstad et al. 2005; Gjerde & Saltkjelvik 2009) and high infection pressure (600 copepodids per fish) produce a high abundance of parasites on fish during settlement (165.8 ± 10.2) (Dawson et al.1997). Another pattern described in the literature is the preference of L. salmonis for settling on fins rather than other areas of the body (Dawson et al. 1997; Tucker, Sommerville & Wootten 2000, 2002; Genna, Mordue, Pike & Mordue 2005). Bron et al. (1991) have suggested that the fin rays may provide protection from currents and make settlement easier; the distribution would be a consequence of the current speed and the ability of the copepodid to occupy a given area. Another pattern was shown by Treasurer and Wadsworth (2004) under laboratory conditions. Under experimental challenges, a high frequency of chalimus was found on fish gills instead of fins. They suggested that this is due to the reduced host swimming speed in tanks that permits copepodids to attach to the gills.
The aim of this study was to assess the effect of experimental challenges on Atlantic salmon with incremental levels of copepodids of sea louse C. rogercresseyi on infestation and early development. Specifically, we compared abundance and prevalence of parasites 4 and 14 days postinfestation. Also, we compared settling preference of copepodids in different areas of the body 4 days postinfestation (chalimus stages) and characterized the parasites in accordance with its developmental stages 14 days postinfestation (adult stages). Experimental challenges with C. rogercresseyi were conducted by collecting egg-bearing females from a salmon farm located in the X Region in Chile (41°28′18″S; 72°56′12″W). In order to obtain the copepodids, the egg sacs were removed from the females and cultivated in filtered and sterilized seawater at a temperature of 12°C with constant aeration. Previously, a total of 180 fish were divided equally (20 fish per tank) into nine 350 L tanks. The fish were acclimatized over a period of 15 days in the tanks with salinity at 30–32 pp/thousand, temperature of 11–14°C and constant aeration of 8–10 mg L−1. Fish were challenged with incremental levels of copepodids per fish (50, 100 and 150) added to each tank (infection pressure), using three replicates for treatment. Prior to adding the copepodids, the water level was decreased and flow was stopped for a period of 6 h. The tanks were also kept covered to decrease light intensity. Subsequent to the challenge, abundance and prevalence were recorded for each treatment on days 4 and 14 postinfestation, evaluating 10 and 3 fish, respectively, per tank. Each fish was killed with a lethal dose of benzocaine, and both weight and length were then recorded. The settlement of the parasites was classified per fin and body zone for the sampling carried out at day 4 postinfestation, similar to the technique used by Tucker et al. (2000). Whereas for the sampling carried out at day 14 postinfestation, a population characterization was made of the parasites per treatment in accordance with the developmental stages defined by González and Carvajal (2003) and with the length of each parasite using a Nikon SMZ 800 (Nikon Inc., Melville, NY, USA) magnifying glass with an Infinity digital camera with image analysis software. The comparison of the number of parasites per fish and the settling areas for all three infection pressures was done using a mixed linear model, where both infestation levels and areas were considered as fixed effects; the tank was regarded as a random effect and the body weight as covariable. A posteriori Tukey test was performed to identify significant differences between treatments. Furthermore, a one-way anova was carried out to compare the parasite size between treatments. The statistical program SAS Institute Inc. (2006) was used for all analyses.
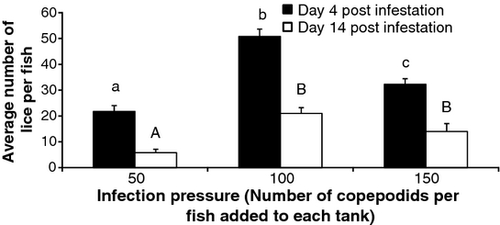
In the challenge test, prevalence of sea lice on fish was 100%, with significant differences of abundance of parasites between treatment (Fig. 1), both for day 4 (F = 30.67, P < 0.05; Tukey test: 50 ≠ 100 ≠ 150, P < 0.05) and day 14 postinfestation (F = 13.20, P < 0.05; Tukey test: 50 ≠ 100 = 150, P < 0.05). A total of 654, 1524 and 969 parasites were recorded on fish 4 days postinfestation for the treatment of 50, 100 and 150 copepodids per fish, respectively, which represents a percentage settling of 43% (654/1500), 51% (1524/3000) and 21% (696/4500) respectively. The existence of a maximum settlement at intermediate levels of infection pressure (100 copepodids by fish) was not described in previous studies with others sea lice (Bron et al. 1991; Johnson & Albright 1992; Finstad et al. 2000; Treasurer & Wadsworth 2004; Kolstad et al. 2005; Gjerde & Saltkjelvik 2009). Our results have shown that others factors (i.e. interference among infective stages) beyond the availability of infective stage in the water were regulating the settlement process. Abundance of parasites strongly decreased from day 4 to 14 postinfestation in each of the infection pressures evaluated (Fig. 1). This has been described previously by González et al. (2000) and may be a consequence of a defensive response of fish against the parasites (Mustafa, MacWilliams, Fernandez, Matchett, Conboy & Burka 2000; Tadiso, Krasnov, Skugor, Afanasyev, Hordvik & Nilsen 2011). Differences in parasite abundance among treatments did not seem to be associated with differences in size between the sampled fish, as no significant differences were found for weight in either of the two samples (day 4 F = 2.35, P > 0.05; day 14 F = 0.89, P > 0.05). On the contrary, significant differences on settlement were found between tanks, although only at day 4 postinfestation (F = 13.15, P < 0.05) and not at day 14 postinfestation (F = 1.98, P > 0.05). The population characterization of the parasites at day 14 postinfestation revealed that most parasites reached adult stage (Table 1). No significant differences were found in total length (in mm) of the parasite between different infection pressures (F = 1.36; P > 0.05), where the variable mean values for levels 50, 100 and 150 were 4.19 mm (DS = 0.4; CV = 9.55; n = 29); 4.22 mm (DS = 0.29; CV = 6.87; n = 129) and 4.20 mm (DS = 0.35; CV = 8.33; n = 81) respectively.
| Infection pressure | Total number of settled parasites per treatment | Stages of development | ||||
|---|---|---|---|---|---|---|
| A | CH4 | CH3 | CH2 | CH1 | ||
| 50 | 42 | 69.0 | 23.8 | 4.8 | 4.8 | 0 |
| 100 | 162 | 79.6 | 19.1 | 0.6 | 0.6 | 0 |
| 150 | 102 | 79.4 | 12.7 | 4.9 | 2 | 1 |
The settling preference of C. rogercresseyi showed a significant variation in the different areas of the body (F = 71.65, P < 0.05), where in total, more than 94% of the parasites settled on fins, with higher settling percentages recorded on the pectoral, dorsal and caudal fins (Table 2). Previous authors have reported this preference for fins in L. salmonis. In the work by Genna et al. (2005), the same settling preferences as those found in this study were recorded. Johnson and Albright (1992) evaluated the settlement of L. salmonis on three salmonid species, reporting a preference for pectoral, pelvic and anal fins. Other authors have also described the settling preferences for the chalimus stage of L. salmonis concurring that these preferences are for the pectoral and dorsal fins (Dawson et al. 1997; Tucker et al. 2000; Tucker et al. 2002; Treasurer & Wadsworth 2004). Settled parasites could not found on the gills, similar to what was reported by Treasurer & Wadsworth (2004) in natural challenges of C. elongates. However, this differed from experimental challenges in studies with L. salmonis, in which copepodid settlement was recorded in this zone (Bron et al. 1991; Johnson & Albright 1992; Tucker et al. 2000; Treasurer & Wadsworth 2004).
| Infection pressure | Number of settled parasites per treatment | Settlement zones | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PF | DF | CF | VF | AF | PZ | VZ | O | ||
| 50 | 654 | 35.8 | 27.2 | 20.0 | 9.9 | 3.7 | 2.3 | 0.5 | 0.6 |
| 100 | 1524 | 36.8 | 23.4 | 20.0 | 9.2 | 5.1 | 2.8 | 1.9 | 0.9 |
| 150 | 969 | 39.5 | 18.4 | 19.7 | 12.7 | 4.5 | 4.8 | 0.4 | 0.0 |
In summary, incremental levels of copepodids of C. rogercresseyi during the infestation did not produce a linear response over the settlement on Atlantic salmon. Indeed the maximum abundance of parasites on fish was found in the intermediate pressure of infection. This has not been previously reported in other sea lice and suggests that other factors determine the success of the settlement under high pressure of infection. Future studies must evaluate if host exposure time to the parasite change the relationship between infection pressure and settlement. Finally, the settling preference of C. rogercresseyi on Atlantic salmon was not modified by the infection pressure and this occurred principally on pectoral, dorsal and caudal fins. These results show that sampling methods considering principally fins will represent the early infection process the C. rogercresseyi on Atlantic salmon.
Acknowledgments
This study was financed by INNOVA-CHILE, CORFO, through the projects: Business Consortium for genetics and biotechnological development for the salmon industry (206-5047) and Development of a new methodology for the identification and selection of salmonids genetically resistant to the ectoparasite Caligus rogercresseyi (07CN13PBT- 61). The authors would like to thank the support of the Salmon Technology Institute (INTESAL) in the execution of these projects. We also thank Dr Simon Jones for his technical assistance on the methodological design of the research and Ing. Michael Filp for his assistance on fish management. The authors would like to thank the anonymous reviewers for their valuable comments and suggestions to improve the quality of our manuscript. ***MM is a CONICYT doctoral scholarship.




