First results of embryonic development, spawning and larval rearing of the Mediterranean spider crab Maja squinado (Herbst †) under laboratory conditions, a candidate species for a restocking program
Abstract
The Mediterranean spider crab, Maja squinado, is depleted due to overfishing. The crab has virtually disappeared from areas where it was abundant, such as the Balearic Islands and the Catalan coast. Maja squinado, is economically and ecologically very valuable, and it is essential to obtain information on its biology and rearing conditions to attempt to repopulate the damaged stocks of the species in the Mediterranean basin. Herein, we describe the first successful rearing of M. squinado under laboratory conditions. Our results show that M. squinado is an excellent candidate for restocking using cultured juveniles. Two consecutive broods with a 1–4 day interbrood period were observed in the laboratory in wild-caught females, the maximum observed duration of embryonic development of the egg mass being 32 days at 18.4 ± 0.9°C, and went through four different stages. The complete larval and first juvenile development was studied in laboratory cultures fed enriched Artemia metanauplius. At 19.6 ± 0.6°C, development from hatching to first crab moult took 17 days, and it comprised two zoeae stages and one megalopa stage. The survival rate at the different stages was monitored, and 7.13 ± 2.3% was achieved at the first crab instar.
Introduction
Programmes for enhancing or improving stocks of several severely depleted crab and lobster species have been implemented in Japan, North America and Europe, using aquaculture techniques as a tool for producing juveniles. Examples are the swimming crab, Portunus trituberculatus, in Japan (Ariyama 2000, 2001; Secor, Hines & Place 2002), the blue crab, Callinectes sapidus, in Chesapeake Bay (Davis, Eckert-Mills, Youg-Williams, Hines & Zohar 2005; Zohar, Hines, Zmora, Johnson, Lipcius, Seitz, Eggleston, Place, Schott, Stubblefield & Chung 2008), the European lobster, Homarus gammarus (Beard, Richards & Wickins 1985; Bannister 1998; Browne & Mercer 1998; van der Meeren 2000; Beal, Mercer & O'Conghaile 2002), the American lobster, Homarus americanus (Van Olst, Carlberg & Ford 1977 and Beal, Chapman, Irvine & Bayer 1998) and the spiny lobster, Jasus edwardsii (Svåsand, Skilbrei, van der meeren & holm 1998; van der Meeren 2000; Oliver, Stewart, Mills, MacDiarmid & Gardner 2005; Mills, Gardner & Johnson 2006) among other crustacean species. We studied preliminary data on embryonic development, spawning and larval rearing to assess whether it is possible to restock the Maja squinado population around the Balearic Islands.
Maja squinado (Herbst 1788) was reported to be widely distributed throughout the Mediterranean and the NE Atlantic to the southern North Sea (Clark 1986 and d'Udekem d'Acoz 1999). Based on certain morphological and biometric characters, Neumann (1998) suggested that the Atlantic and Mediterranean spider crabs were in fact different species: Maja brachydactyla (Balss 1922) and M. squinado respectively. Recently, Sotelo, Morán, Fernández and Posada (2008), who used molecular techniques to study variation in two mitochondrial genes, showed that Atlantic and Mediterranean spider crabs are two distinct species, corroborating the classification previously proposed by Neumann (1996), Neumann (1998). Therefore, it follows that all the records of M. squinado from the NE Atlantic must be considered as M. Brachydactyla. The geographical delimitation should be taken into account for the stock enhancement of the Spanish Mediterranean spider crab, M. squinado, using conspecific crabs from the Mediterranean, and never from the Atlantic coasts (Sotelo et al. 2008).
Spider crabs are species of great commercial interest in many European countries, with annual catches of 5000 tonnes according to FAO (1988), especially in the English Channel and on the north coast of Spain. In spite of this, it was not until the early 90s that the first biological and fishing research on M. brachydactyla was carried out. It was then when studies related to reproduction (González-Gurriarán, Fernández, Freire, Muiño & Parapar 1993; González-Gurriarán, Fernández, Freire & Muiño 1998), growth and moult cycles (González-Gurriarán, Freire, Parapar, Sampedro & Urcera 1995; Sampedro, González-Guarriarán & Freire 2003), population dynamics (Corgos, Bernárdez, Verísimo & Freire 2002) and migratory movements (González-Gurriarán, Freire & Bernárdez 2002; Corgos, Verísimo & Freire 2006) began to be carried out in Galician waters. Others like Clark (1986), Urcera, Arnaiz, Rua and Coo (1993) and Iglesias, Sánchez, Moxica, Fuetes, Otero and Pérez (2002), studied some aspects of M. brachydactyla larval development and larval and juvenile rearing. Andrés, Estévez and Rotllant (2007), Andrés, Estévez, Anger and Rotllant (2008) recently carried out laboratory studies and obtained data on growth, survival and the biochemical composition of the same species.
However, there are only a few studies on the Mediterranean spider crab, M. squinado Herbst 1788; and in some cases these are rather imprecise. This research was mainly carried out by Stevcic (1963), Stevcic (1968a), Stevcic (1968b), Stevcic (1971), Stevcic (1973), Stevcic (1975), Stevcic (1976), who studied the biological, reproductive and migratory behaviour, as well as moulting and fishery characters based on wild and aquarium observations. There are no studies on the reproductive process, embryonic development, spawning or larval and juvenile rearing under intensive culture conditions.
The critical decline of the M. squinado population all over the Mediterranean basin, despite its abundance 50 years ago, makes it difficult to obtain living specimens to work within the Balearic Islands, where LIMIA is carrying out its M. squinado projects. In the last 4 years, only a few specimens have been caught per year around Formentera Island (comments made by Formentera fishermen). In the rest of the Balearic Islands, M. squinado has not been observed for over 20 years. The reasons for this rarefaction remain unclear and have not been specifically studied (García 2007).
Maja squinado is one of the invertebrate species included in the Action Plan for the Mediterranean, and its exploitation in the Mediterranean is regulated according to UNEP 1996 and ZEPIM 1999 (Annex III). In the particular case of the Balearic Islands, catching M. squinado in marine reserves is banned due to its delicate conservation status. The dramatic decline of this population means that fisheries management tools need to be used to recover the stock, and restocking with hatchery-reared juveniles is one possibility; therefore, the viability of rearing this species in captivity needs to be studied. This idea is not new, since Bussani and Zuder 1977 considered that producing M. squinado juveniles could be a solution for recovering this species in the Gulf of Trieste after its decline due to overfishing.
This article describes our experiences in collecting a wild broodstock of M. squinado and its adaptation to captivity conditions. Spawning of the species was achieved for the first time in captivity, and larval rearing took place under hatchery conditions. We obtained preliminary data on egg mass development, larval growth parameters, intermoult period and survival at different larval stages. These experiences represent the first step towards establishing a detailed protocol for successful mass-rearing techniques, which will allow us to obtain an adequate number of juveniles to start a programme for enhancing the M. squinado population around the Balearic Islands.
Material and methods
Broodstock management and embryonic development
In April 2007, 15 wild M. squinado breeders, 10 males and 5 ovigerous females, were caught in the ‘Réserve Naturelle de Scandola, Parc Naturel Régional de la Corse’ (Corsica) by local fishermen using trammel nets. From Corsica to the ‘Laboratori d'Investigacions Marines i Aqüicultura’ (LIMIA), in Port d'Andratx, Mallorca, they were transported in a refrigerated van equipped with two 0.5 m3 tanks and supported with air pumps and biological filters. The water temperature was kept at 16 ± 1°C. The trip took 30 h and the temperature, oxygen and ammonium levels were checked every 3–4 h. The oxygen and temperature parameters did not change significantly during the trip; however, the ammonium values increased quickly after 2 h, and it was necessary to add ammonium neutralizer to the tank water.
As all females were caught carrying a fertilized egg mass and according to Stevcic (1976) M. squinado mates before ovulation, once at the laboratory, males (1.29 ± 0.35 kg) and females (1.33 ± 0.22 kg) were kept separately in two 5 m3 fibreglass tanks with a water renewal rate of 50 L min−1. Ammonium levels were checked daily and maintained at values of 0–0.5 mg L−1. Water temperature and salinity were 18.36 ± 0.94°C and 37 g L−1 respectively. The crabs were fed on fresh mussels ad libitum. The broodstock was kept in a half-light regime with a natural photoperiod.
After the first spawning, the female crabs produced a second egg mass without mating, which indicates that M. Squinado has the ability to store sperm, similar to M. brachydactyla (González-Gurriarán et al. 1998).
Ovigerous females were monitored every 2 days to check the maturity stages of the egg mass and macroscopic and microscopic characters. To collect newly hatched larvae, a larval collector with a 500 μm mesh size was installed, and it received the water that overflowed from the tank with the females in it. Estimations of the number of newly hatched zoeae spawned per female were made volumetrically by counting five 500 mL aliquots of well mixed larvae from an aerated 60 L container.
Larval rearing
At 0 days post hatch (DPH), 6510 newly hatched larvae from one single spawning of one female were individually counted and stocked at an initial density of 70 larvae L−1 in six 15.5 L spherical upwellings with a 150 μm mesh size bottom. The spherical upwellings were hung in a 1 m3 cylinder-conical fibreglass tank. The water renewal rate was 2.4 L min−1 in each upwelling. A recirculation system was used to maintain the desired water quality and temperature (19.66 ± 0.58°C). The recirculation system consisted in a 1000 L fibreglass tank, from which water was pumped through a biological filter and then through a refrigerator equipped with an ultraviolet sterilization lamp. Finally, the water was returned to the tank. Salinity was 37 g L−1, and the photophase 24L/0D. The dissolved oxygen ranged from 7.0 to 7.5 mg L−1.
Phytoplankton (Nannochloropsis gaditana and Isochrysis galbana) was added once a day at a density of 80 000–100 000 cells mL−1 during the entire larval period to maintain a green medium. Zoeae, megalopae and first juveniles were fed 24 h with enriched EG Artemia metanauplii (Easy DHA Selco, INVE, Animal Health S.A., Vigo, Pontevedra, España) distributed in each upwelling twice a day at a rate of 4.3 prey mL−1 (60 prey per larvae). Before providing the food, the remaining Artemia were counted with volumetric procedures to determine the appropriate quantity to add to reach the required concentration.
Growth, dry weight, biometric values and survival determinations
Biometric determinations were performed to the nearest 0.01 mm using an Olympus stereomicroscope (Olympus, Barcelona, España S.A.U) equipped with a calibrated eyepiece micrometre. A sample of 10 eggs from each female was measured at each egg mass developmental stage. Egg mass colour, morphological changes, the duration of the embryonic period and the interbrood period were macroscopically and microscopically observed. Larval measurements were taken according to the guidelines described by Guerao, Pastor, Martin, Andrés, Estévez, Grau, Durán and Rotllant (2008).
Replicates of pre-weighted samples (50 individuals) at each developmental stage, zoea I (ZI), zoea II (ZII), megalopa (MG) and first juvenile (C1), were kept at 110°C for 24 h. The dry weights were determined after cooling in vacuo for 1 h.
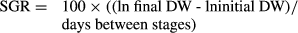
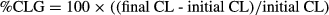

Statistical analysis
The statistical treatment of the data was performed using spss 15.0 for Windows software (IBM España S.A., Santa Hortensia, Madrid, Spain). Data are presented as means ± SD (standard deviation of the mean). Statistical analyses to determine differences in size and growth for each developmental stage were performed using one-way anova at P < 0.05. Growth in DW and body size was analysed by means of regression analyses.
Results
Hatched zoeae per female and embryonic development
The number of zoeae hatched per female was high, and varied from 150 000 to 194 000 newly hatched zoeae per wild M. squinado female (n = 5). Zoeae hatched 4 weeks after the crabs arrived at LIMIA, and four females developed a second egg mass in captivity (not valued) with an interbrood period between 1 and 4 days.
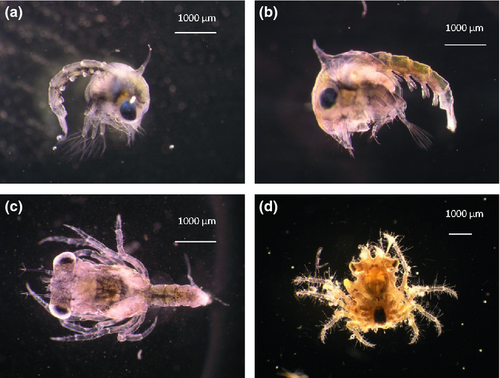
As all the females were carrying a fertilized egg mass when they were caught in Corsica and it was not possible to study the second egg mass in captivity, a maximum duration of the embryonic period of 32 days at 18.36 ± 0.94°C was observed. Four different egg mass developmental stages were observed in all M. squinado females: an early stage (A) (Fig. 1a) with non-pigmented yellow-orange eggs and 90% yolk, which occurred approximately 32 days before hatching (DBH); the second stage (B) occurred 20.5 ± 3.53 DBH, with red eggs in which eye spots and light pigmentation could already be seen (Fig. 1b); the third stage (C) showed 75% pigmented brown eggs in which some movement could be detected (Fig. 1c) and occurred about 9.0 ± 0.0 DBH; and finally the fourth stage (D) prior to hatching with 100% pigmented black eggs (Fig. 1d) occurred 4.0 ± 0.0 DBH. During embryonic development from stage A (741 ± 0.0 μm egg diameter) to stage D (830 ± 30.73 μm egg diameter), a slight increase in egg size, 12% of the initial size, was observed (Table 1). Egg diameters at stage D were significantly larger than at stage A (P ≤ 0.05).

| Stage A | Stage B | Stage C | Stage D | |
|---|---|---|---|---|
| Colour | Yellow-orange | Red | Brown | Black |
| Eggs Ø | 741 ± 0.00 | 785 ± 33.74 | 812.5 ± 25.00 | 830 ± 30.73 |
| Dbh | 32 ± 0.00 | 20.5 ± 3.53 | 9 ± 0.00 | 4 ± 0.00 |
| Character | 90% yolk no pigmentation | Eye spots | Movements 75% pigmentation | 100% pigmentation |
- Colour, egg diameter (μm), days before hatching (Dbh) and microscopic characteristics (Character). Values are given as mean ± SD.
Table 1 shows the results of the macroscopic and microscopic characters, egg diameter and days before hatching of each egg mass developmental stage.
Larval development



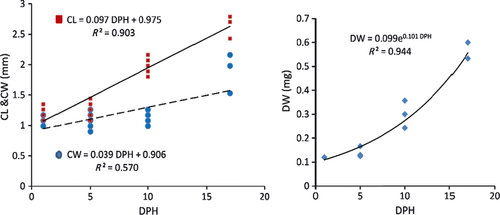
| ZI | ZII | MG | FJ | |
|---|---|---|---|---|
| Dph | 1 | 5 | 10 | 17 |
| Carapace length (mm) | 1.18 ± 0.07a | 1.27 ± 0.10b | 2.01 ± 0.11c | 2.65 ± 0.15d |
| Carapace width (mm) | 1.04 ± 0.06a | 1.06 ± 0.10a | 1.16 ± 0.07a | 1.80 ± 0.32b |
| Dry weight (mg) | 0.12 ± 0.01a | 0.14 ± 0.02a | 0.29 ± 0.05b | 0.55 ± 0.03c |
| Survival (%) | 100 ± 0.00 | 88.26 ± 8.9 | 13.42 ± 2.25 | 7.13 ± 2.33 |
| % CLG | – | 7.6 | 58.2 | 31.8 |
| % DWG | – | 16 | 107 | 89.6 |
| SGR (%) | 3.5 | 14.6 | 9.1 |
- SGR, specific growth ratio; ZI, zoea I; ZII, zoea II; MG, megalopa; FJ, first juvenile; Dph, days post hatch. Values are given as mean ± SD. Means in the same row with different superscripts are significantly different from each other (P ≤ 0.05).
The highest mortality occurred during the megalopa stage, which was the larval rearing phase in which the majority of individuals were lost, as even the last ecdysis to first juvenile did not involve such low survival rates. Carapace length (CL) and width (CW), dry weight (DW), survival (S),%CLG,%DWG and%SGR of M. squinado larvae and first juveniles are shown in Table 2.
Discussion
Our results suggest that M. squinado could be an excellent candidate for rearing in laboratory conditions to achieve a hatchery-reared juvenile population to restock depleted areas, such as the Balearic Islands: metamorphosis to first juvenile was achieved with a mean survival rate of 7.13 ± 2.33%, which is near the survival rate reported by other authors with M. brachydactyla (12.9 ± 1.44%, Andrés et al. 2007; 8–13%, Iglesias et al. 2002), but lower than the one achieved by Urcera et al. (1993) (46%) and by Phena-Lopes, Rhyne, Lin and Narciso (2005) for Mithraculus forceps (60.1 ± 5.1%).
Four egg mass developmental stages with different colourations were observed in M. squinado. Stevcic (1976) noticed a red colour stage during the incubation period of wild Mediterranean spider crabs M. squinado from the Adriatic Sea. However, none of the authors who have studied the Atlantic spider crab M. brachydactyla have mentioned a red colour stage. González-Gurriarán et al. (1993), González-Gurriarán et al. (1998), García-Flórez and Fernández-Rueda (2000) and Iglesias et al. (2002) only described three egg mass developmental stages for M. brachydactyla, with colouration varying from orange to dark orange-grey and finally dark grey in the descriptions by the first two authors, and from orange to orange-brown and brown for the third author.
Egg diameters measured during embryonic development varied between 0.741 mm at stage A (eggs containing 90% yolk) and 0.83 mm at stage D (just before hatching). These measurements are very similar to those previously described by Stevcic (1976) for wild M. squinado caught in the Adriatic Sea (0.76–0.92 mm). However, the eggs of M. brachydactyla seem to have a smaller diameter, between 0.5 and 0.7 mm, at temperatures from 15 to 18°C, according to Iglesias, Sánchez, Moxica, Fuetes, Otero and Pérez (2001).
Stevcic (1967) considered that the time between hatching and the following brood, that is, the interbrood period, was a small unspecified number of days for M. squinado. In our experience, the four females that developed a second egg mass during May, showed an interbrood period of one (three of them) to 4 days. This is shorter than the interbrood period found for M. brachydactyla by different authors on the northwest coast of Spain, for example, the 4–5 day interval described by Iglesias et al. (2002) at 19–22°C or the average of 3.4 days determined by González-Gurriarán et al. (1998) during a complete breeding period; however, García-Flórez and Fernández-Rueda (2000) found a longer period, a mean of 19 days, for M. brachydactyla on the north coast of Spain.
The same authors described an embryonic development duration for M. brachydactyla from 30 to 40 days (Iglesias et al. 2002), 40 to 58 days (González-Gurriarán et al. 1998) and 28 to 77 days (García-Flórez & Fernández-Rueda 2000) depending on the season, which is similar to the duration of the embryonic development we observed (32 days from stage A to D).
We observed two different egg masses in four of the five females. Stevcic (1967) found that M. squinado has three broods a year in the Adriatic Sea, the first between March and May, the second from late May to early July and the last brood from July to August. This agrees with our observations, as our brood female spider crabs were caught in early May, and developed a second brood, but not a third. It is possible then that there was a previous spawning (March) in the wild before they were caught.
The duration of larval development is very similar for all the Majidae species currently described, and agrees with our results: for M. brachydactyla, 15–20 DPH at 18 ± 1°C (Rotllant & Estévez 2005), 6 DPH until the zoea II stage, 12 DPH to the megalopa stage, 22 DPH to first crab (Urcera et al. 1993) at the same temperature, 9 DPH to the megalopa stage and 16 DPH to the first juvenile stage at 19–22°C (Iglesias et al. 2002); and for the newly metamorphosed crabs of Mithraculus forceps, 9 DPH at 28°C (Phena-Lopes, Figueiredo & Narciso 2007).
The preliminary growth data obtained for M. squinado larvae suggest that carapace length and width grow linearly, but that there is an exponential growth pattern for dry weight. Other studies on M. brachydactyla larvae also determined a linear growth pattern for CL and CW and an exponential pattern for DW (Andrés et al. 2008). However, Iglesias et al. (2002) determined an exponential pattern for carapace length.
The feed and feeding schedules are very important for the seed production of any aquatic organism. According to Soundarapandian, Thamizhazhagan and Samuel (2007), the advantage of using Artemia for the last feeding of larval mud crab is that they can contribute to lipids and energy, and thus the feeding efficiency is higher. Using enriched Artemia as live food for rearing, M. brachydactyla larvae has been shown to reduce the development time and significantly increase the viability percentage (Urcera et al. 1993; Iglesias et al. 2002; Andrés et al. 2007). Although we did not use another prey to feed M. squinado larvae, our results showed a good increase in dry weight and carapace length, especially at the megalopa stage. However, the survival rate was the lowest at this instar out of the entire larval process. Andrés et al. (2007) found a significant increase in dry weight at the megalopa stage of M. brachydactyla fed enriched Artemia, and suggested it could be because Artemia enrichments seem to influence the progress of larval development, which is reflected in a higher dry weight rather than higher survival. In our trial, there was always the same amount of prey per volume throughout larval development (4.3 prey mL−1). Due to the considerable reduction in the number of larvae at the megalopa stage, a higher prey number per larva was available at this stage and the feeding rates of megalopa larvae could have been higher than in other stages. An increase in Artemia density has been seen to lead to an increase in dry weight in other decapod crustaceans (Brick 1974; Bigford 1978; Anger & Nair 1979; Minagawa & Murano 1993; and Barros & Valenti 2003). However, necrophagous and cannibalistic behaviour at early developmental stages has been reported for other crab species (Anger & Nair 1979 and Hamasaki 2003) and could be a possible explanation for the high specific growth ratio found at the megalopa stage in M. squinado, because not all larvae showed pelagic behaviour all the time (personal observation), and live larvae could come into contact with dead larvae at the bottom of the rearing tank.
A decrease in survival at the megalopa stage has been observed by several authors in other decapod crustaceans. Urcera et al. (1993) attributed the significant mortality that occurred during the first days of the megalopa stage of M. brachydactyla to the change from predatory pelagic behaviour to benthic behaviour in the larvae, because the inability to adapt to this change could cause high mortalities. Phena-Lopes et al. (2005) described this for the crab Mithraculus forceps, but they attributed it to an increase in interaction (cannibalistic behaviour) between megalopa and zoea II larvae when unsynchronized larval moulting took place. Our observations are more in agreement with the last authors, as it is common to observe the earlier moulted megalopae preying on zoea II larvae, although agonistic behaviour would not be the only cause for the large decrease in survival. More work is required to clarify this issue.
Conclusion
Our results represent the first successful rearing of M. squinado under laboratory conditions. We believe that these results, in terms of the high larval hatching rate, several spawnings during the reproductive season, short larval development, rapid larval growth and good survival rates, are very encouraging and suggest that M. squinado is as an excellent candidate for aquaculture. This would allow us to plan, in the medium term, restocking policies with juveniles in selected zones and protected marine areas around the Balearic Islands. Although there is a broodstock limitation in the Balearic Islands, it is possible to obtain wild breeders of M. squinado from other areas of the Mediterranean Sea where the species is not depleted, or use reared breeders at the laboratory, as long as genetic diversity is maintained. However, much work is needed to understand the basic population dynamics (growth, recruitment, maturation, reproductive behaviour) and to provide the necessary information (management and optimal feeding of breeders, optimal stocking larval densities, feeding regime, prey size, etc.) for designing a hatchery system in the future. The next step is to conduct further investigations to determine the parameters needed to develop a culture protocol that provides a sufficient number of juveniles to restock depleted areas, such as the Balearic Islands.
Acknowledgments
The present work is part of the National Plan for the culture of spider crabs financially supported by JACUMAR.




