Ontogenetic variation of food intake and gut evacuation rate in larvae of the doncella Pseudoplatystoma punctifer, as measured using a non-destructive method
Abstract
Food intake (FI) and gut evacuation (Rg) were measured in larvae of Pseudoplatystoma punctifer (4.5–18.4 mm SL) fed Artemia nauplii, taking advantage of the translucence of their abdominal region to achieve this in a non-destructive way, using digital photographs and mathematical reconstruction of gut volume content (ellipsoidal and cylindrical models for stomach and intestine respectively). The inaccuracy of the method, with reference to counts of nauplii following fish dissection, was low (2.9 ± 1.5%) and independent of fish size (P = 0.6153). Pigmentation hampered measurement in fish >18–19 mm SL. Anaesthesia was needed in fish >9.5 mm SL, thereby preventing the measurement of Rg in individual fish. The FI increased rapidly during the ontogeny, passing from <7% M at 0.6 mg, to 14% M at 1 mg and 21% M at 15–40 mg, and then decreased slightly in larger fish. At 28.5°C, Rg (% M h−1) was modelled as Rg = −8.22 + 12.11 log FI + 6.30 log M – 12.67 (log M)2 (R2 = 0.904, d.f. = 27, with FI in% M and M in mg). Extrapolations of Rg over 24 h gave estimates of daily food rations that fit well with those measured in cannibalistic P. punctifer.
Introduction
The knowledge of how metabolism, growth and food consumption vary between fish species, developmental intervals and environments is essential to understand their adaptive capacities and optimizing their culture. In particular, information on meal size and gut evacuation (Rg) rate is crucial for a correct adjustment of feeding levels and meal frequency (Elliott & Persson 1978; Jobling 1986; Canino & Bailey 1995), which are prerequisites to minimize size heterogeneity and cannibalism (Kubitza & Lovshin 1999; Baras & Jobling 2002). Several methods have been developed to measure the food intake (FI) of fish under controlled conditions (Jobling, Covès, Damsgård, Kristiansen, Koskela, Petursdottir, Kadri & Gudmundsson 2001), with variable accuracy and success, depending on food type and fish size. In particular, numerous methodological restrictions concern fish larvae, which in addition to being small and delicate to handle, generally necessitate live prey (Rønnestad, Rojas-García, Tonheim & Conceição 2001).
The removal method can be accurate for large prey (Baras, Hafsaridewi, Slembrouck, Priyadi, Moreau, Pouyaud & Legendre 2010), but is generally less accurate for small prey, which can decay rapidly, be confused with faeces and pass unnoticed (Kamler 1992), and so it is generally necessary to label small prey. A broad series of tracers have been evaluated (review in Conceição, Morais & Rønnestad 2007). Staining (e.g. with drawing ink or methylene blue) might be inconveniently long (Dendrinos, Dewan & Thorpe 1984), and it is possible that a change in the prey's colour modifies its attractiveness. Radioisotope methods are highly sensitive and leave the prey unaltered (Langar & Guillaume 1994; Morais, Conceição, Dinis & Rønnestad 2004), but they generate contaminated waste and require specific, expensive equipment. Labelling prey with ingested microalgae (Eldridge, Whipple, Eng, Bowers & Jarvis 1981; Yúfera, Pascual & Polo 1993) or fluorescent microsphere particles (McCarter & James 1993; Canino & Bailey 1995) is a ‘clean’ alternative that also facilitates counting the number of prey eaten by fish. However, the accuracy of these techniques depends on whether individual prey equally ingests the markers, which is not systematic (e.g. 81–98% marking efficiency with microsphere particles in 1-day old Artemia; Wuenschel & Werner 2004). In addition, their deployment requires that the prey ingest marked items, which, for example, is not the case of newly hatched brine shrimp Artemia nauplii or decapsulated cysts.
Food intake and Rg rate can also be measured directly with unlabelled prey, but it generally requires the sacrifice and preservation of specimens. Micro-dissection is tedious, and counts of ingested prey can be difficult or inaccurate if digestion is in an advanced stage (Pedersen 1984). Alternatively, the gut content can be weighed on a microbalance (Shoji, Maehara, Aoyama, Fujimoto, Iwamoto & Tanaka 2001), but preservation in chemicals generally induces biases in weight (Giguère, St-Pierre, Bernier, Vézina & Rondeau 1989), and necessitates the use of correction factors that often make FI estimates inaccurate. Recently, a natural marker of Artemia cysts, the ascorbic acid 2-sulphate (AAS), which is neither utilized nor biosynthesized by some fish larvae, has been used to measure FI and Rg in fish fed decapsulated Artemia cysts (García-Ortega, Verreth, Vermis, Nelis, Sorgeloos & Verstegen 2010). This method can be accurate, at least for groups of fish, but it requires sophisticated equipment (HPLC), as well as daily calibrations, as the AAS content of Artemia cysts can be highly variable (García-Ortega et al. 2010). Furthermore, any technique requiring the sacrifice of fish implies that Rg cannot be measured on an individual basis. This might be a shortcoming as FI can vary substantially between individual fish, and Rg is strongly influenced by the degree of gut fullness (Laurence 1971; Jobling 1994).
In many fish species, the body wall is translucent until the late larval or early juvenile stage, especially in the abdominal region. Several studies have taken advantage of this feature for determining the diet of fish (Pryor & Epifanio 1993; Kubitza & Lovshin 1999). In contrast, we are not aware of any study where the volume of the gut content was quantified from such observations, while this information can be obtained from the analysis of digital microphotographs.
This study aimed at evaluating the accuracy and consistency of this method for measuring the ontogenetic variations of FI and Rg dynamics in live fish. It was conducted on larvae of the doncella Pseudoplatysoma punctifer (formerly P. fasciatum; Pimelodidae, Siluriformes), a catfish of major importance for the diversification of South American aquaculture (Kossowski 1996; Nuñez 2009). The development of its culture is still hampered by the massive losses to cannibalism during the larval and early juvenile stages (Padilla Pérez, Alcántara Bocanegra & Ismiño Orbe 2001; Gervásio Leonardo, Romagosa, Borella & Batlouni 2004; Nuñez, Dugué, Corcuy Arana, Duponchelle, Renno, Raynaud & Legendre 2008), partly because information on meal size and frequency is inaccurate or incomplete.
Methods
Fish and rearing conditions
The fish used in this study were half siblings sired from broodstock held captive in the Aquaculture Research Station of the Instituto de Investigaciones de la Amazonía Peruana (IIAP) at Quistococha (Iquitos, Loreto Region, Peru). Hormonally induced ovulation, egg fertilization and incubation were performed following Nuñez et al. (2008). Hatching took place 17 h after fertilization. Hatchlings were 3.1 ± 0.1 mm (notochord length, mean ± SD) and possessed a yolk of about 0.2 mm3 (calculated in the very same way as FI, see next section). During the hours following hatching, fish were transferred in 30-L square tanks (40 × 40 × 19 cm; stocking densities of about 20 fish L−1) in an indoor recirculating system under natural photoperiod (12 h of light and 12 h of darkness). Water temperature was maintained at 28.0 ± 0.5°C, and oxygen was near saturation.
Exogenous feeding was first observed 56 h after hatching (hereafter hah), but was not systematic before 72–80 hah, after the fish had fully absorbed their yolk [standard body length (SL) of 4.6 ± 0.2 mm; total body length (TL) of 5.5 ± 0.2 mm; wet body mass (M) of 0.6 mg; mean ± SD]. Fish were fed live Artemia nauplii, which were distributed every 3 h during the hours of light (five meals per day at 7:00, 10:00; 13:00, 16:00 and 19:00 h). No meal was distributed at night, and uneaten food was siphoned in the evening to ensure that fish had empty guts when experiments started the following morning.
Standard measurement protocol
All observations took place in a room with stable air temperature (about 28°C). On each day of measurement, larvae were collected, rapidly sorted by size to produce a homogenous sample of at least 100 fish, and offered live nauplii in large excess, as the FI of fish larvae does not generally become asymptotic unless food is given in excess (Houde & Schekter 1980, 1981). In the rest of the text, the moment of food distribution is referred to as time zero (t0). Larvae were allowed to feed for 20 min, which was enough for replenishing, in view of the similarity between the gut contents of fish allowed to feed for 20, 30 and 40 min (data not shown). Thereafter, they were transferred in water devoid of food, rapidly examined, and those with gut contents much lower than others to the observer's naked eye were removed with a pipette.
The first observation took place about (nearest 5 min) 30 min after t0. Five to 10 fish were randomly sampled, anaesthetized (2-phenoxy-ethanol, 0.35 mL L−1) and examined successively. Each fish was placed in a lateral recumbent position in a Petri dish filled with the anaesthetic solution under the dissection microscope (magnification ×6 to ×25, depending on fish size). Small larvae of doncella have rounded bodies and short fins, and they generally roll on their side when anaesthetized. Larger larvae possess longer fins and a dorso-ventrally compressed head, which complicate their observation in profile view under the dissection microscope. To place the fish in a lateral recumbent position in a non-destructive way, a small piece of rubber was gently placed with a fine pincer on the fish's caudal peduncle. Thereafter, the stomach and gut regions were photographed by reference to a finely graduated (0.1 mm) scale. Fish size (SL) was measured from digital photographs, under the microscope for small fish and in macrophotography for larger fish. With two operators, the operation took about 30 s per fish. Measured fish were not re-used for any subsequent observation, as anaesthesia might have affected Rg. The same operation was repeated every 30 min on other samples of 5–10 fish, until the guts were emptied.
In addition to the series used for measuring Rg, other fish were sampled on a near daily basis, and examined about 30–40 min after the first morning meal (always before the start of defecation), to further document the ontogenetic variation of FI. The study ended when the pigmentation of the abdominal body wall prevented the accurate observation of gut content.
Protocol for measuring Rg in individual fish
The use of anaesthesia in the standard protocol prevented studying the same individual fish at different stages of digestion. To address this issue, a slightly different protocol involving no anaesthesia was applied. Here, 5–10 fish were randomly sampled about 20 min after t0 and housed in isolation in 300 mL containers. At the time of measurement, a fish was gently captured with a pipette, placed under the dissection microscope, then water was pumped with a pipette and gravity forced the fish in a lateral recumbent position. The fish was photographed rapidly, water was poured again and the fish was returned to its enclosure. The time for processing an individual fish was about the same as with the standard protocol (30 s). The same protocol was applied to the other fish, and was repeated on all fish at 30-min intervals. Fish were always processed in the same sequence to make the intervals between successive observations as similar as possible. After the last measurement, all fish were anaesthetized and photographed for subsequent accurate measurements of body length.
This protocol suffered from some technical and biological limitations. Large larvae could not be observed easily in profile view as their long pectoral fins prevented them from rolling on their side following a sudden drop in water level, and they reacted swiftly when forced in a lateral recumbent position by a pressure on their flank or tail. Small larvae could be observed in profile view, but they did not stay still for more than 2 or 3 s, which was generally too brief for operating a camera in autofocus mode. It required a well-trained operator to take rapid and sharp snapshots in a manual focus mode. Finally, it was uncertain whether repeated handling interfered with Rg. The latter issue was tested by a post hoc comparison between the individual and standard protocols. The observations with the two protocols were done in a row, and so there were never more than 5 min between the observation times for the two series, and the intervals between successive measurements were similar.
Calculation of gut content volume
Digital photographs were processed with the freeware Image J (Abramoff & Magalhaes 2004; http://rsbweb.nih.gov/ij/). The stomach and intestine regions were analysed separately, because of their contrasting shapes. The limit between the two regions was conspicuous in fish of all sizes as the pylorus of doncella develops before the start of exogenous feeding (48 hah, authors’ unpublished data). In each region, the gut content was contoured with a hand-drawn closed polygon, the surface of which was calculated by the freeware. Other measurements, specific to each gut region, were also made (see below).
The intestine (or its content) is shaped as a circumvallated cylinder of irregular section. Its volume (Vi) can thus be estimated as Vi = 0.25 π Li Dim2, where Li is the length and Dim is the mean diameter of the intestine over the regions containing food. The value of Li was obtained by tracing an open polygon passing at mid of the gut content. The value of Dim was deduced from the surface of the contour polygon of the intestine content (Si) and Li, i.e. Dim = Si Li−1. Hence, Vi is given by Vi = 0.25 π Si2 Li−1.
The stomach content is generally shaped as an ellipsoid, the volume (Vs) of which can be calculated as Vs = 0.1667 π Lsm Hsm Wsm, where Lsm, Hsm and Wsm are the mean length, height and width of the stomach content respectively. In a profile view, Ws cannot be measured. Photographs from both profile and bottom views were taken in 20 fish of different sizes (6.0–16.5 mm SL) to compare the dimensions of Ws and Hs. For this comparison, the longest extensions of Ws and Hs (hereafter, WsM and HsM) were preferred, as they could be measured more rapidly than the corresponding means. The WsM:HsM ratio in doncella was independent of fish size (simple linear regression analysis, P = 0.7138; d.f. = 19) and did not differ from 1.00 (mean ± SD of 1.02 ± 0.05). This supported the idea that stomach volume could be measured from profile views. It was thus calculated as Vs = 0.1667 π Le He2, where Le and He are the diameters of a planar ellipse with a surface area equal to that of the contour polygon of the stomach content (Ss), and where the He:Le ratio equals the HsM:LsM ratio between the longest vertical and sagital dimensions of the stomach content.
Validation tests
Additional tests were carried out to verify whether the above protocols and modes of calculation were accurate, consistent and repeatable. Accuracy was evaluated by comparing estimates of gut content volume Vg (= Vi + Vs) to true counts of nauplii, following fish sacrifice (2-phenoxy-ethanol, 3.0 mL L−1) and dissection. The mean volume of newly hatched Artemia nauplii was estimated from their mean wet mass, which was 0.015 mg (volume of 0.015 mm3) in this study, consistent with knowledge on Artemia nauplii (Sorgeloos, Lavens, Léger, Tackaert & Versichele 1986).
To test for consistency, the same photographs were analysed twice by the same operator at several weeks of interval. Fish were analysed at different times after feeding to test whether the digestion stage influenced the consistency of measurements. A second way of evaluating consistency consisted in comparing the gut contents of the same individual fish at different times before the start of defecation, as the gut content remains unchanged in these circumstances. To test for repeatability, the same fish were photographed twice under anaesthesia (right and left profile views).
Data analysis
The estimates of gut content volume (Vg) were obtained by adding the estimated volumes of the intestine (Vi) and stomach regions (Vs), and expressed as a proportion (%) of the fish wet body mass, assuming that 1 mm3 = 1 mg. Fish body mass (M) was not measured straight because of variable degrees of stomach fullness in the fish under study. It was back-calculated from the following models between SL (mm) and M (mg) that were determined in fish with empty guts (E. Baras, unpublished data): Fish > 11.5 mm SL: log M = −1.823 + 2.875 log SL (r2 = 0.992, P < 0.0001, d.f. = 211).
Fish < 11.5 mm SL: log M = 1.731 − 10.472 log SL + 14.721 (log SL)2 − 5.010 (log SL)3 (R2 = 0.988, P < 0.0001, d.f. = 91). The model for small larvae is more complex than most relationships between M and SL in fish, but it accurately reflects the different stages of allometric growth that occur in the early larval stages of doncella.
For validation tests, the relative inaccuracy (Ir,%) was calculated from the difference between the estimated volume Vg and a reference volume (VgR): Ir = 100 (Vg−VgR) VgR−1. The relative inaccuracy indicated whether estimates were on average exaggerated or underestimated. The absolute value of the inaccuracy (Ia = | Ir |) was also calculated to measure the overall degree of discrepancy (inaccuracy or inconsistency, depending on tests). The reference (VgR) was either the volume that was back-calculated from counts of nauplii for tests of accuracy, or the first estimate by the observer for tests of consistency and repeatability. Paired t-tests were used to test for accuracy, consistency and repeatability. Unpaired t-tests were used to compare the inaccuracy when estimating Vg for meals at different stages of digestion.
There has been a considerable debate on which model describes best the rate of gut or stomach evacuation in fish (e.g. Jobling 1986; Jobling 1987). All models are based on the same equation, i.e. dV dt−1 = −Rg VB, where V is the volume of the gut or stomach content, Rg is the rate of evacuation and t is the time, but they use different values for the exponent B. Exponential (B = 1; Persson 1981) and square root models (B = 0.5; Jobling 1981) are widely used in adult or juvenile fish, but linear models (B = 0) have been found to correctly describe the patterns of Rg in fish larvae (Canino & Bailey 1995; Wuenschel & Werner 2004). Hence, in this study, these three types of regression models were tested to analyse the relationships between Vg (% M) and the time since t0. Models were constructed from mean Vg values rather than individual values to enable a comparison (analysis of covariance, ancova) of the values of Rg produced by the two experimental protocols. Stepwise multiple-regression analyses were used to test whether the individual values of Rg obtained with the individual protocol were dependent on fish M and FI.
Experiments on Rg in larvae of different ages took place at slightly different temperatures (26.7–28.5°C). A Q10°C of 2.06 (about the mean value of Q10°C in teleosts for the 25–30°C range; Kamler 1992) was applied to correct raw Rg estimates for thermal discrepancies. Corrected values were calculated for a temperature of 28.5°C, for which information was available on the daily FI of cannibalistic doncella of different sizes (data produced by a removal protocol; E. Baras and D.V. Silva del Aguila, unpublished data). This comparison aimed to test whether the data obtained from the analysis of fish fed discontinuously, as was the case here, might serve to estimate daily food rations. Null hypotheses were rejected at P < 0.05.
Results
Methodological tests
For meals recently ingested (0.5 h), the values of Vg estimated by a well-trained observer never departed significantly from the volumes that were back-calculated from counts of Artemia nauplii (Fig. 1). The inaccuracy at the first observation was low (Ia of 2.9 ± 1.5%; mean ± SD), and independent of fish size (simple linear regression: P = 0.6153, d.f. = 24). Vg was not systematically exaggerated or underestimated (Ir from −6.1 to +4.6%). Repeated measurements of Vg on the same photographs were highly consistent (paired t-test, P = 0.7280, d.f. = 14, Ia of 1.3 ± 0.8%). Analyses of photographs of the same fish from right and left profiles also provided consistent estimates (paired t-test, P = 0.1093, d.f. = 14; Ia of 1.8 ± 0.9%). The inaccuracy was slightly, but not significantly higher when the meal was in more advanced digestion stage (Ia of 3.4 ± 1.6 vs. 2.9 ± 1.5%; unpaired t-test, P = 0.4368, d.f. = 28).
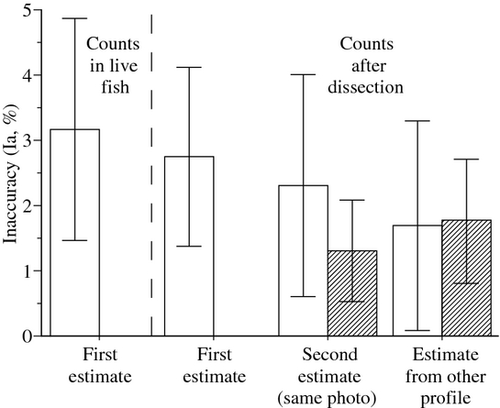
Size limitations
Until a size of 10 mm SL (M of 10 mg), the observation of gut content in doncella was not hampered by pigmentation or fins. Thereafter, the pectoral fins elongated and acquired a dark pigmentation, which rendered anaesthesia indispensable for observing the fish while moving its pectoral fin sideways. In fish larger than 12 mm SL (M of 20 mg), the observation of gut content was further complicated by the development of a dark stripe over the anterior part of the stomach, but this was no longer a problem if the fish was placed in a dorso-lateral recumbent position. This situation persisted until the entire abdominal body wall acquired a yellowish tone, which made the observation of gut content impossible in fish larger than 18–19 mm SL (M of 60–70 mg).
Ontogenetic variation of food intake
Food intake was measured by the contour method in 165 larvae ranging from 4.5 to 18.4 mm SL (0.6–65 mg). At the start of exogenous feeding, larvae of doncella (4.5 mm SL; 0.6 mg) consumed no more than two or three nauplii per meal (4.5–7% M; Fig. 2). Their FI increased rapidly and attained about 14% M in fish of 1 mg. Thereafter, FI continued to increase, but at a slower pace. It was highest (20.5–21.2% M) in fish ranging from 15 to 40 mg (11.2–15.5 mm SL), and then started decreasing slowly in larger fish. The ontogenetic variation of the maximum FI (FIM,% M) was modelled from the highest values of FI in fish of different sizes (Fig. 2), using two separate models for fish smaller and larger than 1 mg:
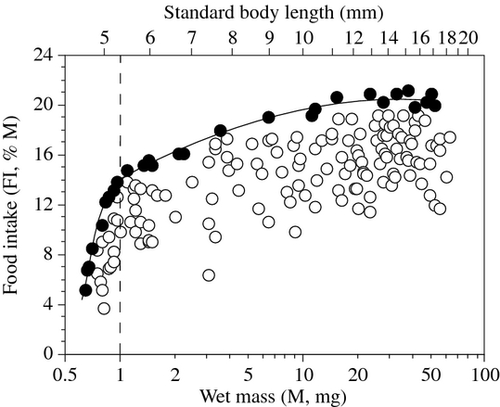
Fish ≤ 1 mg: FIM = 14.25 + 17.87 log M − 137.70 (log M)2 (R2 = 0.987, d.f. = 9, P < 0.0001, P = 0.0156 and 0.0023 for the intercept, first and second order polynomials respectively).
Fish > 1 mg: FIM = 14.14 + 8.07 log M−2.36 (log M)2 (R2 = 0.973, d.f. = 19, P < 0.0001 for all coefficients).
Ontogenetic variation of gut evacuation rate
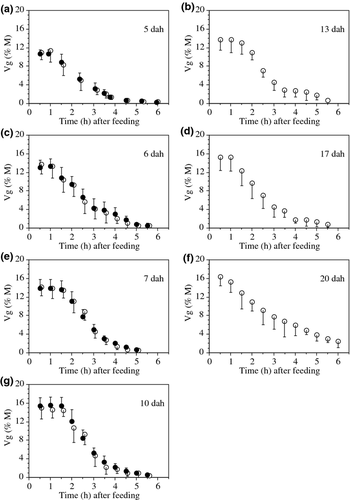
Gut evacuation was studied in larvae aged 5–20 dah (Table 1). The mean FI of these fish were slightly below the FIM values shown in Fig. 2. Anaesthesia was needed for observing fish older than 10 dah and larger than 9–10 mm SL (see Size limitations). In the four series where the individual protocol was used (fish aged 5, 6, 7 and 10 dah), the estimates of Vg at different times before the start of defecation (70–90 min after t0) were highly consistent (Fig. 3), which further supports the idea that Vg was measured accurately.
| Age (dah) | SL (mm) | M (mg) | n | T° (°C) | FI (% M) | Rg (% M h−1) | r2 | d.f. | P | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | 28.5°C | ||||||||||
| 5 | 5.3 ± 0.2 | 0.9 | 5 | Group | 26.9 | 11.4 ± 2.5 | 3.59 | 4.03 | 0.980 | 4 | 0.0012 |
| 5 | 5.3 ± 0.1 | 0.9 | 6 | Ind. | 26.9 | 11.0 ± 0.9 | 3.40 | 3.82 | 0.990 | 4 | 0.0004 |
| 6 | 5.8 ± 0.3 | 1.3 | 5 | Group | 27.3 | 13.7 ± 2.1 | 4.61 | 5.03 | 0.977 | 4 | 0.0015 |
| 6 | 5.8 ± 0.2 | 1.3 | 7 | Ind. | 27.3 | 13.3 ± 1.6 | 4.42 | 4.82 | 0.992 | 4 | 0.0003 |
| 7 | 6.3 ± 0.3 | 1.7 | 5 | Group | 28.5 | 14.2 ± 1.9 | 5.57 | 5.57 | 0.986 | 4 | 0.0006 |
| 7 | 6.1 ± 0.2 | 1.6 | 7 | Ind. | 28.5 | 13.8 ± 2.0 | 5.48 | 5.48 | 0.993 | 4 | 0.0002 |
| 10 | 7.6 ± 0.3 | 3.4 | 5 | Group | 28.4 | 14.8 ± 2.7 | 6.04 | 6.08 | 0.982 | 4 | 0.0010 |
| 10 | 7.6 ± 0.2 | 3.3 | 8 | Ind. | 28.4 | 15.5 ± 1.8 | 6.25 | 6.29 | 0.991 | 4 | 0.0003 |
| 13 | 9.8 ± 0.4 | 10.8 | 5 | Group | 28.4 | 14.1 ± 2.4 | 5.31 | 5.35 | 0.984 | 4 | 0.0008 |
| 17 | 13.6 ± 0.9 | 27.6 | 5 | Group | 27.8 | 15.4 ± 2.7 | 4.44 | 4.67 | 0.980 | 4 | 0.0012 |
| 20 | 17.2 ± 1.0 | 54.1 | 5 | Group | 26.7 | 16.4 ± 1.5 | 3.10 | 3.53 | 0.982 | 4 | 0.0010 |
At all ages, the decline of Vg after the start of defecation was curvilinear, and systematically best described by an exponential model. However, during the first 2 h after the start of defecation, it was equally well or slightly better described by a linear model. This situation was also observed for individual trajectories. The coefficients of determination (r2) of the linear regression analyses for the calculation of Rg were always slightly higher with the individual protocol than with the group protocol (Table 1). Likewise, the SD of the mean Vg at different times were generally lower with the individual than with the group protocol. The differences between the estimates of Rg produced by the two protocols in fish of identical age were always low (≤0.2% M h−1; Table 1) and never differed significantly (ancova, P of 0.6337, 0.6951, 0.8517 and 0.7384 at 5, 6, 7 and 10 dah respectively).
A stepwise multiple-regression analysis of the data obtained with the individual protocol revealed that the Rg rate corrected for 28.5°C (Rg28.5°C,% M h−1) was not only significantly dependent on fish mass (M, mg) but also on FI (% M). The model stood as: Rg28.5°C = −8.22 + 12.11 log FI + 6.30 log M – 12.67 (log M)2 (R2 = 0.904, F = 75.5, d.f. = 27; P of <0.0001, <0.0001, 0.0013 and 0.0003, for the intercept and variables presented in the model).
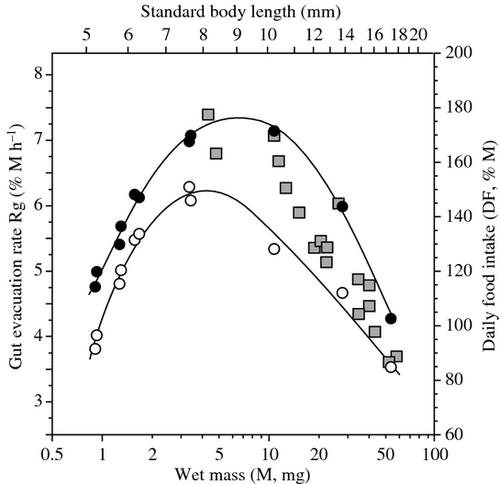
The ontogenetic variation of Rg in doncella was shaped as an asymmetric dome (Fig. 4). The value of Rg increased very rapidly during the early larval stage, attained a maximum at about 9 mm SL (6 mg), and declined in larger fish. This pattern was not modified substantially, and just slightly broadened, after the data were corrected for thermal discrepancies (Rg28.5°C; Fig. 4). It was attempted using the Rg values produced here to estimate the daily FI (DF,% M day−1) of doncella of different sizes, assuming that fish would eat throughout the day and night (i.e. DF = 24 Rg) and compare these estimates with those produced in a parallel study on cannibalism. At all sizes, the DF estimates of fish fed Artemia nauplii were lower than those of cannibals. However, cannibals generally consume very large prey and tend to replenish to a greater extent than fish feeding on small prey. The Rg of doncella larvae depends on FI (see above), and so it is possible that the difference between the two methods originated from different meal sizes. To test for this, DF data for fish feeding on Artemia were transformed, using the multiple-regression model above, as if the fish examined here had fed maximally (using the models of FI against M given in the previous section and shown in Fig. 2). These maximal estimates fell just above those obtained during the study of cannibals (Fig. 4).
Discussion
Methodological aspects
Validation tests indicated that the method could be accurate, consistent and repeatable for measuring the FI and gastric evacuation rate of doncella fed Artemia nauplii. The method is inexpensive, ready-to-use in every laboratory and it leaves no residues. ‘Samples’ are in electronic format, thereby involving no additional cost or problem for storage or transfer. It is a non-destructive method, which enables the repeated examination of the same individual fish (although with some size limitations) and opens interesting perspectives for testing between-individual variability during the ontogeny.
At first sight, the in-depth analysis of photographs might look excessively tedious, but it took no more than 5–10 min, which is not longer than performing micro-dissection and counting. This time might be shorter if the gut content was contoured automatically instead of manually, at least for recently ingested meals. For meals in advanced digestion stage, automatic contouring was evaluated, but judged unsatisfactory in retrospect, as it almost systematically included parts of the gut that had been stained by Artemia pigments, but were obviously empty. This is the main reason why hand-drawn contours were used throughout this study, for the sake of consistency. Measurement is more accurate and can be obtained faster when photographs are sharp and well contrasted, which is the case when using both transmitted and reflected illumination (i.e. light from under and above, respectively). Sharpness is less dependent on the resolution of the digital camera than on the size of its LCD screen.
When performed by an experienced operator, the inaccuracy of the method for measuring FI was always <5% for any individual fish, which is similar to or better than other methods for fish larvae (review in Conceição et al. 2007). It cannot be claimed that the accuracy was equally high for gut contents in an advanced digestion stage, for which there was no absolute reference to compare with in this study. However, the very high r2 values that were obtained when calculating the Rg of individual fish suggest that these estimates were not aberrant. Likewise, the consistency between the gut content estimates of the same individuals at different times before the start of defecation empirically supports the relevance of the models used for back-calculating the volumes of stomach and intestine contents.
The main limitations of the method refer to its incapacity of analysing fish that feed continuously (see below) and to species-specific limitations related to pigmentation. In doncella, the upper size for the measurement of FI was about 18 mm SL (60–70 mg), which nevertheless enabled documenting most of the larval stage in this species. In contrast, for measuring Rg in individual fish, the limit was about 9 mm SL (8–10 mg), which leaves the bulk of the larval stage unexplored. Based on the authors’ experience, the upper size limits for measuring the FI and Rg of individual fish vary substantially between species. In particular, the possibility of analysing non-anaesthetized animals depends not only on morphological factors but also on fish behaviour. The individual protocol was successfully evaluated in larvae of the characid Piaractus brachypomus Cuvier as large as 10 mm SL (about 15 mg), and in those of the cobitid Chromobotia macracanthus Bleeker (up to 12 mm SL and 40 mg; E. Baras and collaborators, unpublished data). In contrast, in the sharptooth catfish Clarias gariepinus Burchell and vundu catfish Heterobranchus longifilis Valenciennes (both from the family Clariidae), the lateral body wall of larvae becomes pigmented at a young age and small size (8–9 mm SL) and the gut content cannot be observed unless the fish is in dorsal recumbent position, which makes anaesthesia indispensable. For species sharing these morphological traits, alternative ways of observation might be worth evaluating, for example, taking photographs from below of live fish held in transparent containers on a glass table.
Ontogenetic variation of food intake
The maximal FI of doncella larvae amounted to 20–21% of their wet body mass. Similar high degrees of gut fullness have been reported in larvae of other species: e.g. 20% in walleye Sander vitreus (L.) (Johnston 1999) and spotted seatrout Cynoscion nebulosus (Cuvier) (Wuenschel & Werner 2004); 25–30% in the striped catfish Pangasianodon hypophthalmus (Sauvage) (Slembrouck, Baras, Subagja, Hung & Legendre 2009). The meal sizes observed here in larvae of 10–15 mm SL are also consistent with the knowledge on cannibalistic doncella, which frequently ingest prey as large as 20–25% M at 8–15 mm SL, but were occasionally found to ingest much larger prey (up to 32% M; E. Baras and D.V. Silva del Aguila, unpublished data). This difference can probably be accounted for by the mechanisms that regulate FI in fish (for a review, see Houlihan, Boujard & Jobling 2001). Appetite in fish is depressed by the degree of gut fullness (Laurence 1971; Bromley 1994; Jobling 1994). Hence, fish that feed on small food items or prey generally halt foraging at a particular degree of stomach fullness or distension, which might be below their maximal capacities of ingesting food. In contrast, for cannibals eating very large prey, the threshold degree of gut fullness that depresses appetite might be passed after a single prey is ingested.
The rapid increase in maximal meal size during the early larval stage of doncella coincides with a period of marked allometric growth. From 4.5 to 12 mm SL, the body depth of doncella passes from 14 to 21% SL (+57%), and body width follows a similar trajectory. During this interval, the length of the body cavity relative to fish size increases by 22%. The volume of the body cavity relative to fish volume is thus about three times larger at 12 mm SL than at 4.5 mm SL. This is about the ratio between the maximal meal sizes of these fish. Similar rapid increases of relative meal size have been reported in growing larvae of several fish species, but they were rarely equated with growth allometries (e.g. vendace Coregonus albula L., Karjalainen & Viljanen 1992; gilthead seabream Sparus aurata (L.) and Senegal sole Solea senegalensis (Kaup), Parra & Yúfera 2001; spotted seatrout, Wuenschel & Werner 2004). In general, beyond a certain fish size, the relative meal size decreases in fish of increasing size, as the developing muscles increasingly restrict the distension of the abdominal region. This was not observed in doncella, but the size range under scrutiny here was probably too narrow to show this.
Ontogenetic variation of gut evacuation and daily food ration
At all ages, Rg in doncella followed a curvilinear trajectory that could be fitted well by an exponential model. This contrasts with the situation observed in many fish species, especially those hatching from small eggs, where there is a two-step dynamics, with a linear evacuation in young larvae, and a different (exponential or square root) trajectory thereafter (Govoni, Boehlert & Watanabe 1986). The transition between the two modes is generally abrupt and often coincides with gut coiling. The eggs and larvae of doncella are quite small on freshwater fish standards, especially by reference to other catfishes of interest to aquaculture. Nevertheless, their gut becomes coiled and their pylorus is well developed at a young age (48 hah), before the start of exogenous feeding. This might account for the consistency of their Rg patterns throughout this study.
The Rg rate increased rapidly in young doncella larvae of increasing size; it peaked at 9 mm SL then and decreased slightly in larger fish. Similar dome-shaped trajectories have been reported in other fish species, and variation in gut residence time was frequently invoked as the main factor behind this pattern (e.g. several marine fish species, Houde & Schekter 1981; vendace and several European cyprinids, Marmulla & Rösch 1990; Troschel & Rösch 1991; spotted seatrout, Wuenschel & Werner 2004; sharptooth catfish, García-Ortega et al. 2010). The rapid increase in Rg rate in young larvae of doncella probably reflected the development of stomach enzymes and muscles, which facilitated the degradation of nauplii and their passage into the intestine. This interpretation was empirically supported by the increasing amplitude of stomach contractions and greater state of digestion of nauplii in the foregut of fish of increasing size. The decrease of the Rg rate in fish larger than 9 mm SL probably corresponds to a combination of slower metabolism and increased gut coiling in fish of increasing size. Similar arguments have been proposed by other authors to account for increased gut residence time in fish of increasing size (Govoni et al. 1986; Yamashita & Bailey 1989; Wuenschel & Werner 2004).
It is generally assumed that the daily FI cannot be inferred from Rg rates measured in fish fed discontinuously, as food passes more rapidly through the gut when fish are fed continuously (Werner & Blaxter 1980; Ruggerone 1989; Bromley 1994; Canino & Bailey 1995; Wuenschel & Werner 2004; García-Ortega et al. 2010). The estimates of daily FI that were obtained here after a straight extrapolation over 24 h of the Rg measured during discontinuous feeding trials were nevertheless close to those obtained during parallel experiments on the daily FI of cannibalistic doncella. There might be several reasons for this. Sensu stricto, cannibals do not feed continuously, especially if they feed on large prey. There is a physical impossibility for a cannibal to ingest another large prey before the previous one has been processed and passes into the intestine. On average, cannibals of doncella (5–10 mg) that (supposedly) fed maximally, consumed a prey every 2–3 h (for prey as large as 15–25% of their body mass; E. Baras and D.V. Silva del Aguila, unpublished data). This was about the same time as the 2-h period used for measuring Rg in this study, and this might account for why the estimates obtained with the two methods did not differ substantially.
Implications for the larviculture of doncella
This study partly accounted for why growth dispersal, which generally triggers or facilitates cannibalism (reviews in Kubitza & Lovshin 1999; Baras & Jobling 2002), is so frequent among doncella larvae. The variations in meal size, Rg rate and daily FI are very high during the early larval stages of doncella. In general, such large differences produce different growth rates, to the benefit of the largest fish. Early size differences are thus prone to amplify genuinely and facilitate cannibalism, even if young doncella are fed adequately. This is more likely to occur if food is quantitatively, spatially or temporally restricted, as food shortage is the first environmental factor behind growth depensation in fish (Kestemont, Jourdan, Houbart, Mélard, Paspatis, Fontaine, Cuvier, Kentouri & Baras 2003). The latter issue can be alleviated by the information on the ontogenetic variation of meal size and Rg rate that is presented here.
Based on Rg values, it is suggested that larvae of doncella raised at 28°C be fed about every 2.5–3.0 h, and more frequently at warmer temperatures. At least, this frequency would maximize FI and growth, possibly to the expense of food conversion, as assimilation is generally proportional to gut residence time, especially in young larvae with incomplete enzyme development (Parra & Yúfera 2001). Longer meal intervals would be worth testing for improving food conversion if growth is not a premium, but their possible benefits should be equated with lower survival, as cannibalism is likely to increase in these circumstances.
Not all fish fed maximally during this study. Appetite is modulated by internal and environmental factors, and so the average FI might be lower or higher in other circumstances. However, for the same type of prey, it is probably not higher than the maximal meal size documented during this study, which might serve as a basis for future feeding charts in doncella larviculture. It is not claimed, however, that this food ration would suffice to maximize the FI and growth of doncella larvae, as the motivation to feed also depends on prey density. Additional experiments are needed to investigate the relationships between fish density, prey density and meal size (see a possible design in Slembrouck et al. 2009), and define the solutions that are best adapted to the particular objectives and constraints of the production (fast growth, low size homogeneity, high survival, low production cost of larva, etc.).
Acknowledgments
The authors are grateful to Jacques Slembrouck and Marc Legendre (IRD, UMR 226) and three anonymous referees for constructive comments on previous versions of this manuscript. This study was supported by an INCAGRO (Innovación y Competitividad para el Agro Peruano; concurso 03-2007-PIEA) funding to a project coordinated by Carmen Garcia-Davila. The authors are indebted to Ir. Salvador Tello, Director of the Aquarec Department of IIAP, who greatly facilitated the scientific collaboration between research institutes, and gave permission for using the biological material in the IIAP Research Station at Quistococha. Special thanks to the technical staff at Quistococha (Edwin Agurto Garcia, Hugo Marichin Ayambo, Cherry Yahuarcani Taminche and Asunción Apuela Guerra). Mrs Dominique Baras-Caseau contributed to improve the English style of the manuscript. Etienne Baras is an honorary research associate of the Belgian FNRS. This is ISEM publication 2011–105.




