Microbial mineralization of organic matter in sinking particles, bottom sediments and seawater in a coastal fish culturing area
Abstract
Microbial mineralization rates in sinking particles, bottom sediments and seawater were determined in a coastal fish (red sea bream Pagrus major) culturing area to clarify the mineralization process of organic matter (OM) in the entire water column. The mineralization rates of 14C-labelled glutamate and glucose per unit volume were highest in the sinking particles and were 131–572 and 7–49 times higher than those of the seawater and bottom sediments respectively. The turnover time of glutamate tended to be shorter than that of glucose at all three sites of the water column. Bacteria appeared to prefer amino acids to monosaccharides because amino acids could be utilized as both energy and nitrogen sources. The sedimentation rate of particulate organic carbon (POC) derived from phytoplankton accounted for 9–61% of the total POC, and it was particularly high in early summer (61% and 50% at fish cage and non-cage stations respectively). The present study clearly shows that sinking particles serve as an important site of microbial mineralization process of OM within the water column, and that phytoplankton production was another serious cause of organic pollution of the seafloor in addition to the organic wastes directly discharged from fish farms.
Introduction
In a coastal fish culturing area, the deposition of organic wastes on the seafloor and subsequent oxygen consumption through the mineralization process sometimes result in formation of anoxic water mass, decline in benthos density and occasional mass mortality of cultured fish (Brown, Gowen & McLusky 1987; Gowen & Bradbury 1987; Wu 1995). The organic wastes derived from aquacultural activities are transported downward to the seafloor as sinking particles. The bacterial community in the sinking particles has considerably higher density, growth rate and metabolic activity than that in the surrounding water (Harvey & Young 1980; Kirchman & Mitchell 1982; Alldredge & Silver 1988; Smith, Simon, Alldredge & Azam 1992; Unanue, Azua & Arrieta 1998; Ploug, Grossart, Azam & Jørgensen 1999; Grossart, Hietanen & Ploug 2003; Grossart, Tang, Kiørboe & Ploug 2007; Kellogg & Deming 2009). Recently, some studies have proposed a possibility that the sinking particles and their solute plumes serve as hotspots of bacterial growth and play an important role in the oceanic carbon cycle (Kiørboe & Jackson 2001; Azam & Long 2001; reviewed by Simon, Grossart, Schweitzer, & Ploug 2002). If a significant part of organic wastes, in the form of sinking particles is mineralized to inorganic carbon within the euphotic zone, where dissolved oxygen (DO) is supplied by phytoplankton photosynthesis and dissolution from the air at the sea surface, the anoxic condition in the bottom water may be partially reduced. Thus, to develop an effective countermeasure for the formation of anoxic water mass in a fish culturing area, it is necessary to gain knowledge on microbial mineralization process of organic matter (OM) at various sites of the water column, namely, seawater, sinking particles and bottom sediments. Many studies have investigated the degradation and oxygen consumption processes of OM deposited on the seafloor in fish cage farms (Kaspar, Hall & Holland 1988; Hall, Anderson, Holby, Kollberg & Samuelsson 1990; Holmer & Kristensen 1992). Also, several studies have measured bacterial mineralization rates in fish pond waters and suspended particles simultaneously (Kirchman & Mitchell 1982; Nedoma, Vrba, Hezlar, Simek & Strakrabová 1994). However, thus far, there has been no study on the examination of bacterial mineralization rates simultaneously in seawater, sinking particles and bottom sediments in aquatic environments.
Using 14C-labelled amino acids and monosaccharides, bacterial mineralization rates can be measured by short time incubation of small volume of samples (Hobbie & Crawford 1969; McKinley, Federle & Vestal 1983); this enables comparison among the various sites of the water column. Amino acids and monosaccharides are the most important components of the labile dissolved organic matter (DOM) and supply a significant portion of the total bacterial requirements for C and N at various sites of the water column (Billen & Fontigny 1987; Fuhrman 1987; Lee & Wakeham 1988; Cowie & Hedges 1992). Glutamic acid is one of the most important amino acids in the marine environment (Cowie & Hedges 1992). Glucose has also been reported to be the most abundant and important neutral monosaccharide in the marine environment (Münster & Chróst 1990; Rich, Ducklow & Kirchman 1996; Skoog, Biddanda & Benner 1999). Thus, we selected two representative substances, namely, glutamate and glucose, for measuring mineralization rates.
Phytoplankton abundance often increases as a result of nitrogen and phosphorus inputs from fish farms (Eloranta & Palomaeki 1986; Gowen & Bradbury 1987). Hence, there is a possibility that phytoplankton production is another important source of OM that accumulates on the seafloor in addition to organic wastes directly released from fish farms. The decomposition and mineralization processes of phytoplankton detritus are probably somewhat different from those of OM derived from aquacultural activities. However, there has been no study that measures the sedimentation rates of them separately in a coastal fish culturing area. We estimated particulate organic carbon (POC) derived from phytoplankton by measuring its specific constituents, namely, chlorophyll a (Chl a) and phaeopigments (Phaeo a) (Strickland 1960).
The primary objective of the present study was to evaluate the relative importance of the sinking particles, bottom sediments and seawater as sites of mineralization of OM in a coastal fish cage farm, by measuring the mineralization rate of glutamate and glucose, bacterial density and POC content at each site of the water column. The secondary aim was to investigate the sedimentation rates of OM derived from phytoplankton and other sources separately and to compare their relative importance as sources of OM deposits on the seafloor.
Materials and methods
Study site and sampling
All observations and measurements were conducted during early summer (June 15–16), midsummer (August 17–18), fall (October 19–20), and winter (December 7–8) in 2004, in the innermost part of Tanabe Bay, Kogaura Inlet (33° 41′ N, 135° 21′E, Fig. 1). Tanabe Bay is a small embayment – 4 km long and 4 km wide – located in the west coast of the Kii Peninsula, Wakayama, Japan and it opens to the northwest Pacific Ocean. Intensive net pen cage aquacultures of the yellowtail Seriola quinqueradiata, the red sea bream Pagrus major and other species are conducted in this bay. Tanabe Bay is a moderately eutrophicated bay and receives organic matter inputs from aquaculture and terrestrial nutrients; this often results in algal bloom and anoxic water mass formation at the bottom during summer (Takeuchi 1994).
Two stations were established during each observation period; the first station was a cage station located near a fish cage (F; closed star, 16 m in depth), and the second station was an adjacent, non-cage station (N1, N2 and N3; open stars) located at tens (N1) and hundreds of metres (N2 and N3) from the fish cages. The location of the non-cage station varied with the sampling period (N1, June 15, 13 m in depth; N2, August 17 & October 19, 14 m in depth; N3, December 7, 16 m in depth) because the fish cages were shifted to other places when there was an occurrence of fish diseases. Sinking particle samples were collected for 24 h using cylindrical sediment traps of 67-mm diameter and 600-mm height that were positioned at a depth of 2 m above the seafloor (bottom–2) at each station. No preservatives were used in the traps. Immediately after collection, the particles present in each trap were added gently into a plastic bottle along with 0.5 L of seawater from the trap. The bottom water samples were obtained at (bottom–2) m depth using a Van Dorn water sampler. Bottom sediment samples were collected using a K-K type core sampler (original model; Kimata, Kawai & Ishida 1960), and the surface layers (0–1 cm) of these sediments samples were used for each analysis. All samples for mineralization measurements were stored at 4 °C until analysis. The measurements were started within 6 h after sampling. Samples for bacterial cell counts were immediately preserved by the addition of neutralized formaldehyde solution to a final concentration of 2% and stored at 4 °C until microscopic analysis. Samples for carbon and nitrogen analysis were immediately frozen and stored at –20 °C.
The measured values of mineralization rates, bacterial density and carbon and nitrogen contents at all three sites of the water column were quantified as rate or amount per unit volume. The volume of the collected sinking particles was quantified using a measuring cylinder after resedimentation in the laboratory on land. Subsamples of the bottom sediments for each analysis were obtained by measuring their wet weight. The wet weight was then converted into volume using the density that was preliminarily determined at each sampling time.
Temperature and salinity profiles were obtained from CTD casts (Alec Electronics, Nishinomiya, Japan). Dissolved Oxygen of the bottom water samples was measured using an electrode DO metre (OM-51, Horiba, Kyoto, Japan).
Carbon and nitrogen analysis
Particulate organic carbon and particulate organic nitrogen (PON) contents of the sinking particles, bottom sediments and seawater were determined using a CHN analyser (MT-6, Yanako, Tokyo, Japan). The sinking particles collected using the sediment traps and the suspended particles in the seawater samples were filtered through 25-mm Whatman GF/F filters precombusted at 450 °C for 1 h and stored below –20 °C until analysis. The filters were dried at 110 °C for 24 h and fumed overnight with conc. HCl to remove the inorganic carbon. Bottom sediment samples were dried at 110 °C for 24 h; the inorganic carbon was removed by the addition of 1 N HCl, and the samples were dried again. Particulate organic carbon and PON contents of these samples were then determined using the CHN analyser.
Particulate organic carbon,POC, derived from phytoplankton was estimated in the sinking particles from pigment analysis assuming a carbon:(Chl a + Phaeo a) ratio of 30 (Strickland 1960). The Chl a and Phaeo a were determined fluorometrically (TD-700, Turner Designs, Sunnyvale, CA, USA) for particles collected on 25-mm Whatman GF/F filters and extracted by N,N-dimethylformamide (Suzuki & Ishimaru 1990).
Seawater sample for dissolved organic carbon (DOC) analysis was passed through a precombusted 25-mm Whatman GF/F filter. The DOC of the filtrate was analysed using a TOC analyser (TOC-500, Shimadzu, Kyoto, Japan) after HCl acidification and 10-min bubbling with 0.22 μm-filtered N2 gas.
Bacterial density
Bacterial cells in the seawater samples were stained with 4′,6-diamidino-2-phenylindole (DAPI); final concentration, 0.5 μg mL−1, collected on a black polycarbonate membrane filter (0.2-μm pore size), and counted under UV radiation using an epifluorescence microscope (Porter & Feig 1980).
Bacterial abundance attached to the sinking particles and bottom sediments was determined by the dual staining method with DAPI and acridine orange (AO) (Kuwae & Hosokawa 1999). Dual staining reduces serious background fluorescence from abiotic particles that mask blue fluorescence from DAPI-stained bacteria under UV radiation. Ten millilitres of 3% NaCl aq. (0.1% Triton X) were added to 0.1 mL of sinking particles or 0.1 g (wet weight) of the surface layer of the bottom sediments. The attached bacteria were removed from the particles and dispersed into the water phase by ultrasonication treatment (18 W, UD-201, Tomy Seiko, Tokyo, Japan) for 7 min. A larger size fraction of abiotic particles was eliminated by centrifugation at 1000 g for 1 min. The supernatant fluid was diluted 50–250 times and dual stained with a combination of DAPI (final concentration, 5 μg mL−1) and AO (final concentration, 1 mg mL−1) for more than 30 min. Preliminary time course experiments showed that the above time conditions of ultrasonication and chemical staining give reasonable results for our samples. Each treated sample (0.5–2 mL) was filtered through black polycarbonate membrane filters (0.2-μm pore size), and the bacterial cells were counted under UV radiation using an epifluorescence microscope.
Mineralization rates of glutamate and glucose
Mineralization rates of 14C-labelled glutamate and glucose were measured from the radioactivity of carbon dioxide produced during microbial mineralization (double-vial radiorespirometry, McKinley et al. 1983). Prior to analysis, the samples of the sinking particles and bottom sediments were suspended in sterilized seawater with dilution factors of 123–286 and 25 respectively. Three subsamples (0.9 mL each) and one KCN-treated sample were poured into precombusted small glass vials (outer diameter, 15 mm; height, 45 mm). The outer wall of the small glass vial was covered with an aluminium foil. To these samples, 0.1 mL of D-[U-14C]glucose (317 mCi mmol−1, Amersham) or L-[U-14C]glutamate (256 mCi mmol−1, Amersham) was added at a final concentration of 3.9 μmol 14C mL−1 (0.2 μCi mL−1). Each small glass vial without a cap was placed in a standard glass scintillation vial (outer diameter, 27.5 mm; height, 57 mm). Prior to this, the scintillation vial was internally lined with a strip of an Advantec No. 2 filter paper (42 × 80 mm) that had been soaked in a scintillation mixture (Schintisol 500 (DOJIN, Kumamoto, Japan), 100 mL; 6 N NaOH, 2 mL; and methanol, 8 mL) and well dried under vacuum. The scintillation vial was then immediately sealed with a cap and incubated in a scintillation counter (LSC-3500, Aloka, Tokyo, Japan) at room temperature. The radioactivity of [14C]-CO2 that was produced by the bacterial respiration of the 14C-labelled organic substances and trapped by the filter paper was continuously counted at approximately 1-h intervals for 24 h. The mineralization rate was obtained as a slope of linear part of the temporal change in the radioactivity (usually 0–12 h) in terms of dpm per small vial per hour and converted to nmol [14C]-CO2 per unit volume [cm3] per day. Turnover times [h] of glutamate and glucose were calculated by dividing the amount of dpm added and dpm respired by bacteria per hour (Williams 1970; Kirchman 1983), assuming that the concentration of added 14C labelled substrates (3.9 mM) was high enough to saturate the uptake systems of bacteria. Thus, turnover times can be calculated without measurement of in situ concentrations of substrates.
Statistical analysis
The differences between the averaged values of mineralization rates, turnover times and the other measured parameters for existence vs. non-existence of fish cage, each site of the water column (sinking particles vs. bottom sediments, bottom sediments vs. seawater, seawater vs. sinking particles), each season (e.g. early summer vs. summer) and glutamate vs. glucose were tested using the one-sample, paired t-test.
Results
Hydrographical conditions of the bottom layer
There was no significant difference in temperature, DO and DOC of the bottom water between the fish cage and non-cage stations (Table 1). The highest and lowest water temperatures at the fish cage station were 26.7 °C in midsummer and 18.5 °C in winter respectively. Dissolved Oxygen was lowest (5.30 mg O2 L−1) in midsummer; however, severe anoxic condition did not occur throughout the observation period. The DOC in the bottom water was highest in winter (7.24 mg C L−1); it decreased in early summer (3.24 mg C L−1) and became relatively low in midsummer and fall (1.17 mg C L−1 each).
| Temperature (°C) | DO (mgO2L−1) | DOC (rngCL−1) | ||
|---|---|---|---|---|
| 15 June | Fish cage | 23.2 | 6.68 | 3.24 |
| Non-cage 1 | 23.4 | 6.76 | 2.19 | |
| 17 August | Fish cage | 26.7 | 5.30 | 1.17 |
| Non-cage 2 | 26.7 | 5.93 | 1.10 | |
| 19 October | Fish cage | 23.5 | 5.79 | 1.17 |
| Non-cage 2 | 23.7 | 5.69 | 0.82 | |
| 7 December | Fish cage | 18.5 | 7.65 | 7.24 |
| Non-cage 3 | 18.5 | 7.24 | 7.42 |
Particulate organic carbon and particulate organic nitrogen contents
The POC content was highest in the bottom sediments (5.6–20.9 mg C cm−3, Table 2), followed by that in the sinking particles (5.1–13.7 mg C cm−3). The POC content of the suspended particles per seawater volume was very low (0.00027–0.00042 mg C cm−3), i.e., 4- to 5-fold lower than those in the bottom sediments and sinking particles.
| POC (mg C cm−3) | C/N ratio (w/w) | ||||||
|---|---|---|---|---|---|---|---|
| Sinking particles | Bottom sediments | Seawater | Sinking particles | Bottom sediments | Seawater | ||
| 15 June | Fish cage | 5.7 | 10.5 | 0.00034 | 9.9 | 8.3 | 6.9 |
| Non-cage 1 | 5.1 | 18.7 | 0.00034 | 9.8 | 6.7 | 13.3 | |
| 17 August | Fish cage | 9.3 | 19.3 | 0.00027 | 10.6 | 7.3 | 15.0 |
| Non-cage 2 | 6.0 | 5.6 | 0.00032 | 16.2 | 7.2 | 30.4 | |
| 19 October | Fish cage | 10.4 | 16.7 | 0.00030 | 11.9 | 7.3 | 16.6 |
| Non-cage 2 | 6.9 | 7.9 | 0.00034 | 11.8 | 7.9 | 13.9 | |
| 7 December | Fish cage | 13.6 | 18.4 | 0.00041 | 14.0 | 8.2 | 15.8 |
| Non-cage 3 | 13.7 | 20.9 | 0.00042 | 12.3 | 7.0 | 17.6 | |
| All (Mean ± SD) | 8.8 ± 3.5 | 14.8 ± 5.9 | 0.00034 ± 0.00005 | 12.0 ± 2.2 | 7.5 ± 0.6 | 16.2 ± 6.6 | |
In midsummer and fall, the POC contents of the sinking particles were higher at the fish cage station (9.3 and 10.4 mg C cm−3 respectively) than those at the non-cage station (6.0 and 6.9 mg C cm−3 respectively). In the same periods, the POC content of the bottom sediments was also higher at the fish cage station (37 and 32 mg C cm−3 respectively) than at the non-cage station (11 and 15 mg C cm−3 respectively). No significant difference was observed in the POC content of the seawater between fish cage and non-cage stations during the observation period. The POC contents of the sinking particles were highest in winter at both fish cage (13.6 mg C cm−3) and non-cage stations (13.7 mg C cm−3). In the bottom sediments and seawater, the seasonal variations in the POC content were inconsistent between the two stations.
The C/N ratio (weight) was highest in the seawater (16.2 ± 6.6, mean ± SD), followed by those in the sinking particles and bottom sediments (12.0 ± 2.2 and 7.5 ± 0.6 respectively. The C/N ratio at each site of the water column did not show any significant differences between the two stations and among the different seasons.
Sedimentation rate of organic matter
Table 3 shows the sedimentation rate of the sinking particles in terms of dry weight, POC and phytoplankton pigments. In midsummer and fall, the sedimentation rate in terms of POC at the fish cage station was 1.5 and 2.2 times, respectively, higher than those at the non-cage station. In fall, no significant difference was observed in the sedimentation rate in terms of phytoplankton pigments between the two stations. Thus, the difference in the sedimentation rate in terms of POC was attributed to other sources of such aquacultural activities. In early summer, the sedimentation rate in terms of phytoplankton pigments was highest at both fish cage and non-cage stations (34.0 and 25.4 mg Chl a + Phaeo a m−2 d−1 respectively), and the POC derived from phytoplankton accounted for approximately half of the total POC (61% and 50% respectively).
| Sedimentation rate per m2 per day | Carbon content (% dry) | Phytoplankton C (% total POC) | ||||
|---|---|---|---|---|---|---|
| (g dry) | (g POC) | (mg Chi a +Phaeo) | ||||
| 15 June | Fish cage | 14.1 | 1.66 | 34.0 | 11.8 | 61 |
| Non-cage 1 | 13.5 | 1.52 | 25.4 | 11.3 | 50 | |
| 17 August | Fish cage | 6.8 | 0.94 | 8.9 | 13.9 | 29 |
| Non-cage 2 | 7.0 | 0.61 | 4.8 | 8.7 | 23 | |
| 19 October | Fish cage | 11.4 | 2.56 | 8.0 | 22.5 | 9 |
| Non-cage 2 | 9.8 | 1.19 | 9.9 | 12.2 | 25 | |
| 7 December | Fish cage | 6.1 | 0.57 | 2.4 | 9.3 | 12 |
| Non-cage 3 | 13.2 | 0.75 | 4.8 | 5.7 | 19 | |
| All (Mean ± SD) | 10.2 ± 3.3 | 1.23 ± 0.67 | 12.3 ± 11.3 | 11.9 ± 4.9 | 29 ± 18 | |
Bacterial density
The bacterial density was highest in the sinking particles (14.0–324 × 107 cells cm−3, Fig. 2), followed by that in the bottom sediments (1.2–29.8 × 107 cells cm−3), and was lowest in the seawater (0.03–0.24 × 107 cells cm−3). The bacterial density in the sinking particles was 145–2650 times higher than that in the surrounding seawater and 1.4–175 times higher than that in the bottom sediments. There was no significant difference in the bacterial density between the fish cage and non-cage stations. The only exception was that in midsummer, the bacterial density in the bottom sediments at the fish cage station (29.8 × 107 cells cm−3) was more than 10 times higher than that at the non-cage station (2.5 × 107 cells cm−3).
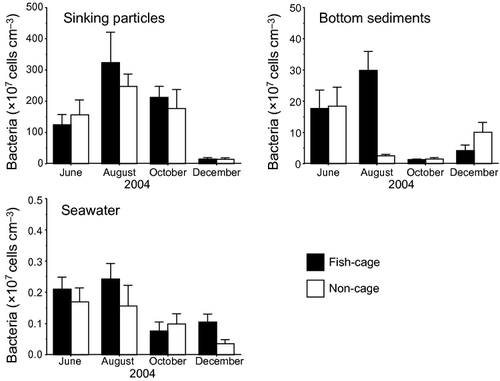
The bacterial density in the sinking particles was lowest (14.5 and 14.0 × 107 cells cm−3) in winter, i.e., one order of magnitude lower than those of the other seasons (177–324 × 107 cells cm−3). Among all seasons, the bacterial density in the bottom sediments was lowest in fall (1.2 and 1.5 × 107 cells cm−3 in fall vs. 2.5–29.8 × 107 cells cm−3 in other seasons). The bacterial density in the seawater was relatively low in fall and winter (0.03–0.10 × 107 cells cm−3) when compared with those in early summer and midsummer (0.16–0.24 × 107 cells cm−3); however, the seasonal difference was not statistically significant.
Mineralization rates of glutamate and glucose
The mineralization rates of 14C-labelled glutamate and glucose per unit volume were highest in the sinking particles (86–343 nmol [14C]-CO2 cm−3 d−1, Table 4), followed by those in the bottom sediments (4.4–28.8 nmol [14C]-CO2 cm−3 d−1), and were lowest in the seawater (0.31–0.86 nmol [14C]-CO2 cm−3 d−1). The mineralization rates in the sinking particles were 131–572 and 7–49 times higher than those in the surrounding seawater and bottom sediments respectively.
| Mineralization rate (nmol [14C]-C02 cm−3 d−1) | Cell-specific mineralization rate (x1O−7 nmol [14C]-CO2 cell−1 d−1) | Turnover time (h) | |
|---|---|---|---|
| Sinking particles (n = 16) | 184 ± 90*,‡ (86–343) | 4.5 ± 7.0 (0.27–23.7) | 0.61 ± 0.27*,‡ (0.26–1.10) |
| Bottom sediments (n = 16) | 11.5 ± 6.9*,† (4.4–28.8) | 2.9 ± 2.6† (0.24–8.4) | 10.2 ± 4.7*,† (3.1–20.6) |
| Seawater (n = 16) | 0.59 ± 0.15†,‡ (0.31–0.86) | 5.7 ± 3.8† (2.7–16.0) | 165 ± 48†,‡ (106–293) |
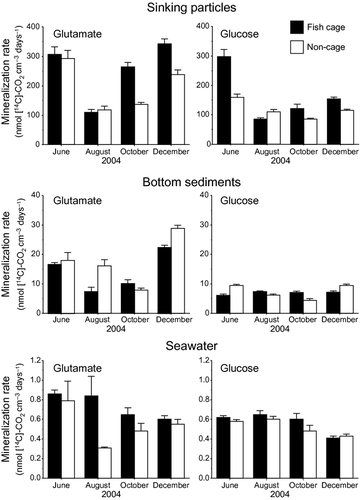
The mineralization rates per unit volume fluctuated seasonally by factors of 2-3 at each site of the water column (Fig. 3), but they did not show any particular seasonal pattern. In the sinking particles, the average mineralization rate at the fish cage station was significantly higher than that at the non-cage station (Table 5). In fall and winter, the mineralization rates in the sinking particles at the fish cage station were 1.3–1.9 times higher than those at the non-cage station (Fig. 3). In the bottom sediments and seawater, no statistically significant differences were observed in the average mineralization rates between the two stations (Table 5).
| Mineralization rate (nmol [14C]-C02 cm−3d−1) | Cell-specific mineralization rate ( × 10−7 nmol [14C]-C02 cell−1 d−1) | Turnover time (h) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sinking particles | Bottom sediments | Seawater | Sinking particles | Bottom sediments | Seawater | Sinking particles | Bottom sediments | Seawater | |
| Fish cage vs. non-cage | |||||||||
| Fish cage (n = 8) | 211* ± 103 | 10.5 ± 5.9 | 0.65 ± 0.14 | 5.2 ± 8.2 | 2.9 ± 3.2 | 4.9 ± 2.3 | 0.55 ± 0.30 | 10.5 ± 3.9 | 146 ± 36 |
| Non-cage (n = 8) | 157* ± 72 | 12.6 ± 8.0 | 0.53 ± 0.14 | 3.8 ± 5.9 | 2.8 ± 2.1 | 6.6 ± 4.9 | 0.67 ± 0.25 | 10.0 ± 5.7 | 184 ± 53 |
| Glutamate vs. glucose | |||||||||
| Glutamate (n = 8) | 226* ± 92 | 15.9* ± 7.4 | 0.64 ± 0.19 | 6.0 ± 9.1 | 3.8*±3.0 | 6.0 ± 4.5 | 0.48*±0.23 | 7.0*±3.5 | 158 ± 62 |
| Glucose (n = 8) | 141* ± 69 | 7.2* ± 1.7 | 0.55 ± 0.09 | 3.0 ± 4.1 | 1.9*±1.9 | 5.4 ± 3.3 | 0.74* ± 0.25 | 13.4* ± 3.5 | 171 ± 32 |
The mineralization rate of 14C-labelled glutamate tended to be at a similar or higher level than that of 14C-labelled glucose at all three sites of the water column (Fig. 3). The differences between the two substrates were particularly greater in winter at all three sites of the water column. The average mineralization rates of 14C-labelled glutamate in the sinking particles and bottom sediments were 2.2 and 1.6 times, respectively, higher than those of 14C-labelled glucose (Table 5). In the seawater, the difference in the mineralization rate between the two substrates was insignificant.
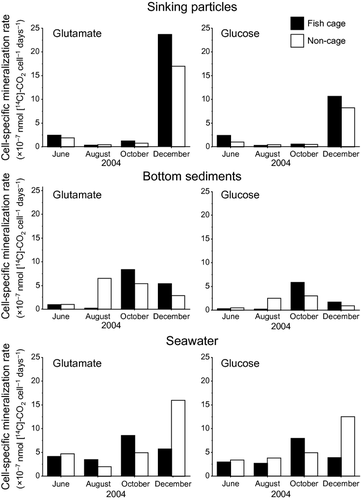
Among the three sites of the water column, the differences in the cell-specific mineralization rates were small as compared to those in the mineralization rates per unit volume (Table 4). The bacterial cell-specific mineralization rates of 14C-labelled glutamate and glucose in the sinking particles did not show any significant difference as compared to those in the bottom sediments and seawater. The average cell-specific mineralization rates in the seawater (5.7 ± 3.8 × 10–7 nmol [14C]-CO2 cell−1 d−1) were twice as those in the bottom sediments (2.9 ± 2.6 × 10–7 nmol [14C]-CO2 cell−1 d−1).
In the sinking particles, the cell-specific mineralization rates showed marked seasonal variation – highest in winter (8.2–23.7 × 10–7 nmol [14C]-CO2 cell−1 d−1) and lowest in midsummer (0.27–0.47 × 10–7 nmol [14C]-CO2 cell−1 d−1 (Fig. 4). In the bottom sediments, the cell-specific mineralization rates were relatively low in early summer (0.35–0.98 × 10–7 nmol [14C]-CO2 cell−1 d−1) as compared to those in other seasons (0.94–8.4 × 10–7 nmol [14C]-CO2 cell−1 d−1). The cell-specific mineralization rate was relatively stable in the seawater.
Turnover of glutamate and glucose
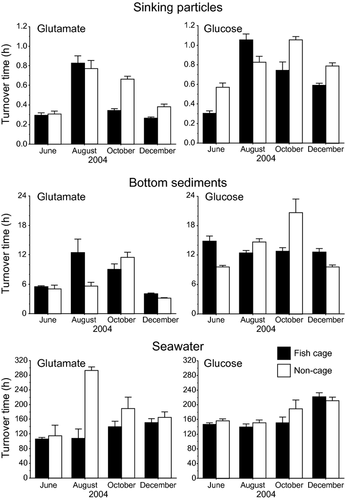
The turnover times of glutamate and glucose were shortest in the sinking particles (0.26–1.1 h), followed by that in the bottom sediments (3.1–26 h), and were longest in the seawater (106–293 h) (Table 4 & Fig. 5). In the sinking particles and bottom sediments, the turnover time of glutamate tended to be shorter than that of glucose (Table 5). In the seawater, no significant difference was observed in the turnover time between glutamate and glucose.
Discussion
Methodological considerations
Sediment traps were deployed for 24 h, which leads to an average residence time of the sinking particles of 12 h. The deployment time of the sediment traps of 24 h was determined to eliminate the effect of diurnal variations in the amount and composition of the sinking particles and to collect enough amount of samples for later analyses. During this time anoxic condition may have occurred inside the traps and a significant part of the labile organic matter, such as amino acids, may have been already utilized by the microbes. However, effects of consumptions of oxygen and the labile organic matter appeared to be small, if any, because the mineralization rates in the sinking particles were very high in the present study and the activities of extracellular enzymes such as peptidase and glucosidase were very high according to the data obtained from the present study area in the following year (Yoshikawa et al., unpublished data).
Shaking of the sinking particles and bottom sediments during the sample preparation may have disturbed DO gradients within and around the particles, which would result in overestimation of the mineralization rates. On the other hand, sedimentation of the sinking particles during measurements may have prevented the water flow around the particles and supply of fresh DO, which would result in underestimation of the mineralization rate (Ploug & Grossart 1999). Despite the above possibility of over- and/or underestimation in mineralization rates, which would rather reduce the difference in mineralization rates of the sinking particles and bottom sediments, the mineralization rate per volume in the sinking particles was one order magnitude higher than that in the bottom sediments. As discussed above, in the present study we measured the potential rates of bacterial mineralization rather than the actual ones.
Calculation of turnover times was based on the assumption that the concentration of added 14C labelled substrates (3.9 mM) was high enough to saturate the uptake systems of bacteria. Dissolved Free Amino Acid (DFAA) concentrations within aggregates were 0.5–27 μM in the Northern Adriatic Sea (Herndl 1992, Kaltenböck & Herndl 1992), and DFAA concentrations are generally one order of magnitude higher than glutamic acid concentrations and its transport constants. Thus, the above assumption appeared to be reasonable. Another reason for adding very high concentration of 14C labelled substrates was to wipeout the isotope dilution effect, resulting from recycling of unlabelled substrates. Actually the production rate of [14C]-CO2 was constant during the 24 h incubation.
Turnover time of glutamate and glucose at various sites of the water column
In the sinking particles, glutamate and glucose were very rapidly (<1 h) taken up and respired by the attached bacteria. Even in a coastal fish culturing area, the sinking particles served as a highly active site of mineralization of OM within the water column. Among the aquatic environments, the turnover times of labile DOM widely range from several to tens of hours in suspended particles in highly eutrophicated pond waters (Kirchman & Mitchell 1982; Nedoma et al. 1994), several to hundreds of hours in estuarine and coastal waters (Coffin 1989; Eguchi & Ishida 1990) and tens to thousands of hours in the open ocean waters (Ishida, Eguchi & Kadota 1986; Eguchi & Ishida 1990; Rich et al. 1996; Skoog, Whitehead, Sperling & Junge 2002). In the present study, the turnover times of glutamate (0.26–0.83 h) and glucose (0.30–1.10 h) in the sinking particles corresponded to those reported for glucose and N-acetylglucosamine in the freshwater in fish ponds (<0.1–0.77 h and <0.1–49 h, respectively, Nedoma et al. 1994), and were rather shorter than those reported in the suspended particles in coastal ponds and marshes (1–4 h and <1–77 h, Kirchman & Mitchell 1982). The turnover times of glutamate and glucose in the bottom sediments (3.1–12.4 h and 9.6–20.6 h, respectively) corresponded to those reported for glutamate and alanine in the coastal bottom sediments (13–40 h and 2–11 h, respectively, Henrichs & Doyle 1986). The turnover times of glutamate (106–293 h) and glucose (140–222 h) in the seawater were within the ranges reported for DFAAs and monosaccharides in estuarine and coastal waters (Coffin 1989; Eguchi & Ishida 1990). These variations in turnover times among the three sites – seawater, sinking particles and bottom sediments – appeared to be dependent on bacterial density and the DOM and nutrient concentrations, as suggested in previous studies (Kirchman & Mitchell 1982; Skoog et al. 2002). Even in the coastal areas, since fish cages are usually positioned in relatively enclosed bays, the turnover of organic substrates attained the same level as that of freshwater fish ponds.
To obtain in situ mineralization rates of glutamate and glucose per unit volume, it is necessary to measure the concentrations of glutamate and glucose in addition to their turnover rates estimated from 14C-substrates. Although the concentrations of glutamate and glucose were not measured in the present study, their approximate concentration ranges could be roughly estimated from POC and DOC measurements and from previously reported POC:DOC ratio. Alldredge (2000) reported that DOC in the pore water of marine snow was approximately 20% of TOC, i.e., DOC corresponded to 25% of POC. By applying this ratio to the present results, the average DOC in the pore water of the sinking particles was estimated to be approximately 2200 mg C L−1, which was 733 times higher than that in the seawater. In the open ocean waters, the labile fraction is only <1% of the total DOM in the seawater, and a large part of DOM consists of refractory and semi-labile fractions (Lee & Wakeham 1988). It has been reported that the ectoenzymatic activity and the percentage of labile fraction in the sinking particles are considerably higher than those in the seawater (Alldredge & Silver 1988; Smith et al. 1992); hence, in the present study, the difference in the labile DOM between the sinking particles and the seawater becomes larger than that in the total DOM. Consequently, the actual mineralization rates of glutamate and glucose in the sinking particles were estimated to be ten to thousand times higher than those in the seawater.
In the present study, the turnover rate of glutamate tended to be slightly higher than that of glucose; however, their values were at a similar level in all three sites of the water column. In contrast, the turnover rates of free amino acids are generally several to hundred times higher than those of monosaccharides in the oligotrophic open ocean waters, where nitrate and ammonium concentrations are very low and bacteria prefer amino acids to monosaccharides (Billen & Fontigny 1987; Eguchi & Ishida 1990; Skoog et al. 2002). Since the present study covered an area containing eutrophicated coastal water, it is possible that both free-living and attached bacteria utilized abundant dissolved inorganic nitrogen (3.6 μM on average, Yoshikawa & Eguchi, unpublished) as nitrogen sources instead of amino acids.
Mineralization and sedimentation of OM in the fish culturing area
In early summer, the sedimentation rate of POC derived from phytoplankton accounted for approximately half of the total POC at both fish cage and non-cage stations. Phytoplankton production in the semi-enclosed bay appeared to be enhanced due to eutrophication resulting from aquacultural activities. In early summer, Chl a concentration in the surface water (9.0 and 7.5 mg m−3 at fish cage and non-cage stations, respectively, Yoshikawa & Eguchi, unpublished) was at the similar level as that in midsummer (8.9 and 7.7 mg m−3 respectively). However, the sedimentation rates of phytoplankton pigments at fish cage and non-cage stations in early summer were three to five times higher (34.0 and 25.4 mg m−2 d−1 respectively) than those in midsummer (8.9 and 4.8 mg m−2 d−1 respectively). This difference may be explained by the species composition of phytoplankton. In early summer, Heterosigma akashiwo (Raphidophyceae) dominated the phytoplankton assemblages in both seawater and trap samples. It is known that H. akashiwo is often lysed by virus infection, leading to formation of aggregates, and resulting in rapid disappearance of the phytoplankton from the water column (Nagasaki, Ando, Itakura, Imai & Ishida 1994). The present results suggest that phytoplankton production was another important cause of organic pollution of the seafloor in addition to the direct impact of organic wastes discharged from fish farms in some seasons.
In early summer and winter, no significant differences were observed between the fish cage and non-cage stations with respect to the sedimentation rates of POC, POC content in the seawater and sinking particles, and DOC in the seawater. This implies that a significant part of OM derived from aquacultural activities dispersed over the entire Kogaura Inlet by tidal currents and did not deposit on the seafloor just beneath the fish cage. Some parts of this result are in agreement with the result of Yokoyama, Abo and Ishii (2006); using stable carbon and nitrogen isotope ratios as a tracer, they showed that the horizontal dispersal of aquaculture-derived organic waste extended to an area up to 300 m. However, they also pointed out that the amount of organic waste decreased exponentially with an increase in the distance from the fish cages.
The C:N ratio of the bottom sediments in the present study (7.5 ± 0.6) was comparable with the values typically reported for coastal waters (7.5-9.0, Holmer & Kristensen 1992). On the other hand, the C:N ratio of the sinking particles in the present study (12.0 ± 2.2) was higher than the typical values (5.7-7.0, Holmer & Kristensen 1992). According to the Redfield Ratio (Redfield 1934), the C:N ratio of the fresh bodies of phytoplankton was 5.7 on average. Yokoyama et al. (2006) reported the high values of C:N ratio (11-14) in the fish faeces in a fish culturing site. The relatively high C:N ratio in the sinking particles in the present study might be attributed to the fish faeces from the fish cage and resuspension of the aged particles from the seafloor.
Although no consistent seasonal patterns were observed in the turnover times of 14C-labelled substrates (Fig. 5), the cell-specific mineralization rates showed clear seasonal variations particularly in the sinking particles (Fig. 4). The cell-specific mineralization rates were highest in winter in spite of the lowest temperature of bottom water. Assuming 2-3 as typical Q10 values for bacterial metabolism (Pomeroy & Wiebe 2001), decline in water temperature by 8.2°C from 26.7°C in summer to 18.5°C in winter corresponds to decrement in the mineralization rate by factors of 0.4-0.6. Meanwhile, both DO and DOC in the bottom water became highest in winter (Table 1), so it appears that abundant oxygen and organic matter accelerated aerobic respiration of bacteria in the water column.
The present study clearly shows the importance of sinking particles as a site of mineralization of OM and suggests the possibility that a significant part of OM was respired during the sinking stage. To evaluate what part of OM in the sinking particles is actually respired until they deposit on the seafloor, it is necessary to investigate: (1) the generation rate of labile DOM, i.e., the decomposition rate of high molecular weight compounds by bacterial ectoenzymatic activity and (2) residence time of the sinking particles in the water column (refer to Anderson & Tang 2010 for a comprehensive model). In the present study, it was expected that DOM concentration and hydrolysis rate as well as POC contents, bacterial density and mineralization rates in the sinking particles were considerably higher than those in the surrounding seawater. However, there is a possibility that the generation of labile DOM may be a limiting step of the entire mineralization process of OM due to their very rapid turnover by respiration (<1 h for glutamate and glucose). Since the sinking velocities of a particle varies from several to hundreds metres per day depending on its size, origin and age as well as water movements (Alldredge & Gottschalk 1988; Turner 2002), it is very difficult to evaluate its residence time in the water column. Thus, in the present study, what part of OM in the sinking particles is respired until they deposit on the sea bottom remained to be clarified. Nevertheless, the attached bacteria in the sinking particles appeared to play an important role in decomposition and mineralization processes of OM even after the sinking particles settled down on the seafloor. In fact, high density and activity of bacteria have been reported at the surface layer of the bottom sediments (Henrichs & Doyle 1986). Furthermore, spring tide and storm in the coastal areas resuspend some parts of the surface layer of the bottom sediments into the water column (Jones, Jago, Bale, Chapman, Howland & Jackson 1998), and this resuspension would reduce size of the particles and enhance decomposition and mineralization processes of OM (Chróst & Riemann 1994). Although no severe anoxic water mass formation occurred during the observation period in the present study, anaerobic decomposition process of OM has been reported to be dominant in the bottom sediments, with sulphate reduction being observed in many cases (Holmer & Kristensen 1992). In contrast, aerobic mineralization of OM in the sinking and resuspended particles was not limited by oxygen supply from the surrounding seawater during sedimentation (Ploug 2001).
In conclusion, the present study shows the importance of sinking particles as a site of mineralization process of OM in the water column in a coastal fish cage farm; further, it reveals that the turnover time of glutamate was shorter than that of glucose, and small seasonal changes occur in the turnover times at each site of the water column. In addition, solute release from the sinking particles through ectoenzymatic hydrolysis processes of the densely attached bacteria appeared to be an important source of DOM in the surrounding seawater, as suggested in various aquatic environments (Alldredge 2000; Kiørboe & Jackson 2001; Azam & Long 2001; Simon, Grossart, Schweitzer, & Ploug 2002). Grossart and Simon (1998) has shown preferential release of amino acids from sedimented particle waters into the surrounding waters. Even in the coastal fish culturing area, sinking particles produce ‘hot spots’ of microbial mineralization process (Azam & Long 2001). The present study suggests that phytoplankton production is another important cause of OM pollution of the seafloor in addition to the direct impact of organic wastes discharged from fish farms in some seasons, and that a significant part of OM derived from aquacultural activities may be dispersed over the bay. For more accurate and concrete evaluation of microbial decomposition and mineralization processes of OM in a coastal fish cage farm, further studies are required on the bacterial ectoenzymatic hydrolysis activities at each site of the water column and the role of resuspension of the bottom sediments.
Acknowledgments
We are grateful to O. Murata, S. Miyashita, S. Miyano, K. Kato and the other staff of the Fisheries Laboratory, Kinki University, for their co-operation at sea. We are also grateful to S. Yamochi and S. Fujiwara for their co-operation with CHN analysis. Financial support was provided by 21st Century COE Program (the Center of Aquaculture Science and Technology for Bluefin Tuna and Other Cultivated Fish) and Global COE Program (International Education and Research Center for Aquaculture Science of Bluefin Tuna and other Cultured Fish), Kinki University.




