Seasonal patterns in the community of gill monogeneans on wild versus cultured orange-spotted grouper, Epinephelus coioides Hamilton, 1822 in Daya Bay, South China Sea
Abstract
Epinephelus is among the most important fish genera in the Southeast Asian Pacific coastal systems. In Chinese coast, the orange-spotted grouper Epinephelus coioides is one of the most significant species based on culture volume, and it is consumed all over the world. Very little information exists on this species’ parasitofauna. Composition and temporal variation in the community structure of the gill monogeneans on wild versus cultured E. coioides from Daya Bay (23°25′N, 117°2′E), South China Sea were determined using seasonal samples taken between April 2008 and January 2009. Eight species of monogeneans of three families and four genera on the gills of E. coioides were found: Neobenedenia melleni, Haliotrema cromileptis, Diplectanum grouperi, Pseudorhabdosynochus justinei, P. lantauensis, P. coioidesis, P. serrani and P. shenzhenensis. Eight of the monogenean species exhibited seasonal variation in their infection dynamics associated with environmental changes during seasons. The variations in the infection dynamics generated changes in the community structure during the sampling periods. Moreover, in the three different host living environments (wild, monocultured and polycultured), the monogenean communities exhibited the different seasonal patterns.
Introduction
The orange-spotted grouper, Epinephelus coioides Hamilton, is an important teleost for mariculture in Southeast Asian countries, and with a relatively high market value (Liao, Su & Chang 2001). Disease outbreaks have been obstacles to the development of effective culture programmes (Zeng 2006; Do & Phan 2007). Lethal pathogens reported included viruses, bacteria and parasites (Fukuda, Nguyen, Furuhashi & Nakai 1996; Leong 1997; Cribb, Bray, Wright & Pichelin 2002; Nagasawa & Cruz-Lacierda 2004; Yuki, Ikuo, Toyohiko & Mamoru 2008). Monogeneans as a group have been considered to be harassing pathogens (Do & Phan 2007), which are difficult to eradicate in open culture systems. Some species such as benedeniines and diplectanids may cause heavy casualties to cultured E. coioides or even the death of entire populations held in net cages or ponds (Leong 1997; Zeng 2006). Insufficient research, however, has been focused on the ecological aspects of monogeneans on E. coioides.
Studies on the community ecology of monogenean parasites in wild and cultured fishes have increased in the last decade (e.g. Janovy, Mcdowell & Ferdig 1991; Koskivaara, Valtonen & Prost 1991a,b; Reversat, Silan & Maillard 1992; Guegan & Hugueny 1994; Luque 1994; Rohde, Hayward, Heap & Gosper 1994; Rohde, Hayward & Heap 1995; Gutiérrez & Martorelli 1999; Gutiérrez 2001; Cordeiro & Luque 2004; Knopf, Krieger & Hölker 2007; Yamada, Takemoto & Pavanelli 2007). This has increased our knowledge of the composition and structure of infracommunities as well as component communities of monogeneans parasitizing different fish species. Groups have received less attention in this context, although there were studies on monogenean communities of wild Epinephelus spp. in Asian (Zeng 2006). Moreover, there has not been any report to date focusing on seasonal dynamics of the community of monogeneans on polycultured hosts within a confined habitat, where the parasite can potentially choose between related host species. Polyculture of groupers in net cage is, however, a common commercial aquaculture practice in Southeast Asian countries. Many of fish farmers often place every species of groupers caught in natural water in any time into a stable grouper-cultured system. We therefore investigated monogenean community variations in mixed species groupings of potential host species focusing on those in the dominant grouper species, Epinephelus coioides, in wild versus cultured conditions in Daya Bay, South China Sea.
The structure of the monogenean community is influenced by numerous factors including the seasonality of parasitic infection (Chubb 1977; Koskivaara et al. 1991b; Rauque, Semenas & Viozzi 2006), host living environment modifications (Yamada et al. 2007), variability of the host specificity of the parasites (Blazek, Bagge & Valtonen 2008) and interspecific interactions between parasites. Knowledge of these ecological characteristics is an important component of the understanding of community structure and dynamics of monogeneans on a host.
The aim of this study was to investigate seasonal patterns in the community of monogeneans on the gills of wild versus cultured E. coioides in Daya Bay, South China Sea, and to analyse the influence of the existence of potential susceptible hosts in the polycultured environment on the community structure of monogeneans on gills of the fish.
Materials and methods
Experimental design and host collection
In Daya Bay, the months of the spring season include March to May. Summer is from June to August, autumn occurs from September through November and winter from December to the following February. This investigation was carried out over a period of 10 months from April 2008 to January 2009.
A plastic tank of 1175 L (0.52 × π × 1.0 m3) and a cement pond of 54 000 L of water (6 × 6 × 1.5 m3) were, respectively, set up for monoculture of E. coioides and polyculture of several species of Epinephelus spp. to investigate the effect of host living conditions on the community structure of monogeneans on the gills of E. coioides. The experimental tank and pond, in the Mariculture Research Center, Guangdong Marine and Fishery Bureau at Huizhou, Guangdong Province, were supplied with continuously flowing (10 m3 h−1) sand-filtered sea water pumped from the bottom of the adjacent small estuary.
Wild samples of Epinephelus spp., collected monthly from Daya Bay (23°25′N, 117°2′E) by trapping, were identified to species according to previous descriptions (Cheng & Zhen 1987; Heemstra & Randall 1993). Some of the captured fishes were immediately placed into the experimental circular tank or pond, respectively, where they were cultured over 25 days. Seasonal samples of the wild, monocultured and polycultured fish were examined at the end of each month between April 2008 and January 2009. Thirty individuals of E. coioides were always kept in the monoculture tank by replenishing the same number of individuals as monthly sampled. Probably, a total of 199 individual fishes were maintained in the pond, including 35 individuals for each of the five main Epinephelus spp.: E. awoara (Temminck and Schlegel), E. coioides (Hamilton), E. bruneus (Bloch), E. quoyanus (Valenciennes) and E. bleekeri (Vaillant), and six individuals for each of the four rare Epinephelus species: E. fasciatomaculosus (Peters), E. fuscoguttatus (Forsskål), E. areolatus (Forsskål) and Epinephelus sp. Fishes were fed small frozen trash fishes. The number of the five common Epinephelus spp. sampled in this study and their standard lengths are shown in Table 1. Three individuals, with the standard length ranges from 12.8 to 20.7 cm, of each species of the four rare Epinephelus spp. (E. fasciatomaculosus, E. fuscoguttatus, E. areolatus and Epinephelus sp.) under both wild and polycultured conditions were monthly examined. The salinity and water surface temperature were measured daily.
| No. fish sampled | Length of fish (mean ± SE) (cm) | |||||
|---|---|---|---|---|---|---|
| Wild | Monocultured | Polycultured | Wild | Monocultured | Polycultured | |
| E. coioides | ||||||
| Spring (23.8°C) | 15 | 6 | 20 | 13.5–25.8 (18.3 ± 1.5) | 13.4–26.9 (18.9 ± 1.2) | 14.5–25.8 (21.5 ± 0.8) |
| Summer (28.9°C) | 25 | 9 | 61 | 12.8–24.5 (19.0 ± 0.8) | 23.2–29.2 (25.8 ± 0.7) | 14.5–28.6 (23.4 ± 0.4) |
| Autumn (24.7°C) | 17 | 8 | 20 | 17.8–28.0 (20.7 ± 1.2) | 19.9–25.5 (21.8 ± 0.7) | 17.5–26.5 (20.2 ± 0.5) |
| Winter (18.2°C) | 12 | 9 | 34 | 20.3–29.8 (25.4 ± 2.0) | 20.1–31.0 (23.8 ± 1.1) | 11.8–28.3 (22.9 ± 0.6) |
| E. awoara | ||||||
| Spring (23.8°C) | 15 | – | 20 | 13.3–22.4 (18.1 ± 0.7) | – | 15.2–26.3 (20.9 ± 0.5) |
| Summer (28.9°C) | 26 | – | 68 | 12.2–24.5 (19.1 ± 0.6) | – | 14.2–24.6 (20.3 ± 0.3) |
| Autumn (24.7°C) | 24 | – | 44 | 12.1–27.2 (17.9 ± 1.0) | – | 11.3–27.5 (20.8 ± 0.6) |
| Winter (18.2°C) | 17 | – | 34 | 10.8–23.3 (17.4 ± 1.1) | – | 10.6–25.0 (20.7 ± 0.6) |
| E. bruneus | ||||||
| Spring (23.8°C) | 3 | – | 7 | 27.0–29.3 (28.2 ± 1.2) | – | 22.2–27.1 (25.1 ± 0.7) |
| Summer (28.9°C) | 17 | – | 33 | 11.8–24.4 (16.7 ± 0.7) | – | 11.9–27.9 (18.8 ± 0.8) |
| Autumn (24.7°C) | 3 | – | 9 | 22.1–25.0 (23.9 ± 0.9) | – | 17.6–27.5 (23.8 ± 1.1) |
| Winter (18.2°C) | 5 | – | 14 | 19.4–26.2 (22.5 ± 1.3) | – | 20.5–28.4 (24.3 ± 0.6) |
| E. quoyanus | ||||||
| Spring (23.8°C) | 4 | – | 14 | 15.4–18.8 (17.0 ± 0.7) | – | 15.7–19.7 (17.9 ± 0.3) |
| Summer (28.9°C) | 10 | – | 17 | 18.2–25.4 (22.1 ± 0.6) | – | 15.7–25.7 (21.1 ± 0.7) |
| Autumn (24.7°C) | 5 | – | 6 | 17.8–26.0 (22.5 ± 1.4) | – | 19.3–25.8 (23.9 ± 1.0) |
| Winter (18.2°C) | 3 | – | 7 | 21.2–25.3 (23.8 ± 1.3) | – | 18.4–26.5 (24.2 ± 1.2) |
| E. bleekeri | ||||||
| Spring (23.8°C) | 3 | – | 3 | 14.2–17.0 (15.6 ± 1.4) | – | 19.0–26.2 (21.6 ± 2.3) |
| Summer (28.9°C) | 8 | – | 12 | 16.2–28.0 (23.9 ± 1.6) | – | 14.6–29.9 (22.0 ± 1.5) |
| Autumn (24.7°C) | 4 | – | 3 | 13.1–24.4 (20.8 ± 2.6) | – | 22.1–27.0 (25.1 ± 1.5) |
| Winter (18.2°C) | 3 | – | 10 | 17.6–27.2 (21.5 ± 3.0) | – | 13.1–28.7 (20.0 ± 1.4) |
Parasite examination
Fish sampled for parasite examination were sacrificed by pithing. Standard lengths and body weights were determined for each individual fish. The gills were excised immediately after death and placed in separate Petri dishes containing seawater. The epithelial lining of the gill filaments were scraped with a needle and the scrapings poured into a beaker filled with seawater. The contents of the beaker were stirred, allowed to settle and had the supernatant decanted. This procedure was repeated until a clear suspension was obtained. Sediments were examined under a dissecting microscope for the presence of parasites. Specimens of the parasites were temporarily preserved in ammonium picrate glycerine on a slide. Monogeneans were examined on each slide microscopically using an Olympus BX41 (Olympus Corporation, Tokyo, Japan), and each specimen was immediately numbered. Different species were distinguished by morphology and measurements of haptoral sclerites and male copulatory organs previously proposed by Bu, Leong, Wong, Woo and Foo (1999), Kritsky and Beverley-Burton (1986), Yang, Zeng and Gibson (2005) and Justine (2005, 2007, 2008).
Statistical analyses
Variation in species composition during the sampling period was described using prevalence (per cent of hosts infected), mean abundance (mean number of parasites per examined fish) and the maximum value of intensity (Bush, Lafferty, Lotz & Shostak 1997) for each monogenean species per sampling month.
The monogeneans of each host are considered to comprise an infracommunity, and all infracommunities on one species of host are considered as a component community (Bush & Holmes 1986). The component community parameters included total number of parasite species, the Shannon–Wiener Index as a measure of diversity, species evenness (equitability; Krebs 1999) and the Berger–Parker Index as a measure of numerical dominance. The Kruskal–Wallis tests were used to determine significant differences in the component community parameters between seasons at this level. The infra-communities were described by the mean number of monogenean species per fish, the Berger–Parker Index value, the mean value of the Brillouin Diversity Index per fish and species evenness (equitability). An one-way analysis of variance was employed to identify differences in prevalence, Berger–Parker Index and Brillouin Diversity Index between seasons. The Kruskall–Wallis test compared differences in abundance of the gill monogeneans on E. coioides between seasons, and the influence of three environments (natural water, monocultured tank and polycultured pond) on parasite infection levels (Zar 1996). Prevalence of 33.3% was adopted as the lowest limit in identifying satellite species; species with prevalences 33.3% < P < 66.6% were assigned as secondary species; species with prevalences of 66.6% or higher were considered as core species (Caswell 1978; Hanski 1982; Bush & Holmes 1986).
All statistical analyses were executed using spss 11.5 (SPSS, Chicago, IL, USA), at a significance level of 0.05.
Results
Community characterization
In this study, the following 13 species of monogeneans belonging to three families (Capsalidae, Dactylogyridae, Diplectanidae) and representatives of four genera were found from Epinephelus spp.: Neobenedenia melleni (MacCallum 1927), Yamaguti 1963; Haliotrema cromileptis Young 1968; H. epinepheli Young 1968; Diplectanum grouperi Bu et al. 1999; Pseudorhabdosynochus justinei Zeng & Yang 2006; P. epinepheli, (Yamaguti 1938), Kritsky & Beverley-Burton 1986; P. lantauensis (Beverley-Burton & Suriano 1981), Kritsky & Beverley-Burton 1986; P. coioidesis Bu et al. 1999; P. serrani (Yamaguti 1953), Kritsky & Beverley-Burton 1986; P. shenzhenensis Yang et al. 2005; P. cupatus (Young 1969), Kritsky & Beverley-Burton 1986; P. vagampullum (Young 1969), Kritsky & Beverley-Burton 1986 and Pseudorhabdosynochus sp. Among them, the following monogenean species were found on the gills of E. coioides: N. melleni, H. cromileptis, D. grouperi, P. justinei, P. lantauensis, P. coioidesis, P. serrani and P. shenzhenensis. The frequency distributions of the monogenean species on the gills of all E. coioides sampled showed the community was made up of two groups: common parasites, with prevalence >33.3% (D. grouperi, P. coioidesis and P. serrani); and rare parasites, with prevalence <33.3%. The most frequent species were also the most abundant (mean abundance >10 parasites) except for those on wild E. coioides.
Monogenean infection dynamics
There were evidently different seasonal fluctuation patterns of monogeneans on wild versus cultured E. coioides in the Daya bay, South China Sea. Neobenedenia melleni and P. shenzhenensis were only found in summer under both wild and polycultured conditions, whereas H. cromileptis was only seen in winter under both conditions (Table 2). Pseudorhabdosynochus justinei was only observed in the spring sampling months on wild E. coioides (Table 2). The four species of parasites did not occur at all during the sampling period on the monocultured hosts (Table 2).
| Wild | Monocultured | Polycultured | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Prevalence (%) | Abundance (SE) MI | SC | Prevalence (%) | Abundance (SE) MI | SC | Prevalence (%) | Abundance (SE) MI | SC | |
| N. melleni | |||||||||
| Total pooled data | 5.0 | 0.1 (0.1) 1 | Sa | 0 | 0 | 14.8 | 0.4 (0.2) 9 | Sa | |
| Spring | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Summer | 11.1 | 0.1 (0.1) 1 | Sa | 0 | 0 | 31.2 | 0.8 (0.2) 9 | Sa | |
| Autumn | 0 | 0 | 0 | 0 | 2.9 | 0 | |||
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |||
| P. shenzhenensis | |||||||||
| Total pooled data | 2.5 | 0.1 (0.1) 1 | Sa | 0 | 0 | 1.0 | 0.1 (0.1) 2 | Sa | |
| Spring | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Summer | 5.6 | 0.1 (0.1) 1 | Sa | 0 | 0 | 1.6 | 0.1 (0.1) 2 | Sa | |
| Autumn | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |||
| D. grouperi | |||||||||
| Total pooled data | 72.5 | 9.0 (1.9) 615 | Co | 94.1 | 53.7 (31.4) 615 | Co | 83.0 | 270.2 (190.6) 2744 | Co |
| Spring | 60.0 | 5.7 (2.5) 35 | Se | 83.3 | 12.0 (5.4) 35 | Co | 60.0 | 3.6 (0.9) 13 | Se |
| Summer | 96.0 | 65.8 (29.4) 615 | Co | 100.0 | 161.8 (73.3) 615 | Co | 100.0 | 585.0 (98.7) 2744 | Co |
| Autumn | 94.1 | 14.1 (2.9) 38 | Co | 100.0 | 18.5 (4.1) 36 | Co | 85.0 | 28.1 (8.9) 126 | Co |
| Winter | 75.0 | 4.9 (1.6) 17 | Co | 88.9 | 4.7 (1.3) 13 | Co | 76.5 | 4.5 (0.8) 22 | Co |
| P. coioidesis | |||||||||
| Total pooled data | 20.0 | 4.2 (3.2) 37 | Sa | 46.9 | 48.7 (13.6) 582 | Se | 45.2 | 16.2 (6.9) 292 | Se |
| Spring | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Summer | 0 | 0 | 0 | 0 | 41.0 | 10.1 (2.9) 122 | Se | ||
| Autumn | 44.4 | 2.0 (1.0) 8 | Se | 100.0 | 164.3 (66.5) 582 | Co | 55.0 | 42.1 (16.9) 292 | Se |
| Winter | 50.0 | 11.4 (8.9) 37 | Se | 77.8 | 27.0 (9.3) 80 | Co | 73.5 | 21.4 (4.4) 94 | Co |
| P. serrani | |||||||||
| Total pooled data | 15.0 | 2.3 (1.1) 32 | Sa | 50.0 | 19.4 (6.7) 232 | Se | 52.6 | 12.1 (5.4) 122 | Se |
| Spring | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Summer | 0 | 0 | 0 | 0 | 55.7 | 7.7 (1.4) 45 | Se | ||
| Autumn | 33.3 | 3.6 (2.1) 18 | Sa | 87.5 | 51.1 (26.8) 232 | Co | 55.5 | 23.6 (7.8) 122 | Se |
| Winter | 60.0 | 11.8 (8.6) 32 | Se | 100.0 | 23.7 (13.7) 125 | Co | 79.4 | 20.5 (4.5) 119 | Co |
| P. lantauensis | |||||||||
| Total pooled data | 12.5 | 0.2 (0.1) 5 | Sa | 15.6 | 0.3 (0.2) 5 | Sa | 19.3 | 0.8 (0.4) 26 | Sa |
| Spring | 6.7 | 0.1 (0.1) 1 | Sa | 0 | 0 | 0 | 0 | ||
| Summer | 16.0 | 0.3 (0.1) 2 | Sa | 22.2 | 0.3 (0.2) 2 | Sa | 29.5 | 1.7 (0.6) 26 | Sa |
| Autumn | 11.8 | 0.3 (0.2) 2 | Sa | 0 | 0 | 10.0 | 0.2 (0.1) 2 | Sa | |
| Winter | 25.0 | 0.8 (0.6) 5 | Sa | 33.3 | 0.8 (0.5) 5 | Sa | 17.7 | 0.2 (0.1) 2 | Sa |
| P. justinei | |||||||||
| Total pooled data | 2.5 | 0.1 (0.1) 2 | Sa | 0 | 0 | 0 | 0 | ||
| Spring | 11.1 | 0.2 (0.2) 2 | Sa | 0 | 0 | 0 | 0 | ||
| Summer | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Autumn | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Winter | 0 | 0 | 0 | 0 | 0 | 0 | |||
| H. cromileptis | |||||||||
| Total pooled data | 2.5 | 0.1 (0.1) 1 | Sa | 0 | 0 | 1.0 | 0.1 (0.1) 3 | Sa | |
| Spring | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Summer | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Autumn | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Winter | 25.0 | 0.3 (0.3) 1 | Sa | 0 | 0 | 2.9 | 0.1 (0.1) 3 | Sa | |
The seasonal dynamics in the prevalence and abundance of D. grouperi on E. coioides were similar under wild, monocultured and polycultured conditions (Table 2). Higher prevalence were all found in summer and autumn and relatively lower values in spring and winter; the highest abundances also were all found in summer, and the lowest abundances in winter or spring (Table 2). Diplectanum grouperi infections on E. coioides under both wild and polycultured conditions exhibited significant seasonal changes in prevalence (wild: F = 32.01, P < 0.05; polycultured: F = 58.67, P < 0.05). Significant seasonal differences in abundances of the parasite under both cultured conditions were seen (monocultured: H = 16.88, P < 0.05; polycultured: H = 77.64, P < 0.05).
Under both wild and monocultured conditions, E. coioides was found to be infected with P. coioidesis and P. serrani during autumn and winter in Daya Bay during the sampling period, whereas no specimens of these two parasites were collected from the hosts during the sampling period (Table 2). In the wild, there were significant seasonal differences in prevalence of these two parasites (for P. coioidesis: F = 25.83, P < 0.05; for P. serrani: F = 11.54, P < 0.05) and in their abundances (for P. coioidesis: H = 11.54, P < 0.05; for P. serrani: H = 11.18, P < 0.05). On monocultured E. coioides, there were also significant seasonal differences in prevalence of both parasites (for P. coioidesis: F = 41.18, P < 0.05; for P. serrani: F = 81.39, P < 0.05) and in their abundances (for P. coioidesis: H = 24.93, P < 0.05; for P. serrani: H = 24.73, P < 0.05). On polycultured E. coioides, the prevalence of P. coioidesis increased during summer and autumn, and reached a peak in winter (Table 2). The prevalence of P. serrani in summer levelled out during the autumn and likewise attained its highest values in winter. In the case of abundance, in both parasite species, it reached a peak in autumn (Table 2). Significant seasonal differences of the abundance of the two parasites were found (for P. coioidesis: H = 7.64, P < 0.05; for P. serrani: H = 11.18, P < 0.05).
The maximum values of prevalence of P. lantauensis under three conditions were observed in the summer and winter, and the minimum ones in the spring and autumn (Table 2). The seasonal changes of the abundance showed the different patterns in different conditions; the abundance reached a peak in winter in wild or monocultured conditions, whereas it peaked in summer in the polycultured environment (Table 2). Under wild and polycultured conditions, the seasonal differences in the parasite prevalence on E. coioides were significant (wild: F = 34.80, P < 0.05; polycultured: F = 51.83, P < 0.05).
Infracommunity
Seasonal changes of the species composition of the monogenean communities on the gills of E. coioides under wild, monocultured and polycultured conditions were apparent in Daya Bay (Table 1). The monogenean species richness at infracommunity level on wild, monocultured and polycultured E. coioides was highest in the autumn, winter and summer, respectively, whereas under both cultured conditions the infracommunity diversity was highest in the winter, but the highest degree of diversity was seen on wild hosts in the autumn (Table 3). Significant seasonal differences appeared in species richness of monogenean infracommunities on the wild, monocultured and polycultured hosts (wild: F = 7.47, P < 0.05; monocultured: F = 50.89, P < 0.05; polycultured: F = 13.85, P < 0.05), and in the infracommunity diversity under three conditions (wild: F = 6.27, P < 0.05; monocultured: F = 171.89, P < 0.05; polycultured: F = 24.59, P < 0.05).
| Infracommunity (mean ± SD) | Component community | |||||||
|---|---|---|---|---|---|---|---|---|
| Richness | Brillouin Diversity Index | Evenness | Berger–Parker Index | Richness | Shannon's Diversity Index | Evenness | Berger–Parker Index | |
| Total pooled data | ||||||||
| Wild | 1.33 ± 1.19 | 0.20 ± 0.32 | 0.21 ± 0.31 | 0.85 ± 0.20 | 8 | 1.06 | 0.51 | 0.72 |
| Monocultured | 2.12 ± 1.08 | 0.37 ± 0.35 | 0.35 ± 0.33 | 0.83 ± 0.16 | 4 | 1.04 | 0.75 | 0.44 |
| Polycultured | 2.19 ± 1.40 | 0.31 ± 0.35 | 0.29 ± 0.32 | 0.81 ± 0.22 | 7 | 0.41 | 0.21 | 0.90 |
| Spring | ||||||||
| Wild | 0.67 ± 0.87 | 0.08 ± 0.16 | 0.11 ± 0.23 | 0.79 ± 0.25 | 3 | 0.60 | 0.55 | 0.81 |
| Monocultured | 0.83 ± 0.41 | 0 | 0 | 1.00 ± 0.00 | 1 | 0 | 0 | 1.00 |
| Polycultured | 0.60 ± 0.50 | 0 | 0 | 1.00 ± 0.00 | 1 | 0 | 0 | 1.00 |
| Summer | ||||||||
| Wild | 1.11 ± 0.68 | 0.06 ± 0.11 | 0.09 ± 0.16 | 0.96 ± 0.09 | 4 | 0.15 | 0.11 | 0.97 |
| Monocultured | 1.22 ± 0.44 | 0.01 ± 0.03 | 0.02 ± 0.04 | 1.00 ± 0.00 | 2 | 0.01 | 0.02 | 1.00 |
| Polycultured | 2.61 ± 1.13 | 0.20 ± 0.21 | 0.21 ± 0.23 | 0.92 ± 0.12 | 6 | 0.18 | 0.10 | 0.97 |
| Autumn | ||||||||
| Wild | 2.22 ± 1.39 | 0.45 ± 0.38 | 0.43 ± 0.35 | 0.71 ± 0.24 | 4 | 1.00 | 0.72 | 0.66 |
| Monocultured | 2.88 ± 0.35 | 0.70 ± 0.13 | 0.66 ± 0.08 | 0.64 ± 0.18 | 3 | 0.78 | 0.71 | 0.70 |
| Polycultured | 1.85 ± 1.57 | 0.50 ± 0.47 | 0.44 ± 0.41 | 0.33 ± 0.23 | 4 | 1.08 | 0.78 | 0.45 |
| Winter | ||||||||
| Wild | 1.75 ± 2.06 | 0.49 ± 0.57 | 0.40 ± 0.46 | 0.45 ± 0.21 | 4 | 1.03 | 0.74 | 0.53 |
| Monocultured | 3.00 ± 0.50 | 0.67 ± 0.09 | 0.63 ± 0.10 | 0.48 ± 0.31 | 4 | 0.98 | 0.71 | 0.48 |
| Polycultured | 2.50 ± 1.31 | 0.58 ± 0.33 | 0.54 ± 0.29 | 0.40 ± 0.25 | 6 | 0.99 | 0.55 | 0.46 |
The number of parasite species per infected host ranged from one to five. In winter, all individuals of E. coioides sampled under three conditions harboured two or more species of monogeneans. However, 22.2%, 83.3% and 60.0% of wild, monocultured and polycultured E. coioides in spring, 55.0%, 77.8% and 19.7% in summer, and 22.2%, 0.0% and 10.0% in autumn harboured only one species of monogeneans (Fig. 1).
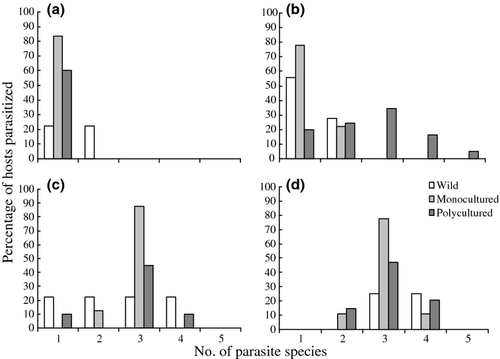
No significant correlation was detected between the host's standard length and the parasite species richness at infracommunity level (Spearman P > 0.05). The correlation between the total number of gill monogeneans and the species number in the infracommunities was significant (on wild E. coioides, Spearman P < 0.05; on polycultured E. coioides, Spearman P < 0.05).
Component community
There were also clear seasonal variations in the monogenean species richness and diversities at component community level on the gills of E. coioides under three conditions in Daya Bay (Table 3). The species richness at this level on cultured E. coioides were highest both in the winter, but the highest degree of richness was seen on the wild hosts in the summer (Table 3). The monogenean component communities on E. coioides under wild and monocultured conditions displayed highest diversities in the winter, but on polycultured E. coioides in the autumn (Table 3). Comparison between seasons indicated that both species richness and diversity did not vary between seasons (P > 0.05).
Diplectanum grouperi was an evident dominant species on both wild and cultured E. coioides during all seasons during the sampling period in Daya Bay (Table 2); P. coioidesis and P. serrani coexisted on wild and monocultured E. coioides in autumn and winter. On polycultured hosts from summer to winter, these two species displayed the high infection levels and were therefore considered as core (prevalence over 33.3%) or secondary species (Table 2). Pseudorhabdosynochus lantauensis always appeared at relatively low infection levels and was considered to be a satellite species, although it could occur in different seasons ranging from spring to autumn on wild E. coioides and from summer to winter on polycultured hosts (Table 2). The other four monogenean species, N. melleni, H. cromileptis, P. justinei and P. shenzhenensis, were all satellites (Table 2).
Impact of host living environments on the monogenean community
With regard to the infections of monogeneans on E. coioides in three living environments in Daya Bay, D. grouperi, P. coioidesis and P. serrani presented significant differences in the intensity of infection in relation to the environment, with significantly higher infections in the monoculture (highest for P. coioidesis; H = 11.56, P < 0.05 and highest for P. serrani; H = 16.89, P < 0.05) and polyculture (highest for D. grouperi; H = 11.19, P < 0.05) environment and lower infections in the estuary (Table 2).
There were also significant differences in species richness (H = 11.30, P < 0.05) of the monogenean infracommunities on the host E. coioides in Daya Bay in relation to the host living environment, with the highest degree of richness in natural water of the bay (Table 3).
Discussion
Epinephelus coioides is highly susceptible to infestation by numerous species of monogeneans. Zeng (2006) found 13 species of gill monogeneans on E. coioides in Daya Bay, eight of which were collected in the present study. It was demonstrated that D. grouperi was the dominant species in all seasons, whereas P. coioidesis and P. serrani were mainly prevalent in autumn and winter. The number of core species (prevalence higher than 66.6%) like D. grouperi, P. coioidesis and P. serrani could be one, two or as many as three in a season. The concurrence of two or three core species on the gills of hosts in autumn and/or winter might imply that there was no apparent competition among these monogenean species, which was in accordance with the previous report that interspecific interactions of few monogenean species on the gills of Pimelodus maculates in the Río de la Plata, Argentina were detected, and some of these were positive (Gutiérrez & Martorelli 1999).
Many of the most abundance and frequent monogenean species, such as D. grouperi, P. coioidesis and P. serrani on both wild and cultured E. coioides exhibited clear seasonal variations in their infection dynamics associated with environmental changes during seasons. Both prevalence and mean abundance of the core species D. grouperi on both wild and cultured hosts were significantly higher in summer than in spring, and the other two core species P. coioidesis and P. serrani co-occurred in autumn and winter with high infection levels, but disappeared from the hosts in the sampling months of spring. The prevalence and abundance of gill monogeneans at the infracommunity level on either wild or polycultured E. coioides demonstrated significant seasonal differences. These results are similar to those of Chubb (1977) and Koskivaara and Valtonen (1992) for monogeneans in the northern hemisphere. Several factors are considered to affect the seasonal community dynamics of monogeneans and may explain the higher infection levels of the monogeneans such as D. grouperi and the species richness of monogeneans in summer than those in other three seasons. The major abiotic factor affecting reproduction and population growth of monogeneans is water temperature, which affects the transmission (Soleng, Jansen & Bakke 1999) as well as birth and mortality rates directly, and probably also affects the host immune system (Scott & Nokes 1984; Jansen & Bakke 1991; Andersen & Buchmann 1998; Bakke, Cable & Harris 2007; Winger, Marte, Roar & Rune 2008). However, in this study, the fact that P. coioidesis and P. serrani in Daya Bay performed better on E. coioides in autumn and winter than in summer may have been a result of their better tolerance of lower water temperature. The higher water temperature in summer may result in a higher host resistance than in autumn and winter (Jansen & Bakke 1993). In addition, the factors impacting water quality, such as dissolved oxygen, salinity and pH values of water also probably play an important role in determining seasonal variations in prevalence and abundance for some of the monogeneans studied. Further research need to be performed to illustrate the mechanism of their impacts on infection dynamics of monogeneans on the gills of E. coioides.
It is known that infrapopulational growth and infection duration of monogeneans often differ between related host species (e.g. Mo 1997; Boeger, Kritsky, Pie & Engers 2005; Moen & Stockwell 2006; Olstad, Robertsen, Bachmann & Bakke 2007), and the chemical structure of the epidermis and mucus of related fish can differ substantially (Buchmann & Uldal 1997). This may explain different abundances of parasites on different species of hosts. Sympatric fish hosts unsuitable for parasite reproduction could, on the other hand, be used as transient hosts (Bakke, Harris & Cable 2002). When related hosts were polycultured in a system, such as the polycultured pond with several species of Epinephelus in this study, parasites switched readily to related host species, and some host species would increase susceptibility of other species of hosts to the parasites. Therefore, host specificity is important intrinsic factors in determining differences in infection dynamics and community structure of monogeneans, especially those on related host species in a polyculture system.
The variations in the infection dynamics generated changes in the community structure over the sampling time. Significant seasonal differences in the species richness and diversity of the monogenean infracommunities on either wild or cultured E. coioides were seen. The component communities presented the same seasonal pattern as the infracommunities, that is, they were also species rich and highly diverse in autumn and winter, and highly and numerically dominated in spring and summer. The positive significant correlation of mean number of parasite species and mean parasite intensity with total host length indicates that larger hosts harboured more parasite species and more parasite burden than smaller ones. This is attributed to the fact that larger (i.e. older) fish have had more time to accumulate parasites than younger (smaller) ones (Golder, Chndra & Rahman 1987; Koskivaara & Valtonen 1992; Gutiérrez & Martorelli 1999; Gutiérrez 2001).
Hosts living environments can affect both the parasite community structure and the suite of parasites available to it mainly by changing parasite transmission abilities (Grutter 1998; Yamada et al. 2007; Blazek et al. 2008). A significant difference in the species richness of monogeneans on the gills of E. coioides was found between the environments (natural water, monocultured tank and polycultured pond) in Daya Bay. Similarly, significant differences for the mean abundance of both D. grouperi and P. coioidesis were observed between the environments. These results are in accordance with the hypothesis that species richness and abundance of the monogeneans with the monoxenic cycle can be influenced by the environment. Higher abundance and species richness of the mongeneans in polycultured pond conditions, when compared with the wild, may be attributed to the increased transmission rates between hosts and the relative ease in finding the suitable hosts.
In this study, the infracommunities presented the different patterns with the component communities in natural water and monocultured tank; for example, there were the lower species richness in the wild and the higher diversity under monocultured conditions at infracommunity levels, whereas the higher degree of richness on wild E. coioides and the lower diversity on monocultured hosts were found at component community levels. However, in the polycultured pond, the infracommunities presented the same pattern as the component communities, that is, they were species-rich, highly numerically dominated and not highly diverse. This showed that a cage or pond where related fish species were polycultured was an unstable culture system where rational management measures, such as choice of fish species, methods pretreating the fish caught from natural water in different seasons, needed to been taken to prevent the outbreak of monogenean diseases.
Acknowledgments
This research was financially supported by National Natural Science Foundation of China (No. 30771659). We thank Wang Yunxin and Zhang Haifa in the Mariculture Research Center, Guangdong Marine and Fishery Bureau at Ao'tou, Huizhou, Guangdong Province for their kind help during the experiment.




