Influence of dietary garlic (Allium sativum) on the antioxidative status of rainbow trout (Oncorhynchus mykiss)
Abstract
Oxidative damage by free radicals has been implicated in the pathogenesis of several diseases. In this study, the antioxidative effect of dietary garlic on rainbow trout was examined. Trout fingerlings were fed on diets containing 10, 20, 30, 40 and 50 g garlic powder kg−1 diet. Serum lipid peroxides and activities of antioxidant enzymes were measured. Serum thiobarbituric acid reactive substances (TBARS) assay showed that garlic consumption that resulted in a significant decrease in lipid peroxidation. The lowest levels of TBARS were observed in fish fed diet supplemented with 30 g kg−1 garlic. However, higher doses of garlic (40 and 50 g kg−1 diet) caused no further reduction in serum TBARS. The results showed a significant increase in superoxide dismutase in all of the garlic-treated groups compared with the control. Ingestion of 10, 20 and 30 g kg−1 dietary garlic resulted in a significant reduction in the catalase activity compared with all but the 10 g kg−1 group. There was no significant difference in glutathione peroxidase activity among the different groups. Serum alanine aminotransferase and aspartate aminotransferase levels increased significantly in trout-fed diets containing 40 and 50 g kg−1 garlic powder. These results suggest that dietary garlic may improve the antioxidant status of rainbow trout. However, undesirable effects of higher doses of garlic should be considered.
Introduction
Formation of reactive oxygen species (ROS) is an inevitable consequence in most organisms due to aerobic metabolism. Oxygen supports life for organisms; however, oxygen consumption generates cytotoxic byproducts (Li, Zlabek, Velisek, Grabic, Machova & Randak 2010). Conversely, there is a multilayered strategy of defence against oxidative damage including enzymatic and nonenzymatic antioxidants as well as adaptive responses (Sies 1993). Oxidative stress refers to the imbalance between generation of ROS and antioxidative defence mechanisms (Nematollahi, Decostere, Pasmans & Haesebrouck 2003; Ece, Gurkan, Kervancioglu, Kocamaz, Gunes, Atamer & Selek 2006). ROS formation can be enhanced by xenobiotics, including drugs and environmental chemicals. ROS can play physiological roles in signal transduction, but in excess can contribute to the mechanisms of disease by dysregulation of signal transduction and/or by oxidative damage to cellular macromolecules such as lipids, proteins and nucleic acids that exceeds the cellular capacity for regeneration or repair (Wells, Mccallum, Chen, Henderson, Lee, Perstin, Preston, Wiley & Wong 2009). It is well established that oxidative stress is an important cause of cell damage associated with the initiation and progression of many diseases (Beck, Handy & Levander 2004; Willcox, Ash & Catignani 2004), and there is evidence in which oxidative stress may play a role in the pathology of fish disease and that fish tissues are particularly susceptible to oxidative damage (Welker & Congleton 2005). In both mammals and fish, insufficient dietary antioxidants have been followed by a decrease in antioxidative defence and increased susceptibility to oxidative stress (Sies, Stahl & Sevanian 2005; Welker & Congleton 2009).
The antioxidative defence system capacity of cultured fish has been found insufficient (Nakano, Kanmuri, Sato & Takeuchi 1999). With regard to increasing water pollution and its stimulating effects on oxidative stress, improving antioxidative status of fish seems to be necessary (Welker & Congleton 2005). Therefore, any attempt to enhance this system may be associated with beneficial effects on health status of fish.
Recent trends in prevention and treating diseases tend to prefer to use natural-based antioxidants rather than synthetic ones in degradation and scavenging of ROS (Hassan, Hafez & Zeghebar 2010). In this context, garlic has been extensively studied and shown to antioxidant activity including the ability to lower oxidative stress (Drobiova, Thomson, Al-Qattan, Peltonen-Shalaby, Al-Amin & Ali 2011). Some research groups have reported beneficial effects of garlic and its components consumption in prevention and treatment of fish diseases (Nya, Dawood & Austin 2010) and toxicosis (Shahsavani, Baghshani & Alishahi 2010). However, to our knowledge there have been no reports about the effect of steady-state dietary garlic on the antioxidant status of farmed rainbow trout. Therefore, the purpose of the present study was to investigate the antioxidant effect of garlic on rainbow trout under controlled conditions.
Materials and methods
Chemicals
Nitroblue tetrazolium (NBT), xanthine, xanthine oxidase, thiobarbituric acid, glutathione reductase and reduced glutathione were purchased from Sigma Chemical Co. (St Louis, MO, USA). EDTA, sodium azide, NADPH, ammonium molybdate and hydrogen peroxide, trichloroacetic acid and hydrochloric acid were purchased from Merck & Co. (Darmstadt, Germany). Lipid profile and serum aminotransferase assay kits were purchased from Darmankav (Isfahan, Iran). All other reagents were analytical grade.
Animals and breeding
Rainbow trout (Oncorhynchus mykiss) fingerlings were obtained from a commercial fish farm (Sharekord, Iran). Fish were kept in 100 L flow-through tanks receiving dechlorinated Isfahan city water at a rate of 1 L min−1 kg−1 of fish biomass under constant aeration to maintain dissolved oxygen values at 6.8–8.5 mg L−1. The water continuously passed through a sand filter to remove sediments and zeolite filter to maintain ammonium < 0.01 ppm. Temperature was 16 ± 1°C and pH was 7.4 ± 0.2. Photoperiod was a 14:10 light–dark cycle. Fish were acclimatized for 7 days before the start of the experiments. Fish were fed commercial food at a ration of 4.1% of body weight twice daily (Table 1).
| Ingredient (% dry weight) | Control | Dietay garlic (g kg−1) | ||||
|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | ||
| Fish meala | 47 | 47 | 47 | 47 | 47 | 47 |
| Soybean meal | 25 | 25 | 25 | 25 | 25 | 25 |
| Wheat meal | 18.5 | 17.5 | 16.5 | 15.5 | 14.5 | 13.5 |
| Fish oil | 2 | 2 | 2 | 2 | 2 | 2 |
| Sunflower oil | 2 | 2 | 2 | 2 | 2 | 2 |
| Lysin | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| Methionin | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Vitamin premixb | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 |
| Mineral premixc | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Cellulose | 2 | 2 | 2 | 2 | 2 | 2 |
| Garlic powder | 0 | 1 | 2 | 3 | 4 | 5 |
- a Pars Kilka Co; Iran, based on 100% dry weight included 72% crude protein, 16.2% crude lipid.
- b Unit kg−1 of mineral mixture: Fe, 4500 mg; Cu, 500 mg; Se, 50 mg; Zn, 6000 mg; Mn, 5000 mg; I, 150 mg; choline chloride, 150 000 mg; carrier up to 1 kg.
- c Unit kg−1 of vitamin mixture: retinol acetate (A), 1 200 000 IU; cholecalciferol (D3), 400 000; DL-α-tocopheryl acetate (E), 30 IU; menadione sodium bisulphite (K3), 1200 mg; l-ascorbic acid (C), 5400 mg; D-biotin (H2), 200 mg; thiamin mononitrate (B1), 200 mg; riboflavin (B2), 3600 mg; calcium d-pantothenate (B3), 7200 mg; niacinamide (B5), 9000 mg; pyridoxine hydrochloride (B6), 2400 mg; folic acid (B9), 600 mg; cyanocobalamin (B12), 4 mg; antioxidant 500 mg; Carrier up to 1 kg.
Garlic powder preparation
Garlic (Allium sativum) was purchased from a local market in Isfahan, Iran. The peeled cloves were cut into thin slices and dried in the oven in 50°C for 72 h. Dried slices were ground to a powder in a blender and passed through a 40-mesh screen.
Experimental design and diets
The fish (7 ± 1 g) were randomly distributed among 12 tanks with 30 fish per tank, giving duplicate tanks per diet. Trout in control group was fed with basic diet. The fish in remaining groups were fed daily one of the five concentrations of garlic powder namely 10, 20, 30, 40, 50 g kg−1 diet, respectively, for 8 weeks. The composition of the experimental diets is shown in Table 1. Briefly, diet ingredients were blended and thoroughly stirred by adding distilled water. Pellets were made using an electric grinder equipped with a disc of 3 mm hole diameter. After drying the pellets at room temperature for 24 h, they were stored at −20°C until further use.
At the end of the feeding trial, trout were fasted for 1 day and then weighed individually. Blood samples were collected from 10 fish per tank from the caudal vessels under 40 mg L−1 MS-222 (tricaine methanesulfonate) anaesthesia. Samples were kept on ice, centrifuged at 4°C (4000 g) for 15 min, and the serum was stored at −40°C, pending further analysis.
Biochemical analysis
The activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), levels of triglycerides, cholesterol and HDL in sera were measured using commercial kits and automated chemistry analyzer Selectra E (Vital Scientific NV, Dieren, the Netherlands). Serum LDL-cholesterol was calculated from serum triglyceride, total cholesterol and HDL-cholesterol levels using the Friedewald formula (Friedewald, Levy & Fredrickson 1972). Serum total protein levels were measured using a Bio-Rad protein assay kit.
Serum superoxide dismutase (SOD) activity was estimated using the NBT dye reduction test (Yasuda, Takesue, Inutsuka, Honda, Nozoe & Korenaga 2002). In brief, 0.1 mL trout serum was added to 2 mL reaction mixture, which contained 0.20 mM xanthine, 0.12 mM NBT, 0.049 IU xanthine oxidase and 0.1 M phosphate buffer (pH 7.0). The mixture was incubated for 20 min at 37°C. In the reaction mixture, the superoxide radical is produced, which reduces NBT to a blue colour dye, NBTH2. The rate of reduction of NBT was measured at 560 nm. The results for SOD activity were expressed as the per cent inhibition of reduction of NBT by SOD.
Serum glutathione peroxidase (GPX) activity was determined according to the method of Lawrence and Burk (1976). Briefly, 0.9 mL of the reaction mixture containing 50 mM potassium phosphate buffer pH 7.0, 1 mM EDTA, 1 mM sodium azide, 0.2 mM NADPH, 20 mU glutathione reductase and 1 mM GSH was incubated for 5 min at 25°C. Next, 100 μL 0.25 mM H2O2 and 50 μL serum were added to the assay mixture. The change in absorbance at 340 nm (Unico 2100, United Products Instruments, Inc., Dayton, NJ, USA) was monitored against a blank (serum was replaced with distilled water) for 1 min. One unit of GPX activity was reported as μmol NADPH consumed per min per mg serum protein, using the appropriate molar absorptivity coefficient for NADPH (6220 M cm−1).
The serum catalase activity was assayed according to the method of Goth (1991). Briefly, 0.1 mL of serum sample was incubated in 1 mL reaction mixture that contained 50 mM potassium phosphate buffer (pH 7.0) and 10.6 mM H2O2 freshly prepared at 37°C for 60 s. The reaction was terminated by adding 0.5 mL of 32.4 mM ammonium molybdate solution. A yellow complex of ammonium molybdate and hydrogen peroxide was formed. The absorbance of this yellow colour was measured at 405 nm with a spectrophotometer (Unico 2100) against a blank (serum was replaced with distilled water). One unit of catalase activity was defined as the amount of enzyme that catalyses the decomposition of 1 μmol of hydrogen peroxide per minute.
Serum malondialdehyde concentrations, also known as thiobarbituric acid reactive substances (TBARS), were determined colorimetrically using the method of Buege and Aust (1978). In brief, 0.1 mL of serum was treated with 2 mL of TBA–TCA–HCl reagent (thiobarbituric acid 0.37%, 0.25 N HCl and 15% TCA) and placed in water bath for 15 min. After cooling, the flocculent precipitate was removed by centrifugation at 112 g for 10 min. The absorbance of supernatant was measured against reference blank at 535 nm. Concentration was calculated using an extinction coefficient of 1.56 × 105 M−1 cm−1 and expressed as nmol L−1.
Statistical analysis
The data were expressed as the mean ± SD. One-way analysis of variance followed by Tukey's post hoc test was used and differences were considered significant when P < 0.05. All statistical analyses were performed using the SIGMAPLOT11 (SYSTAT Software, Chicago, IL, USA).
Results
Lipid peroxidation
The effects of various doses of diet garlic content on lipid peroxidation, as indicated by serum TBARS levels, are shown in Fig. 1. A significant (P < 0.05) decrease in TBARS concentration in all experimental groups that received 10, 20, 30, 40 or 50 mg kg−1 of dietary garlic in comparison to control group was observed (−22%, −33%, −39%, −21% and −24% respectively). Maximum reduction of lipid peroxidation was observed in fish that was fed 30 g kg−1 dietary garlic followed by the group fed 20 g kg−1, which was significantly lower than fish fed all diets except 30 g kg−1. Fish fed the 10, 40 or 50 mg kg−1 garlic diet had TBARS levels significantly lower and higher, respectively, than the control group and the 20 and 30 g kg−1 diets.
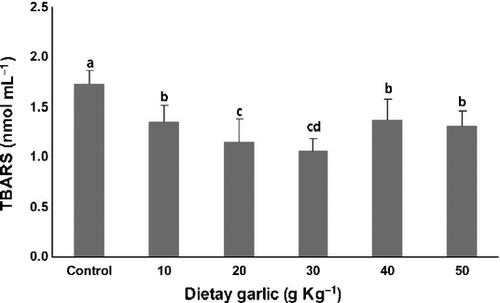
Antioxidant enzymes
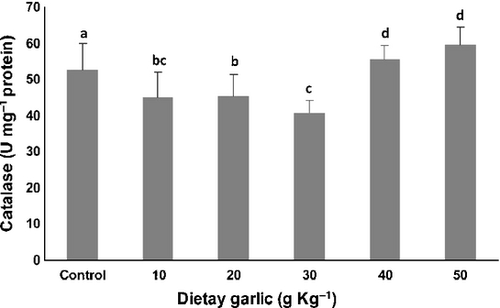
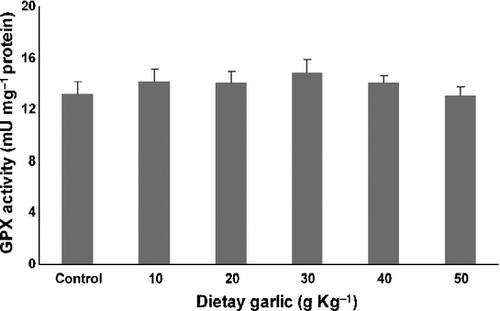
Figures 2-4 show the effects of 8 weeks consumption of different amount of dietary garlic on activities of antioxidant enzymes in serum of rainbow trout.
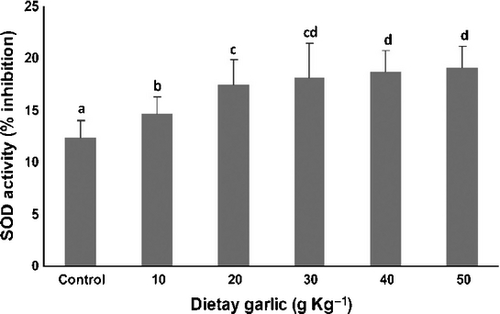
The results showed a significant (P < 0.001) increase in SOD in all of the treated groups. Fish fed the 40 and 50 g kg−1 garlic diets had SOD values that were significantly lower than those fed the other garlic supplemented diets. The 20 g kg−1 garlic diet produced SOD activity similar to the 30 g kg−1 diet, but significantly higher than the 10 mg kg−1 diet.
As shown in Fig. 3, consumption of 10, 20 and 30 g kg−1 dietary garlic resulted in significant reduction (P < 0.001) in the catalase activity compared with fish which received diet containing 40 and 50 g kg−1 garlic and also control group. The 30 g kg−1 garlic diet produced the lowest catalase activity, which was significantly different from the activity in fish fed all but the 10 g kg−1 diet. Fish fed the 40 and 50 mg kg−1 garlic diets had catalase activities that were significantly higher than all the garlic supplemented diets and the control group.
There were no significant changes in GPX activity in any of trout groups.
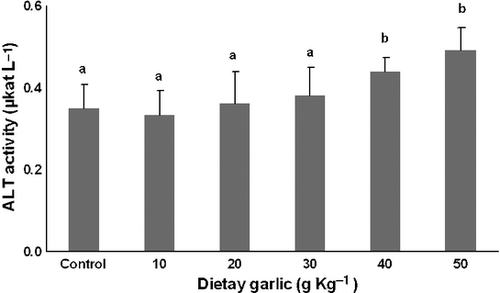
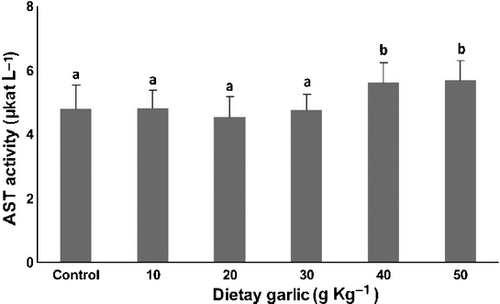
Aminotransferases
The serum activities of ALT and AST for the six groups of fish are shown in Figs 5, 6. AST and ALT activity were significantly (P < 0.05) greater in the groups of fish that received 40 and 50 g kg−1 dietary garlic than other groups. No significant differences in AST and ALT activities occurred among the remaining diets.
Serum lipid profile
The change in lipid profile is demonstrated in Table 2. Serum total cholesterol and triglyceride levels showed a significant (P < 0.05) decrease in trout-fed diets containing 20, 30, 40 and 50 g kg−1 garlic. However, there were no significant differences in serum triglyceride and total cholesterol between fish fed the 20, 30, 40 and 50 g kg−1 garlic diets. Serum triglyceride and total cholesterol were not affected by 10 g kg−1 dietary garlic.
| Control | Dietay garlic (g kg−1) | |||||
|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | ||
| Triglyceride(mg dL−1) | 330.48 ± 5.65a | 321.15 ± 6.42ab | 301.15 ± 6.09b | 290.9 ± 6.55c | 305.85 ± 7.27bc | 288.75 ± 5.51c |
| Total cholesterol (mg dL−1) | 372.35 ± 4.02a | 359.7 ± 4.6ab | 349.5 ± 5.48b | 340.65 ± 5.4bc | 335.65 ± 6.12c | 337 ± 6.95c |
| HDL-C (mg dL−1) | 131.65 ± 6.27a | 137.5 ± 10.34ab | 144.8 ± 6.33ab | 164.25 ± 5.5bc | 183.25 ± 5.64c | 189.45 ± 8.94c |
| LDL-C (mg dL−1) | 174.61 ± 8.17a | 157.97 ± 9.54a | 144.47 ± 9.09ab | 118.22 ± 9.37bc | 91.23 ± 6.03cd | 89.8 ± 10.81cd |
Dietary garlic in 30, 40 and 50 g kg−1 doses resulted in significant (P < 0.05) reduce in serum HDL-C and LDL-C. Feeding 10 and 20 g kg−1 of garlic did not affect the serum concentration of these lipoproteins.
Discussion
During the last decade, it has become increasingly evident that many chronic diseases are accompanied by increased levels of oxidative stress exacerbated by decreased antioxidant levels (Klaunig & Kamendulis 2004; Dalle-Donne, Rossi, Colombo, Giustarini & Milzani 2006). These observations have precipitated much interest in study of the correlations between oxidative stress, antioxidant potential and development of diseases in both humans and animal models (Marnewick, Rautenbach, Venter, Neethling, Blackhurst, Wolmarans & Macharia 2010; Mukherjee, Roy, Bandyopadhyay, Chattopadhyay, Basu, Mitra, Ghosh, Reiter & Bandyopadhyay 2010; Negre-Salvayre, Auge, Ayala, Basaga, Boada, Brenke, Chapple, Cohen, Feher, Grune, Lengyel, Mann, Pamplona, Poli, Portero-Otin, Riahi, Salvayre, Sasson, Serrano, Shamni, Siems, Siow, Wiswedel, Zarkovic & Zarkovic 2010). In this context, several studies have been carried out to investigate the oxidative stress and effects of antioxidants on the different tissues of the fish (Nakano et al. 1999; Nematollahi, Pasmans, Haesebrouck & Decostere 2005; Li et al. 2010). In this regard, assay of circulatory biomarkers of oxidative stress has emerged as a reliable method for screening putative chemopreventive agents (Balasenthil, Arivazhagan & Nagini 2000).
In this study, 8 weeks feeding of rainbow trout with 10–50 g kg−1 dietary garlic caused a significant decreasing in serum TBARS levels compared with control fish. Several studies have reported decreasing effects of garlic in lipid peroxidation in human (Durak, Aytac, Atmaca, Devrim, Avci, Erol & Oral 2004; Jabbari, Argani, Ghorbanihaghjo & Mahdavi 2005; Duda, Suliburska & Pupek-Musialik 2008) and animal models (Hfaiedh, Murat & Elfeki 2010; Nahdi, Hammami, Kouidhi, Chargui, Ben Ammar, Hamdaoui, El May & El May 2010; Vidyashankar, Sambaiah & Srinivasan 2010).Our finding is in agreement with Lee, Gweon, Seo, Im, Kang, Kim and Kim (2009) and Hassan et al. (2010) who reported such effects in the diabetic mice and rats after exposure to sodium nitrite respectively. Kumar, Prasad, Patra, Ranjan, Swarup, Patra and Pal (2009) also have shown ameliorative effect of garlic on lipid peroxidation in freshwater catfish (Clarias batrachus) during exposure to cadmium. Lipid peroxidation has been reported to be a major contributor to the loss of cell function under oxidative stress (Pichaud, Pellerin, Fournier, Gauthier-Clerc, Rioux & Pelletier 2008; Toni, De Menezes, Loro, Clasen, Cattaneo, Santi, Pretto, Zanella & Leitemperger 2010). Lipid peroxidation cause profound alterations in the structural organization and functions of the cell membrane including decreased membrane fluidity, increased membrane permeability, inactivation of membrane-bound enzymes and loss of essential fatty acids. Peroxidation of lipids is particularly important for aquatic animals, as these species body normally contain greater amounts of highly unsaturated fatty acids than other species (Li et al. 2010). Certain pollutants, such as polychlorinated biphenyl, paraquat (methyl viologen) and heavy metals induce lipid peroxidation in fish tissues (Nakano et al. 1999). Furthermore, some fish diseases, such as muscular dystrophy, haemolysis and jaundice are known to be closely related to lipid peroxidation and thought to be catalysed by oxidative damage to tissue (Tanaka, Higo, Shibata, Suzuki, Hatate, Nagayama & Nakamura 2002). In this regard, finding effective and safe antioxidant with minimum undesirable side effects is important in preventing oxidative stress-mediated disease in fish.
Two major classes of antioxidant components are identified in garlic, namely flavonoids and sulphur-containing compounds [diallyl disulphide (DADS) and s-allyl cysteine] (Sharma, Sharma & Kansal 2010). Flavonoids belong to a group of plant-based substances that is known as natural antioxidant. Flavonoids are oxidized by free radicals, resulting in a more stable, less-reactive radical (Nijveldt, Van Nood, Van Hoorn, Boelens, Van Norren & Van Leeuwen 2001). The main flavonoids in garlic are nobiletin, tangeretin and rutin (Berginc, Milisav & Kristl 2010). S-allyl cysteine is known as a suppressor of the formation of superoxides, whereas DADS scavenges hydroxyl radicals (Chung 2006).
Based on our findings, dietary garlic decreased serum triglyceride, total cholesterol and LDL-cholesterol, whereas the serum HDL-cholesterol was increased in a dose-dependent manner. There is a general belief that garlic is an effective and harmless mode of lowering cholesterol. This effect has not been linked to inhibition of HMG CoA reductase, regulatory enzyme in cholesterol biosynthesis, and is presumably due to a different mechanism of action (Peleg, Hershcovici, Lipa, Anbar, Redler & Beigel 2003). Dietary garlic may exert its hypolipidaemic effect through reducing intestinal microsomal triglyceride transfer protein gene expression, thus suppressing the assembly and secretion of chylomicrons from intestine to the blood circulation (Lin, Wang, Lee, Chin, Liu, Chiu & Kung 2002).On the other hand, it was reported that most of serum lipid peroxides are in association with LDL-cholesterol (Nourooz-Zadeh, Tajaddini-Sarmadi, Ling & Wolff 1996; Nakano et al. 1999). Thus, it seems that in addition to direct reducing effects of dietary garlic on serum lipid peroxides, decreasing of LDL-cholesterol, lipid peroxidation main substrate, may lead to decreased serum levels of lipid peroxides.
Among the enzymes involved in antioxidative defence, particularly well studied are the antioxidative properties of the SOD, GPX and catalase (Karbownik & Reiter 2000). SOD converts superoxide anions into H2O2, which is then further degraded into H2O by catalase or GPX. Catalase is also responsible for the reduction of hydrogen peroxide produced from the metabolism of long chain fatty acids in peroxisomes; however, GPX catalyses the reduction of both hydrogen peroxide and lipid peroxide (Li et al. 2010). Our finding revealed that dietary garlic affects serum activity of SOD and catalase in different ways. SOD activity showed a dose-dependent increase, whereas serum catalase activity was decreased in fish that received 10, 20 or 30 g kg−1 of dietary garlic compared with the control group. However, the GPX activity was not significantly different among six dietary groups. There are contradictory data in the literature on the effect of garlic on the antioxidant enzymes systems in different tissues, cells and species. For example, Banerjee, Maulik, Mancahanda, Dinda, Gupta and Maulik (2002) found increased SOD and decreased GPX activities in rat heart tissue by administration of garlic. Chen, Tsai, Yang, Liu, Kuo and Lii (2003) also reported increased SOD and decreased GPX activities in hepatic tissues from rats fed a garlic oil rich diet. However, Durak et al. (2004) have reported that ingestion of garlic extract caused no increases in the serum activities of SOD and catalase and GSH levels in atherosclerotic patients. In another study, Lee et al. (2009) observed increasing effects of garlic in SOD activity accompanied by an increase in GPX activity. Meanwhile, several studies have reported presence of enzyme SOD in garlic (He, Li, Sun & Ling 2008). However, it is not clear whether serum activity of SOD could be affected directly by the garlic enzyme content. Excessive amount of hydrogen peroxide in cells induces an elevation in antioxidant enzymes activity particularly catalase and GPX, which are contributed to an adoptive response of organism to oxidative stress. In contrast, decreasing the hydrogen peroxide and severity of oxidative stress may result in the reduction of antioxidant enzyme activity (Hussain, Amstad, He, Robles, Lupold, Kaneko, Ichimiya, Sengupta, Mechanic, Okamura, Hofseth, Moake, Nagashima, Forrester & Harris 2004). The observed decrease in serum catalase activity in the present study agrees with the findings of Pedraza-Chaverri, Granados-Silvestre, Medina-Campos, Maldonado, Olivares-Corichi and Ibarra-Rubio (2001) who suggested that the reduced catalase expression in garlic fed rats was mediated by post-transcriptional changes, which could represent an adaptation to the low hydrogen peroxide due to a direct antioxidant effect of garlic. In this regard, our data showed an increase in catalase activity with a corresponding increase in serum TBARS in fish that received 40 or 50 g kg−1 dietary garlic.
The relative increase of TBARS and catalase levels was accompanied by increased activity of serum AST and ALT. These aminotransferase enzymes are known as indicators of liver injury (Nakano et al. 1999). Based on these findings, it seems that dietary garlic in doses of 40 and 50g kg−1 diet may have cytotoxic effects on liver cells of the trout in association with increased oxidative stress. In another study (Joseph, Rao & Sundaresh 1989), rats fed with a garlic extract of 200 g L−1 in drinking water for 10 days showed a significant rise in hepatic enzyme activities and focal injury of hepatocytes, with inflammatory cells infiltration. Banerjee, Maulik, Manchanda, Dinda, Das and Maulik (2001) have reported toxic effects of high doses of garlic (500 and1000 mg kg−1 day−1) in kidney and liver of rats associated with histopathological signs of cell injury and elevated levels of TBARS in those organs. These undesirable effects of garlic may be attributed to allicin and other oil-soluble organosulphur constituents (Amagase, Petesch, Matsuura, Kasuga & Itakura 2001). Intact garlic cloves do not contain allicin, but only its odourless precursor alliin. Alliin is converted to allicin by alliinase that is enclosed in compartments within the garlic clove cells. Allicin reacts rapidly with free thiol groups and penetrates biological membranes with ease (Miron, Rabinkov, Mirelman, Wilchek & Weiner 2000; Amagase et al. 2001). Based on in vitro studies, cytotoxic effects of allicin are attributed to its ability to react with thiol groups to suppress formation and polymerization of tubulin in cells (Prager-Khoutorsky, Goncharov, Rabinkov, Mirelman, Geiger & Bershadsky 2007).
Conclusion
Our results demonstrated that ingestion of garlic decreases the oxidative stress by reducing lipid peroxidation levels in rainbow trout. The observed decrease in circulatory lipid peroxides levels may be a reflection of beneficial effects of garlic to improve antioxidant status of different tissues of the rainbow trout. However, high levels of garlic supplementation in diets of trout may have negative physiological consequences.
Acknowledgment
This research was funded by Shahrekord University, Shahrekord and Isfahan University of Technology, Isfahan, Iran.




