Aflatoxin B1 induced alterations in the stability of the lysosomal membrane in Oreochromis mossambicus (Peters, 1852)
Abstract
This article elucidates the effect of crude dietary aflatoxin B1 (AFB1) on the stability of the lysosomal membrane in tilapia (Oreochromis mossambicus), and the analysis was made at the biochemical and cellular level. The liver lysosomal acid phosphatase activity was used to evaluate the functional integrity of the lysosome. Three experimental groups were fed on diets containing 0.375, 2.5 and 6 mg kg−1 of AFB1 for a time period of 6 weeks, whereas a fourth group fed on the semi-purified diet formed the control. Significant changes were observed in the stability of the lysosomal membrane in aflatoxin exposed groups. This is the first report, so far, of changes induced by AFB1 on the stability of lysosomal membrane in tilapia (O. mossambicus).
Introduction
Aflatoxins are toxic metabolites produced by certain fungi Aspergillus flavus and Aspergillus parasiticus, which generally gain entry into a variety of feeds and feed stuffs when stored improperly at high temperatures and moisture conditions (Diener & Davis 1969). Tilapias are very hardy fish, resistant to diseases, and tolerant to overcrowded production conditions and various environmental stressors (Alceste 2000a). Intensive culture of tilapia has gained popularity, and nutritionally complete feeds have become a necessity, when fish are stocked at high densities in tanks, raceways, net peps and ponds, and natural feeds is absent or insignificant (Alceste 2000b). Many fish species currently cultured have a high dietary protein requirement (30–50%), which vary for each species and with each particular life stage (Alceste 2000c). In tilapia culture, the most commonly used protein sources include ingredients like fishmeal, soybean meal, cottonseed meal, cornmeal and wheat (Alceste 2000b). Aflatoxins can contaminate animal feeds before harvest, during harvest and also when developing crops are exposed to drought conditions. The presence of aflatoxin residues or toxic metabolites in animal tissues can end up in food for human use (Haumann 1995). Nearly all of the interest in aflatoxins has focused on aflatoxin B1 (AFB1), primarily due to its extreme acute and chronic toxicity and its carcinogenic activity in animals, in addition to its potential effects in humans (Sharma & Salunkhe 1991). The carcinogenic effect of aflatoxins has been studied in different species of fish, such as salmonids, rainbow trout, channel catfish, guppy, Nile tilapia and Indian major carps (Jantrarotai & Lovell 1990; Lovell 1992; Tacon 1992; Chavez-Sanchez, Martinez Palacios & Osorio Mareno 1994; Murjani 2003). Several studies on the effects of aflatoxin on rat liver lysosome have been undertaken and reported (Balasubramaniam, Wijesundera, Arseculeratne & Tennekoon 1969; Pokrovsky, Kravchenko & Tutelyan 1972; Pitout & Schabort 1973 and Toskulkao & Glinsukon 1990). Earlier reports on the effects of aflatoxin on the lysosomal membrane in fish are not available and therefore this study was undertaken to investigate the effects of AFB1 on the lysosomal membrane of the liver in tilapia (Oreochromis mossambicus).
Materials and methods
Test animals and their maintenance
A total of 72 fingerlings of tilapia, O. mossambicus of size 10 ± 3 g, were collected from the culture ponds of Rice Research Institute, Vytilla, Kerala. Prior to the experiment, the fish were acclimatized to the fresh water condition in the laboratory for 2 weeks in polypropylene tanks. Continuous aeration was provided to all the tanks. Feeding was carried out twice daily at the rate of 2% of the body weight. Water temperature, pH and dissolved oxygen were recorded daily. Water carbon dioxide, ammonia-N, nitrite-N, nitrate-N and salinity were recorded once a week following the standard method of APHA 1998.
Preparation of aflatoxin B1
In this study, AFB1 was produced by A. flavus (MTCC No: 277) in the laboratory using polished raw rice as substrate for growth, and Czapek Yeast Extract Agar as growth medium. The cultured filtrates were extracted with chloroform, decanted through anhydrous sodium sulphate, and evaporated to dryness. The dried aflatoxin extract was dissolved in 15 mL of chloroform and preserved under refrigeration at 4°C in an amber-coloured bottle sealed with teflon tape until qualitative analysis using thin-layer silica gel chromatography to confirm the presence or absence of AFB1 (Reddy, Reddy, Kumar, Laha & Muralidharan 2004). Aflatoxin B1 thus obtained was quantified using the Velasco Fluorotoxinmeter (this facility was availed at Veterinary College, Kerala Agricultural University, Mannuthy). Before addition of toxin to experimental feeds, the required amount of toxin dissolved in chloroform was taken in a glass beaker, evaporated in a water bath, and replaced with equal volumes of ethanol.
Feed formulation and preparation
Feed formulation was carried out as suggested by Jauncey and Ross 1982 for fresh water tilapia with minor modifications. The composition of the control diet is given in Table 1.
| Ingredients | Percentage of diet (g kg−1) |
|---|---|
| Fish meal | 350 |
| Soybean flour | 250 |
| Coconut oil cake | 100 |
| Tapioca starch | 200 |
| Gelatin | 30 |
| Vegetable oil | 20 |
| Fish oil | 20 |
| Mineral premixa | 10 |
| Vitamin premix b | 20 |
- a Mineral mixture (mg kg−1) (Ossopan tablets): Each film-coated tablet contains: microcrystalline hydroxyapatite complex (MCHC) providing calcium 33 mg and phosphorus 15 mg.
- b Vitamin mixture (mg kg−1): vitamin B1 (10 mg), vitamin B2 (10 mg), vitamin B6 (3 mg), nicotonamide (100 mg), calcium pantothenate (50 mg), folic acid (1500 μg), vitamin B12 (15 μg) and vitamin C (150 μg).
Fish oil and vegetable oil were subjected to the peroxide value test (AOAC 1984) to ensure that there were no oxidized oils in the diets. All the dry ingredients except tapioca flour were powdered, sieved and blended thoroughly. Tapioca flour was gelatinized, cooled and added to the dry mixture to make dough of uniform consistency. The dough so made was extruded through a kitchen noodle maker with a 3-mm die, dried at 45°C overnight and stored in airtight containers. The prepared feed was divided into 500 g each of four types of feed viz. control diet and three experimental diets: 0.375, 2.5 and 6 mg kg−1.
Experimental design
For the experimental trials, six fishes were stocked into each of 12 polypropylene circular tanks containing 40 L of water in a re-circulated water system with a constant inflow of 1 L min−1. One control and three test groups were selected for the experiment of 6 week duration. The trials were carried out in triplicates in the control group and the aflatoxin exposed groups. After a 6-week experimental period, liver samples were collected from the control and aflatoxin exposed groups for studies on the lysosomal membrane and histopathological studies.
Lysosomal lability index
Liver tissue from the control and aflatoxin exposed groups were excised and homogenized separately in 10 volumes of ice-cold isotonic sucrose (0.33 M) in an electric mortar (sucrose fraction). The homogenate was centrifuged at 600 g at 4°C in a refrigerated centrifuge for 10 min. The pellet was dissolved in 10 volumes citrate buffer (nuclear fraction). The supernatant was centrifuged for 30 min at 15 000 g to remove intact lysosome. The supernatant was saved (soluble fraction). Lysosomal pellet was dissolved in 10 volumes citrate buffer containing 0.2% Brij – 35 (lysosomal fraction). Acid phosphatase activity (measured as the amount of p-nitrophenol formed in mg h−1 mg−1 protein) measured in the various fractions at wavelength of 410 nm in a UV-visible spectrophotometer (Hitachi, Ambala Cantt, Haryana, India) was estimated following the method of Colowick and Kaplan 1957. Protein content was determined using the method of Lowry, Rosenberg, Fan and Randall 1951.
Lysosomal enzyme release assay (in vivo studies)
Liver lysosomal fractions from control and the 6-week aflatoxin exposed fishes were washed, centrifuged at 15 000 g for 10 min and dissolved in isotonic sucrose. 0.5 mL of each of these fractions was incubated at room temperature, and 0.1 mL each was withdrawn at various time intervals of 10, 15 and 30 min and stored in the refrigerator at 0°C. Fractions were centrifuged at 15 000 g for 30 min to separate unbroken lysosome, and the acid phosphatase activity (measured as the amount of p-nitrophenol formed in mg h−1 mg−1 protein) in the supernatant was determined as above. Protein content was determined using the method of Lowry et al. 1951.
Histopathological analysis
Liver samples from fishes exposed to a 6-week aflatoxin stress were fixed in 10% formalin. The protocol used to make paraffin wax blocks for histopathological studies was as follows: tissues were dehydrated in ascending grades of alcohol: 50% – 1/2 h; 80% and 90% – 3/4th hour each. Tissues were given two changes in absolute alcohol of 1 h each. Tissues were cleared in xylene until they became translucent. Tissues were transferred to molten paraffin wax for 1 h to remove xylene completely, and then embedded in paraffin wax of melting point 60–62°C. Paraffin blocks were cut in a rotary microtome to prepare sections of thickness 4–6 microns. The tissues were stained using haematoxylin–eosin stain and examined under the light microscope (Humason 1979).
Statistical analysis
For statistical analysis, all data are presented as mean ± SD for six fishes in each group. Comparisons between enzyme activity in the control and different aflatoxin concentrations in the different subcellular fractions of the liver were done using one-way analysis of variance (anova). Effects of the release of acid phosphatase with time and between different aflatoxin concentrations were studied using two-way anova. Subsequent comparisons between different aflatoxin concentrations were done using least significant difference (LSD) analysis.
Results
Sub-cellular activity of acid phosphatase
An overall significant change (P < 0.01) as revealed using one-way anova was seen in the enzyme activity in the nuclear fraction, soluble fraction and lysosomal fraction in the test groups (Table 2). Subsequent comparisons between different concentrations were done by LSD analysis. A significant increase (P < 0.01) was observed in nuclear and soluble acid phosphatase activities in all the three aflatoxin concentrations when compared with the control. A significant decrease was noticed on the other hand in lysosomal acid phosphatase activity of the aflatoxin-exposed groups when compared with the control. Comparisons between the various aflatoxin concentrations revealed no significant difference in the soluble acid phosphatase activity between control and 2.5 mg kg−1, but significant difference (P < 0.01) was noticed between control and the other aflatoxin concentrations and also between concentrations. In the case of nuclear and lysosomal acid phosphatase activity, significant differences (P < 0.01) were noticed between the control and the different aflatoxin concentrations and also between concentrations. The lysosomal acid phosphatase activity in the aflatoxin-dosed groups was lower than that of the control unlike the soluble acid phosphatase activity that recorded an increase. The lysosomal stability index that is the ratio of the lysosomal acid phosphatase activity to the soluble acid phosphatase activity was the least in the highest concentration (6 mg kg−1) of aflatoxin followed by 2.5 and 0.375 mg kg−1. This result reflects the damage inflicted on the lysosomal membrane with increasing concentrations of aflatoxin.
| Fractions | Groups | |||
|---|---|---|---|---|
| Control | 0.375 mg kg−1 | 2.5 mg kg−1 | 6 mg kg−1 | |
| 1 | ||||
| Nuclear | 6.8 ± 0.35 | 13.379 ± 0.28aa | 15.033 ± 1.7ab | 8.166 ± 0.85ac |
| Lysosomal | 12.08 ± 1.29 | 4.01 ± 1.08aa | 6.714 ± 0.53ab | 5.392 ± 0.57ac |
| Soluble | 6.3 ± 0.68 | 4.5 ± 1.4aa | 7.545 ± 1.5 | 9.703 ± 1.49ac |
| Ratio lysosomal/soluble (LSI) | 1.92 | 0.89 | 0.89 | 0.56 |
- Mean ± SD of different aflatoxin concentrations between concentrations having different superscript letters are significantly different (P < 0.01).
- a Mean ± SD of control and different aflatoxin concentrations are significantly different (P < 0.01).
Lysosomal enzyme release assay (in vivo)
There was no significant difference in the release of acid phosphatase with time as revealed using two-way anova (Table 3). A significant difference (P < 0.05) was, however, observed between the different aflatoxin concentrations with respect to the release of acid phosphatase. Subsequent LSD analysis reflected significant differences (P < 0.05) between concentrations, control and 6, 0.375 mg kg−1 and 6, 2.5 mg kg−1 and 6 mg kg−1 in the release of acid phosphatase.
| Time (min) | Control | 0.375 mg kg−1 | 2.5 mg kg−1 | 6 mg kg−1 |
|---|---|---|---|---|
| 0 | 0.521 ± 1.44 (4.17) | 0.53 ± 1.1 (4.24) | 0.904 ± 1.4 (7.23) | 1.261 ± 1.56 (10.09) |
| 15 | 0.698 ± 1.56 (5.58) | 0.733 ± 0.78 (5.86) | 1.222 ± 0.89 (9.78) | 3.915 ± 1.61 (31.32) |
| 30 | 0.741 ± 1.99 (5.93) | 0.764 ± 0.57 (6.11) | 1.247 ± 0.76 (9.982) | 4.271 ± 1.44 (34.17) |
- Values are the mean ± SD.
- Values within brackets represent acid phosphatase release as % of total activity (considering the values of enzyme activity in the sucrose fraction as total).
Histopathological analysis
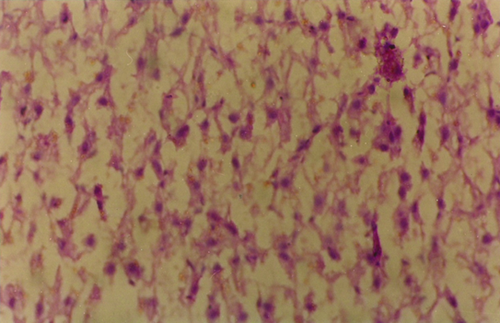
A section of the liver tissue of fish exposed to 2.5 mg kg−1 of AFB1 at 6 weeks showed complete necrosis of the hepatic cells (Fig. 1).
Discussion
Lysosomes are present in the endoplasmic reticulum enclosed by a membrane and enclose several kinds of hydrolases such as acid phosphatase and β-glucuronidase. When the membrane of a lysosome is destabilized by chemical action, resident enzymes are released (Dean 1981). The migration of the enzymes of the lysosomes to the external medium has been used as one of the safest parameters for the evaluation of the physicochemical stability of their membranes (Petrovic, Semencic, Ozretic & Ozretic 2004). The measurement of the integrity of the lysosomal membrane, first established in mammals and then used in marine invertebrate studies (Moore 1988), was transferred to analysis of the liver of different fish species (Kohler 1991; Kohler, Diesemann & Lauritzen 1992). Acid phosphatase is a hydrolytic lysosomal enzyme, and is released by the lysosome during stress induced tissue/cell damage. It is also concerned with the process of transphosphorylation with an important role in the general energetics of the organism (Shoba Rani, Sudharsan, Reddy, Reddy & Raju 2001). Acid phosphatase is very important for tissue reorganization and tissue repair. Intracellularly, acid phosphatase activity is restricted to the lysosome (Verma & Nair 2001). In this study, the lysosomal acid phosphatase activity in the aflatoxin-dosed groups was lower than that of the control unlike the soluble acid phosphatase activity, which recorded an increase. Pokrovsky et al. 1972 in their study on the effect of aflatoxin, one of the most potent hepatotoxic carcinogen and antitumour antibiotics – mitomycin C and rubomycin C on the activities of five rat liver lysosomal enzymes – have also reported similar changes. These results also corroborate that of Pitout and Schabort 1973; who showed that in the in vivo experiment with AFB1, the specific activity of acid DNase in liver lysosomes was markedly decreased in the rats dosed AFB1, whereas the specific activity of the cytoplasmic acid DNases or nonsedimental acid DNase was dramatically increased. As further supporting evidence, Toskulkao and Glinsukon 1990 in their study on hepatic mitochondrial function and lysosomal activity in ethanol-potentiated AFB1 hepatotoxicity have reported that both total and free activities of hepatic lysosomal enzymes (glucuronidase, arylsulfatase and acid phosphatase) were significantly increased in rats at 24–30 h after AFB1 administration. Increased acid phosphatase activity in the testis of aflatoxin-treated mice was reported to be due to an increase in the leakage of the enzyme (Verma & Nair 2001).
Lysosomal enzyme release assay is generally considered as a bioindicator of the presence of pollutants in the ambient environment and organisms (Jayakumar, Jothivel & Paul 2008). The property of lysosomes requiring membrane damage for enzyme expression is usually referred to as lability (Baccino & Zuretti 1975). In this study, the results of lysosomal enzyme release assay, in vivo, indicate that although a significant difference (P < 0.05) was seen between the different aflatoxin concentrations with respect to release of acid phosphatase, the increase in the release of enzyme noticed with time was not statistically significant.
Each cellular organelle has the ability to sense stressful and pathogenic alterations and to initiate local or global responses to stress. This can lead ultimately to adaptation or once a critical threshold of insult or damage has been reached, cell death. When the extrinsic or intrinsic death cascades are induced to full throttle, most organelles of the dying cell manifest biochemical alterations such as partial proteolysis and the permeabilization/rupture of membranes (Syntichaki & Tavernarakis 2002). Lysosomes metaphorically also called the cell's ‘suicide bag’ contain over 80 types of hydrolytic enzymes, including the cathepsin class of nonspecific protease. Leakage of these enzymes into the cytoplasm due to lysosomal membrane injury or rupture has been implicated in necrotic cell death after both heart and brain ischaemic injuries (Adamec, Mohan, Cataldo, Vonsattel & Nixon 2000). The liver is the primary target of aflatoxin action, and becomes infiltrated with fatty deposits when sufficiently high levels of aflatoxin are administered. Breakdown of lysosome and the release of degradative lysosomal enzymes into the surrounding tissues are previously implicated in the hepatic necrosis and haemorrhage, consistently observed with acutely toxic doses of AFB1 (Shank 1981). Histopathological examination of the liver section (Fig. 1) of the fish exposed to 2.5 mg kg−1 of aflatoxin in this study revealed areas of massive necrosis.
It can be concluded from the above results that aflatoxin causes rupture of the lysosomal membrane in tilapia with increasing concentration of the toxin, thereby damaging it. Hence, this study signifies the importance of implementing strict regulations governing the testing of food products for aflatoxin.




