Reproductive performance, survival and development of nauplii and copepodites, sex ratio and adult life expectancy of the harpacticoid copepod, Euterpina acutifrons, fed different microalgal diets
Abstract
A series of laboratory experiments were conducted on the harpacticoid copepod, Euterpina acutifrons, to assess the influence of 10 different microalgal diets (four monoalgal and six mixed algal diets) on several parameters related to its productivity in culture. The four monoalgal diets were the Tahitian strain of Isochrysis sp. (T-Iso), Pavlova salina (Pav), Tetraselmis chuii (Tet) and the diatom Chaetoceros muelleri (Chaet), the five binary diets were T-Iso+Tet, Pav+Tet, T-Iso+Pav, Tet+Chaet and Pav+Chaet, while the tri-algal diet was T-Iso+Tet+Pav. All diets were fed to copepods at 1500 μ gC L−1 and in the case of binary or trialgal diets, carbon concentration was divided equally between the two or three algae offered. Among monoalgal diets, the diatom Chaet was excellent for E. acutifrons. Out of the 10 diets tested, the binary diet Tet+Chaet, which contained the diatom Chaet, was the best for naupliar production of single pair E. acutifrons (19.5±1.7 nauplii female−1 day−1), significantly (P<0.05) higher than all other treatments except for the Chaet treatment (P>0.05). Similarly, in the group naupliar production trial (50 adult E. acutifrons per replicate), Tet+Chaet produced a significantly higher number of nauplii (405.8±18.6 nauplii day−1) than the other treatments (P<0.05). Tet+Chaet further supported the highest naupliar survival (82.0±2.8%) and copepodite survival (89.0±2.8%), while the mono-algal diet Chaet produced the second highest naupliar (76.7±2.6%) and copepodite survival (83.5±2.6%). In contrast, Pav produced the lowest overall survival at the naupliar stage (30.0±2.9%), significantly lower than all other treatments (P<0.05). While development from newly hatched nauplii to copepodites was not significantly affected by diets, mean development time from nauplius to adult was significantly different among treatments. Mean development time from hatching (naupliar I stage; NI) to the adult stage was the fastest with Tet+Chaet and Chaet (6.8±0.0 days for both treatments), which was significantly faster than that of Pav, T-Iso Pav+Tet and T-Iso+Pav+Te treatment (P<0.05). E. acutifrons sex ratio was significantly affected by diets, and always skewed in favour of males. Feeding on Pav resulted in the lowest proportion of females (23.7±1.2%), significantly lower than for six of the other treatments (P<0.05). Adult females had longer average life expectancy than males for all treatments, and were the longest when fed Tet+Chaet (9.5±0.4 days), which was more than twice as long as the shortest lifespan recorded for the Pav treatment (4.2±0.6 days) (P<0.05). In summary, among 10 diets tested, the binary diet Tet+Chaet appeared to support the highest culture productivity of E. acutifrons while the diatom Chaet also performed well as a monoalgal diet.
Introduction
Copepods are the natural food items for the early larvae of many marine fish (Houde 1973; McKinnon & Duggan 2001; Payne & Rippingale 2001) and have been proposed as suitable alternatives for larvae that do not perform well on traditional hatchery live feeds, such as rotifers and Artemia nauplii (Lee, Park, Lee & Kang 2006; Buttino, Ianora, Buono, Vitello, Sansone & Miralto 2009). Copepods as larval diets typically provide better survival, growth and improved stress resistance to cultured larvae when compared to commonly used live prey (Kraul, Ako, Nelson, Brittain & Ogasawara 1992; Hernandez Molejon & Alvarez-Lajonchere 2003; Chen, Sheng, Lin, Gao & Lv 2006; Peck & Holste 2006). Such superior performances have been attributed to their higher nutritional value compared with rotifers or Artemia (e.g. Teshima, Kanazawa, Horinouchi, Yamasaki & Hirata 1987; Støttrup 2000; Hernandez Molejon & Alvarez-Lajonchere 2003; van der Meeren, Olsen, Hamre & Fyhn 2008), and their ability to supply digestive enzymes benefiting the larvae that ingest them (e.g. Munilla-Morana, Stark & Barbourb 1990).
To date, more than 10 000 copepod species have been identified and this number could easily double in the near future (Huys & Boxshall 1991). Owing to their high species diversity and multiple developmental stages, copepods offer a great variety of size ranges, habitat types, swimming patterns and biochemical contents, which make it likely that some of them would meet the nutritional demands of a particular fish larvae (Shansudin, Yusof & Shukri 1997). Yet, despite all their advantages, until now, copepods are only used sporadically in aquaculture hatcheries. This could partly be attributed to the fact that relatively few copepod species have been studied with the clear objective of utilizing them as live prey (Marcus & Murray 2001; Lee, O'Bryen & Marcus 2005). More research is therefore needed to assess the culture potential of thus far un-investigated copepod species, as they represent a great untapped pool for alternative live prey for today's aquaculture.
Harpacticoid copepods are generally small (predominantly <1mm), and are among the most abundant and species-rich (well over 3000 species) invertebrate groups (Huys, Gee, Moore & Hamond 1996; Ólafsson, Ingolfsson & Steinarsdottir 2001). Harpacticoids possess different biological traits as compared with calanoids (Cutts 2001), such as the capacity to sustain higher stocking densities (Kahan 1992; Ohman & Hirche 2001; The Big Fish Bang 2003) and higher tolerances to environmental fluctuations (Olivotto, Capriotti, Buttino, Avella, Vitiello, Maradonna & Carnevali 2008), which make them good potential candidates for aquaculture. Furthermore, harpacticoid copepods consume a wide variety of food sources (Souza-Santos, Santos & Castel 1999; De Troch, Chepurnov, Gheerardyn, Vanreusel & Ólafsson 2006; Wyckmans, Chepurnov, Vanreusel & De Troch 2007) and have been shown to possess the ability to synthesize several important essential fatty acids de novo (Cutts 2001). As a result of their benthic/epibenthic feeding habit, they are also known to keep relatively better tank hygiene (Olivotto et al. 2008).
Most harpacticoid species are benthic or epibenthic and are associated with benthic meiofaunal communities. Euterpina acutifrons is the sole species of the Euterpinidae family and is considered an atypical harpacticoid as it is distributed in the plankton community. It is an egg-carrying species that has a cosmopolitan distribution in shallow coastal waters worldwide over wide range of temperature and salinity conditions (Haq 1972; Boxshall & Halsey 2004). Moreover, movement patterns of E. acutifrons are semi-continuous, which was suggested to stimulate strong foraging behaviour in cultured larvae (Titelman & Kiørboe 2003).
Most of past studies on E. acutifrons were field studies, including population dynamics (Sciandra 1986; Carlotti & Sciandra 1989; Ara 2001) and insitu feeding trials (Guisande, Riveiro & Maneiro 2000; Díaz, Cotano & Villate 2003). As microalgal diets have been shown to play a crucial role in copepod culture productivity (Milione & Zeng 2007; Camus, Zeng & McKinnon 2009), the present study was conducted to investigate the effects of 10 single or mixed microalgal diets on reproductive performance, survival and development of nauplii and copepodites, sex ratio and adult lifespan of E. acutifrons.
Materials and methods
Algal culture
Microalgal species used in all trials are commonly used in aquaculture hatcheries and were all inoculated from starter cultures supplied by the Commonwealth Scientific and Industrial Research Organization (CSIRO) Microalgae Supply Service, Hobart, Tas., Australia. Algal species, including the Tahitian strain of Isochrysis sp. (T-Iso; CS-177), Tetraselmis chuii (Tet; CS-26), Pavlova salina (Pav; CS-49) and the diatom Chaetoceros muelleri (Chaet; CS-176) were cultured in a temperature-controlled room, using 20 L polycarbonate carboys filled with 1 μM filtered, autoclaved and UV-sterilized seawater (salinity 30±1). All microalgae species were cultured using f/2 medium (Guillard & Ryther 1962), with addition of silicates for the diatom C. muelleri. Room temperature was maintained at 25±1 °C and vigorous aeration (0.1 μM filtered air) was provided to all algal cultures. Photoperiod was set at light:dark cycle of 12 h:12 h. Microalgae were fed to copepods during their exponential growth phase, which started after 20–25 h after they were inoculated from stocks.
E. acutifrons stock culture
E. acutifrons used in present experiments were initially obtained from a plankton tow performed at the mouth of the Ross River in Townsville, Northern Qld, Australia, on 5 December 2008. Immediately after collection, plankton samples were transported back to a laboratory at James Cook University where E. acutifrons were isolated from the rest of the zooplankton and gradually scaled up over a month to four 20 L carboys filled with 1 μm filtered seawater and gently aerated. Salinity was 30±1 and the culture temperature was maintained at 27±1 °C. Light intensity was about 700 lx and the photoperiod was set as light:dark cycle of 12 h:12 h. About 20% of the culture water was exchanged daily using a siphon with a 22 μM mesh attached to the end to prevent removal of copepods. E. acutifrons were fed daily with a mixture of three microalgae: the Tahitian strain of Isochrysis sp. (Isochrysis galbana; T-Iso), T. chuii (Tet) and P. salina (Pav) at an equal ratio of biomass (i.e. T-Iso: Tet: Pav=1:1:1). Carboys containing copepod stock cultures were completely drained approximately every 10 days to remove detritus, while copepods were retained on a 150 μM sieve. Carboys were then cleaned and sterilized with chlorine before cultures were restarted with fresh food and seawater.
Experimental design and setup
A series of experiments was carried out to assess the influence of various microalgal diets on several important parameters related to E. acutifrons culture productivity, i.e. daily nauplii production, both by a single female or by a group of 50 adults; survival and development of nauplii and copepodites; time of first female sexual maturity and complete population sexual maturity; adult sex ratio and life expectancy.
Ten experimental microalgal diets were used in these trials, which include four monoalgal diets and six mixed diets (five binary and one tri-algal diets). Details of diet treatments were as follows:
-
Diet 1: I. galbana (T-Iso) (5±0.8 μM, Prymnesiophyceae)
-
Diet 2: T. chuii (Tet) (13±2 μM, Prasinophyceae)
-
Diet 3: P. salina (Pav) (4.5±0.7 μM, Prymnesiophyceae)
-
Diet 4: C. muelleri (Chaet) (7±1.3 μM Bacillariophyceae)
-
Diet 5: I. galbana+T. chuii (T-Iso+Tet) (1:1)
-
Diet 6: P. salina+T. chuii (Pav+Tet) (1:1)
-
Diet 7: I. galbana+P. salina (T-Iso+Pav) (1:1)
-
Diet 8: T. chuii+C. muelleri (Tet+Chaet) (1:1)
-
Diet 9: P. salina+C. muelleri (Pav+Chaet) (1:1)
-
Diet 10: I. galbana+T. chuii+P. salina (T-Iso+Tet+Pav=1:1:1)
All feeding experiments were carried out under similar conditions as described for the stock cultures (i.e. 27±1 °C; salinity 30±1 and photoperiod L:D=12 h:12 h). Cell concentrations of microalgae were determined daily using a haemocytometer and a compound microscope (Leica CME, North Ryde, NSW, Australia). For all experiments, E. acutifrons were fed with different microalgal diets on an equal biomass basis, which was based on carbon concentrations calculated for each species based on Strathmann (1967). Algae were fed to copepod cultures at a level of 1500 μgC L−1, a carbon concentration known to saturate copepod feeding (Kiørboe, Mohlenberg & Hamburger 1985). When E. acutifrons were fed with a binary or a tri-algal diet, carbon concentration was divided equally between the two or three algae offered based on their carbon equivalency.
Nauplii production
Two separate experiments were conducted to assess E. acutifrons naupliar production when fed 10 microalgal diets. In the first experiment, single pairs of mature female and male were maintained separately for recording nauplii production of individual female. In the second experiment, average naupliar production by groups of 25 adult pairs was recorded for each diet treatment. They were termed ‘individual’ and ‘group’ nauplii production experiment respectively.
Before both experiments, at least three 1 L pre-conditioning culture beakers were set up for each algal diet treatment. Each beaker contained a mixed population of E. acutifrons (i.e. containing all sexes and developmental stages) obtained from the stock cultures with an initial density of approximately 1 individual mL−1. Populations of E. acutifrons inside the pre-conditioning beakers were daily fed in satiated condition (1500 μgC L−1) with a designated microalgal diet for five consecutive days to acclimatize them to the experimental diets.
For the individual nauplii production trial, pairs of sexually mature E. acutifrons were randomly selected from the pre-conditioning beakers following 5 days of acclimatization. Single pairs were gently transferred with a broad mouthed pipette to a 30 mL Petri dish filled with 25 mL of filtered seawater and experimental diet. There were six pairs as replicates for each diet treatment, hence a total of 60 Petri dishes were set up. For the subsequent seven consecutive days, pairs of E. acutifrons were gently transferred into a new Petri dish containing fresh seawater and the treatment diet. Twenty-four-hour nauplii production of the individual female was then determined daily by counting the number of nauplii produced.
For the group nauplii production experiment, after 5 days of pre-conditioning, groups of 50 mature adults were randomly selected from the pre-conditioning beakers and gently introduced into a 600 mL round enclosures sat within a 1 L beaker, which contained designated food. The 600 mL round enclosure had a 100 μM mesh attached to its bottom that allowed newly hatched nauplii to pass through. Each 1 L beaker formed a replicate and three replicates were set up for each diet treatment. Groups of adult E. acutifrons were subsequently cultured for 5 days on designated diets and their daily nauplii production recorded by gently lifting the 600 mL enclosures, which retained all adults, and immediately transferring them to a new set of beakers containing fresh food and seawater. When the enclosures were lifted, newly produced nauplii passed through the bottom mesh of the enclosures and remained inside the original beakers. They were then collected on a 25 μM mesh and counted under a dissecting microscope (Leica CME) to obtain daily naupliar production for each replicate.
Survival, development, sex ratio and adult life expectancy experiment
As for the naupliar production experiments, E. acutifrons were first pre-conditioned to respective microalgal diets for 5 days. The entire copepod population inside each pre-acclimatization beaker was then gently sieved using a 150 μM mesh, this resulted in only adults being retained while all eggs, nauplii and copepodites were removed. Adults retained on the mesh were promptly returned to each beaker, which had been filled with fresh food and water. These adults were then left for 12 h to produce nauplii. After 12 h, newly appeared nauplii (i.e. all nauplii were produced within past 12 h) were isolated from the adult population using a 150 μM sieve. Early hatched nauplii (NI and NII) were then sorted in groups of 100 under a dissecting microscope and introduced into a set of thirty 500 mL beakers with three beakers assigned to one of 10 diet treatments as replicates.
Every day, all replicates from each diet treatment were checked under a dissecting microscope and mortality was recorded while live nauplii were gently transferred using a broad tip pipette to a new Petri dish filled with filtered, sterilized seawater and the designated diet of the particular treatment, This ensures that optimal water quality parameters were maintained throughout the trial. When experimental nauplii started to develop into copepodites, newly appearing copepodites found on each day were transferred to a new Petri dish containing filtered, sterilized seawater and treatment food before being recorded for development data. A new Petri dish was setup each day for newly moulted copepodites found within a given replicate, which allowed precise record on individual development duration of copepodites. All new Petri dishes were labelled with date when copepodite culture started (i.e. the day they became copepodite) as well as diet treatment and replicate number. This continued until all nauplii either died or moulted to copepodites.
Similar procedure as described earlier for nauplii was followed to record copepodite survival and development to adults (confirmed by presence of exuviates in Petri dishes) in all replicates. When copepodites reached the adult stage, their sex was determined and the adults were transferred to a separate Petri dish to continue their culture in order to obtain adult life expectancy data. The experiment lasted until all adults died.
Based on data collected, overall survival for naupliar stage was calculated for each replicate by dividing the total number of nauplii that moulted successfully to copepodites by the number of nauplii initially introduced. Copepodite survival was calculated the similar way.
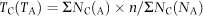
Timing of first female sexual maturity, as well as total population maturity was also determined. First sexual maturity was defined as the first appearance of a gravid female within a replicate, while population maturity was determined by the time when no free swimming copepodite was observed and all females within the population were gravid (Rhyne, Ohs & Stenn 2009).
Adult life expectancy of males and females was calculated separately by averaging within a given replicate the lifespans of males and females. The day when a C-5 copepodite moulted to become an adult (C-6) was considered as Day 0 of its adult phase. Mean adult life expectancy for males and females in a given diet treatment was then obtained by averaging respective data for males and females from all replicates.
Statistics
Sex ratio data was analysed as percentage of females. Data from all trials were analysed using one-way anova. Data were first verified to meet the parametric test assumptions (i.e. normally distributed, homogeneity of variance, independent and randomness of the data) and all percentage data were arcsine-transformed before analysis. When significant differences (P<0.05) were found among treatments by the anova analysis, Tukey's multiple comparisons test was used to test for specific differences among treatments. All statistical analyses were applied to data using Statistica, version 7. Data are presented as mean±standard error (SE).
Results
Nauplii production
Microalgal diets significantly (P<0.001) affected daily nauplii production of E. acutifrons in both the ‘individual’ and ‘group’ nauplii production experiment (1, 2). The copepods feeding on the binary diet Tet+Chaet produced significantly (P<0.05) higher numbers of nauplii than the copepods on the remaining diets, both as single pairs (19.5±1.7 nauplii day−1) and as groups of 25 pairs of adults (405.8±18.6 nauplii day−1) with the only exception that no significant difference was detected for the Chaet treatment in the ‘individual’ trial (13.5±1.1 nauplii day−1) (P>0.05) (1, 2).
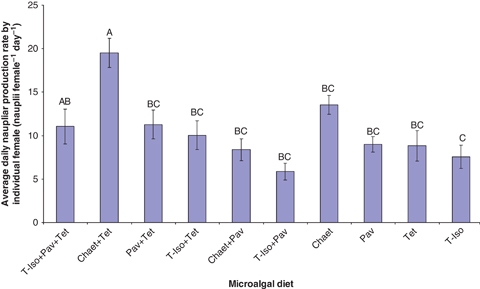
Mean nauplii production of paired adult Euterpina acutifrons fed different microalgal diets. Data are presented as mean±standard error. Different letters above the bars indicate significant differences (P<0.05).
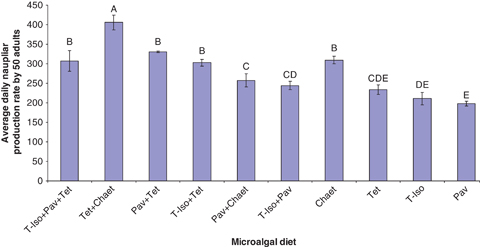
Mean nauplii production of 50 adult Euterpina acutifrons fed different microalgal diets. Data are presented as mean±standard error. Different letters above the bars indicate significant differences (P<0.05).
Conversely, pairs feeding on T-Iso+Pav produced the lowest number of nauplii for the ‘individual’ experiment (5.9±1.0 nauplii day−1), which was significantly (P<0.05) lower than the naupliar production of pairs feeding on Tet+Chaet and Chaet treatments (Fig. 1) but not significantly different from the rest of treatments (P>0.05) (Fig. 1). In the ‘group’ trial, naupliar production of E. acutifrons fed monoalgal diet Chaet (309.7±9.7 nauplii day−1), the binary diets Pav+Tet (330.6±2.4 nauplii day−1) and T-Iso+Tet (302.6±9.1 nauplii day−1), as well as the tri-algal diet T-Iso+Pav+Tet (307.1±26.8 nauplii day−1) were not significant different from each another (P>0.05) but they were all significantly lower than the naupliar production of the Tet+Chaet treatment (Fig. 2). The least productive diets for the ‘group’ trial were Pav (198.2±6.3 nauplii day−1), T-Iso (210.8±15.8 nauplii day−1) and Tet (234.1±12.2 nauplii day−1) and they were not significantly different from each other (P>0.05) (Fig. 2).
Survival and development of nauplii and copepodites
Microalgal diets significantly influenced survival of both nauplii (P<0.001) and copepodites of E. acutifrons (P<0.001) (Table 1). Again, Tet+Chaet supported the highest naupliar (82.0±2.8%) and copepodite survival (89.0±2.8%). However, for naupliar survival, it was not significantly different from the Chaet (76.7±2.6%) and Tet (70.1±0.9%) treatment (P>0.05) while for copepodite survival, it was not significantly different from Chaet (83.5±2.6%), T-Iso+Tet (81.8±4.1%), Tet (76.7±2.4%) and T-Iso+Pav (75.5±2.4%) treatments (P>0.05) (Table 1). The tri-algal diet T-Iso+Pav+Tet only produced intermediate survival for both nauplii (61.6±3.9%) and copepodites (59.9±3.9%), which were significantly lower than Tet+Chaet (P<0.05) but not significantly different from most other diets (P>0.05). The lowest naupliar survival (30.0±2.9%) was recorded for Pav and was significantly lower than the remaining diets (P<0.05) (Table 1). The lowest copepodite survival (52.0±2.9%) was found in the Pav treatment, although not significantly different from T-Iso+Pav (75.5±2.4%), Pav+Chaet (67.4±2.9%), T-Iso (61.2±2.8%), T-Iso+Pav+Tet (59.9±3.9%) and Pav+Tet (54.5±6.7%) (P>0.05) (Table 1). Overall, with the exceptions of the T-Iso and T-Iso+Pav+Tet diets, survival at the copepodite stages were generally higher when compared with those observed at the naupliar stages (Table 1).
| Treatment | Overall naupliar survival | Overall copepodite survival |
|---|---|---|
| Chaet+Tet | 82.0±2.8C | 89.0±2.8C |
| Chaet | 76.7±2.6BC | 83.5±2.6BC |
| T-Iso+Pav+Tet | 61.6±3.9AB | 59.9±3.9AB |
| Pav+Tet | 48.7±6.7A | 54.5±6.7A |
| Tet | 70.1±0.9BC | 76.7±2.4ABC |
| T-Iso+Tet | 64.0±4.1AB | 81.8±4.1BC |
| Chaet+Pav | 55.1±2.9A | 67.4±2.9ABC |
| Pav | 30.0±2.9E | 52.0±2.9A |
| T-Iso | 65.5±2.8ABC | 61.2±2.8AB |
| T-Iso+Pav | 55.1±2.4A | 75.5±2.4ABC |
- Different superscripts indicate significant difference between treatments (P<0.05).
Interestingly, while the mean development time from newly hatched nauplii (NI) to copepodite was not significantly influenced by diet (P>0.05), mean development duration from nauplii to adults was significantly affected (P<0.05) (Table 2). The fastest development from nauplii to adults was recorded in both the Tet+Chaet (6.8±0.0 days) and the Chaet treatments (6.8±0.0 days), which was significantly faster than that of T-Iso+Pav+Tet (7.3±0.0 days), Pav+Tet (7.3±0.1 days), T-Iso (7.3±0.1 days) and Pav (7.4±0.1 days) (P<0.05). The longest development time from nauplii to the adults was recorded when E. acutifrons was fed Pav as the sole diet (7.4±0.1 days).
| Treatment | Mean development time from early nauplii to copepodites | Mean development time from early nauplii to adults | Average male life expectancy | Average female life expectancy | Adult sex ratio (% females) | First female maturity | Population maturity |
|---|---|---|---|---|---|---|---|
| Chaet+Tet | 4.9±0.0 | 6.8±0.0CD | 8.6±0.3d | 9.5±0.4d | 39.8±3.3a | Day 6 | Day 8 |
| Chaet | 4.8±0.1 | 6.8±0.0C | 7.4±0.4bcd | 8.4±0.4abd | 42.9±3.1a | Day 6 | Day 9 |
| T-Iso+Pav+Tet | 5.1±0.1 | 7.3±0.0AB | 6.2±0.4ab | 7.2±0.7abc | 38.8±2.1a | Day 7 | Day 9 |
| Pav+Tet | 5.1±0.0 | 7.3±0.1AB | 6.8±0.5abc | 7.0±0.6abc | 41.0±2.9a | Day 7 | Day 9 |
| Tet | 5.0±0.1 | 7.0±0.1ABC | 7.2±0.7bcd | 8.7±0.4bd | 40.7±1.9a | Day 7 | Day 9 |
| T-Iso+Tet | 4.9±0.1 | 7.1±0.0ABCD | 7.8±0.3cd | 9.1±0.3bd | 43.4±1.0a | Day 7 | Day 10 |
| Chaet+Pav | 5.2±0.1 | 7.1±0.1ABCD | 6.1±0.5abc | 7.5±1.1abcd | 32.3±2.4ab | Day 7 | Day 9 |
| Pav | 5.2±0.1 | 7.4±0.1B | 3.5±0.3e | 4.1±0.6c | 23.7±1.2b | Day 8 | Day 10 |
| T-Iso | 5.1±0.1 | 7.3±0.1AB | 5.2±0.3ae | 5.5±0.7ac | 32.1±1.9ab | Day 7 | Day 9 |
| T-Iso+Pav | 5.0±0.1 | 7.2±0.1ABD | 6.2±0.2ab | 7.3±0.5ab | 35.8±1.6ab | Day 7 | Day 9 |
- First female maturation, population maturation time, adult sex ratio and adult life expectancy for both E. acutifrons males and females are also indicated. Durations are indicated in days. Both uppercase and lowercase superscripts indicate significant differences between treatments (P<0.05).
The first female maturity was observed on Day 6 in both Tet+Chaet and Chaet treatments (Table 2). Among all diets tested, population maturity was first observed in the Tet+Chaet treatment after 8 days of culture (Table 2).
Sex ratio, male and female live expectancy
Sex ratios were skewed in favour of males in all treatments (Table 2). Proportions of females ranged from 23.7±1.2% (Pav) to 43.4±1.0% (T-Iso+Tet) (Table 2). Pav treatment produced significantly lower percentage of females than the rest of the treatments (P<0.05) except T-Iso, Pav+Chaet and T-Iso+Pav (P>0.05) (Table 2). No significant differences were found among other treatments (Table 2).
Significant differences (P<0.05) were found in average lifespan of both male and female E. acutifrons fed different microalgal diets. Female life expectancy was maximized when fed Tet+Chaet (9.5±0.4 days), which was significantly longer than T-Iso+Pav, T-Iso+Pav+Tet, Pav+Tet and T-Iso (P<0.05) and more than twice longer than the shortest lifespan recorded for Pav (4.2±0.6 days) (P<0.05) (Table 2). Similarly, average male life expectancy was the longest when fed Tet+Chaet (8.6±0.3 days) and shortest when fed Pav (3.5±0.3 days) and the differences among various diet treatments were often significant (Table 2). Mean life expectancy of females was always longer than males, with differences ranging from 0.3 days (Pav+Tet) to 1.4 days (Pav+Chaet) (Table 2).
Discussion
E. acutifrons is a small (<100 μM for early nauplii and <700 μM for adult) and atypical harpacticoid copepod that is distributed in the plankton community but also often associated with the benthic environment (Boxshall & Halsey 2004). It is hence suitable for the rearing of planktonic fish larvae as well as for early stages of benthic fish species, such as the mudskipper Boleophthalmus pectinirostris (Zhang, Hong, Dai, Zhang & Cai 1989) and the mandarinfish Synchiropus splendidus (Sadovy, Mitcheson & Rasotto 2001). Results of the current study demonstrate that when fed any of the 10 microalgal diets tested in the present experiments, E. acutifrons was able to produce nauplii that successfully developed to the adult stage. Except for the mean development time from newly hatched nauplii to copepodites, all parameters investigated in the current experiments were significantly affected by algal diets. This demonstrates microalgal diets play a crucial role in the culture productivity of E. acutifrons and the need for the laboratory experiments such as the present one to identify suitable diets by screening a wide range of candidate species.
Interestingly, in our previous experiment (Camus et al. 2009), the paracalanid copepod Bestiolina similis was found unable to sustain proper development when offered some of the diets used in present study. In particular, B. similis produced low numbers of eggs, sometimes deformed, when fed diets containing the diatom C. muelleri. Similarly, deleterious effect of diatoms on copepod reproduction have also been reported on other calanoid or planktonic copepod species, such as Temora stylifera (Turner, Ianora, Miralto, Laabir & Esposito 2001), Acartia tonsa (Jones & Flynn 2005), Acartia clausi and Calanus helgolandicus (Miralto, Barone, Romano, Poulet, Ianora, Russo, Buttino, Mazzarella, Laabir, Cabrini & Glacobbe 1999). Yet our present results showed that among the 10 diets tested, the binary Tet+Chaet, containing the diatom C. muelleri, performed best while as the monoalgal diet, the diatom Chaet was the second best for naupliar production as well as naupliar and copepodite survival. Both diets also supported the faster development from nauplii to adults. Superior performance of these two diets suggests that the diatom C. muelleri is an excellent diet for E. acutifrons. This is in agreement with previous studies that showed harpacticoid copepods readily accepted diatoms (e.g. Sellner 1976; Vander Berghe & Bergmans 1981; Decho 1986; Souza-Santos et al. 1999; De Troch et al. 2006; Wyckmans et al. 2007). The present results confirm that harpacticoid copepods can survive and develop on a wider range of food sources as compared with other copepods, such as calanoids or paracalanids (Cutts 2001; Marinho da Costa, Franco, Cacho & Fernandez 2005).
In contrast, while the Pav was found to be a good diet for the rearing of B. similis (Camus et al. 2009), it was proved to be the worst diet for E. acutifrons, responsible for low nauplii production, the lowest naupliar and copepodite survival and the longest development duration to adults, as well as the shortest lifespan for both adult males and females. It was further noticed that when fed solely on Pav, copepodite and adult E. acutifrons were often lethargic with small, narrow bodies and impaired displacement patterns. When Pav was mixed with other algal species (e.g. Pav+Tet or T-Iso+Pav), its insufficiency as the diet for E. acutifrons was still exhibited, although somewhat alleviated with fewer animals showing less severe malformations.
It is generally true that feeding copepods with multiple algal species provides better nutritional balance and therefore improves culture productivity. However, there are exceptions if the combinations are inappropriate for the specie requirements. For example, the tri-algal diet T-Iso+Tet+Pav was found to be the best diet for B. similis (Camus et al. 2009), however, in the case of E. acutifrons, the overall performance of the algal combination was only mediocre. Buttino et al. (2009) also reported that for the calanoid copepod T. stylifera, adding extra algal species to their diets was either useless or even detrimental. Such a clear difference observed between various copepod species justifies the needs for species-specific research approach with regard to their dietary requirements.
Dietary fatty acid composition is known to significantly influence egg reproduction of some copepod species (e.g. Arendt, Jónasdóttir, Hansen & Gärtner 2005). While most past studies have correlated copepod chemical compositions with their microalgal diets (e.g. Delbare, Dhert & Lavens 1996), including a report for harpacticoid copepod Tisbe sp. (Nanton & Castell 1998), others have reported no significant relationship between the two (e.g. Watanabe, Kitajima & Fujita 1983). As copepod species are hugely diverse (Saiz, Calbet & Broglio 2003), it is not surprising that species-specific differences are found (Hernandez Molejon & Alvarez-Lajonchere 2003). In the case of E. acutifrons, Guisande et al. (2000) has demonstrated that the adults of the species were capable of stabilizing their amino acid (AA) profile regardless of their nutritional conditions. However, AA contents in their eggs did vary significantly with different food supply. In a field study, Díaz et al. (2003) also reported that variations in nutritional environments caused noticeable differences in E. acutifrons reproductive performance.
In the present study, female to male sex ratios were observed to be always lower than 1:1 in all diet treatments. This is contrary to the cases of both A. sinjiensis (Camus & Zeng 2008) and B. similis (Camus et al. 2009) as sex ratios of the two species were found strongly skewed toward having more females than males in a population. Another interesting finding of the present study is that diets significantly affected sex ratio of E. acutifrons, which is in contrast to the results of our previous studies on A. sinjiensis and B. similis, in which sex ratio was found to be a rather conservative parameter of the two species that was not significantly affected by a range of dietary and environmental factors, including photoperiod, stocking density and diets (Camus & Zeng 2008; Camus & Zeng 2009; Camus et al. 2009). This again suggests highly species-specific feature in copepod biological traits. Present results also show that average lifespan of male E. acutifrons was consistently shorter than that of females when fed the same diet. Haq (1972) also reported a shorter lifespan of E. acutifrons males and speculate that it could be a result of their lower tolerance to environmental variations than that of the females.
In conclusion, the present study showed that among 10 diets tested, the binary diet Tet+Chaet appeared to support the highest culture productivity of E. acutifrons while the monoalgal Chaet also performed well. In contrast, the performance of the monoalgal diet Pav was the poorest while the tri-algal diet T-Iso+Tet+Pav was also sub-standard. Several major differences of the present study in comparison to previous research suggest that more species-specific studies are needed if we were to fully understand the effects of microalgal diet on culture productivity and biological traits of different copepod species, and to discover more potential candidate species as prey for larval culture.




