Effect of diet on the lipid composition of the commercial clam Donax trunculus (Mollusca: bivalvia): sex-related differences
Abstract
This paper describes the lipid composition of the commercial bivalve Donax trunculus and the differences originated when the animals were fed with two phytoplankton species (Tetraselmis suecica and Chaetoceros sp.) in a hatchery. We also analysed sex-related differences in lipid classes and fatty acid profile. Total lipids were higher in females than in males. Triglycerides and phospholipids were the major lipid components, and the former dominated in females and the latter in males. The main fatty acids in both sexes were 16:0, 20:5n-3 (eicosapentaenoic acid) and 22:6n-3 (docosahexaenoic acid). Females showed higher percentages of saturated and monounsaturated fatty acids and lower levels of polyunsaturated fatty acids than males. Significant differences in total lipid, lipid classes and fatty acid profiles were also found due to diet. Docosahexaenoic acid decreased and total lipids, free fatty acids, arachidonic acid and EPA increased in both sexes. Despite these differences, the condition of the species was maintained and the broodstock even maturated.
Introduction
Donax trunculus, known as wedge shell, is an appreciated commercial bivalve species, which spreads in the West Atlantic coast from Senegal to France, and in the Mediterranean Sea. There are several exhaustive studies about its biology in the North African coast, especially in Algeria, in Morocco and in Tunisia (Ansell, Frenkiel & Mouëza 1980; Bayed 1990; Guillou & Bayed 1991; Dahoui-Ben-Khedher, Aloui-Bejaoui & Le Pennec 2003; Moukrin, El Hamidi, Lagbouri, Kaaya, Zekhnini, Bouhaimi & Narbonne 2004). In France, the population of D. trunculus was described in the Mediterranean and Atlantic coasts (Ansell & Lagardère 1980; Bodoy 1982). The seasonal gonadal cycle was studied in Italy (Badino & Marchionni 1972; Manca Zeichen, Agnesi, Mariani, Maccaroni & Ardizzone 2002) and the growth and reproductive cycle were described in Portugal (Gaspar, Ferreira & Monteiro 1999). In Spain, wedge shell has been little studied despite its gastronomic interest. Only two published studies exist: one of them studied the population structure and growth in the Western Mediterranean (Ramón, Abelló & Richardson 1995); the other one was carried out in Southern Mediterranean and analysed the reproductive cycle and fecundity (Tirado & Salas 1998).
In the last years, Spanish wedge shell population has been overexploited and a sharp decrease has been detected (P. Serván, pers. comm.). The fishing of this clam is not regulated and many people illegally take it from the beach without control. So, its commercial interest together with its population decrease has led to an increase in the interest of developing its culture in hatcheries.
This study is the first one to our knowledge, which focused on adults of D. trunculus. Only Ruíz-Azcona, Rodríguez-Sierra and Martín (1996) carried out several experiments to successfully rear the larvae under different conditions of food and temperature in a hatchery from South-western Spain.
Mollusc reproduction in hatchery is influenced by dietary components of the food given to brood stock, mainly lipids. These lipids are accumulated during gametogenesis and play an important role during embryonic and larval development, affecting larval growth and survival. Many studies have been carried out in this sense during the last years (Gallager, Mann & Sasaki 1986; Soudant, Marty, Moal, Robert, Quere, Lecoz & Samain 1996a, b; Berntsson, Jonsson, Wangberg & Carlsson 1997; Soudant, Van Ryckeghem, Marty, Moal, Samain & Sorgeloos 1999; Leonardos & Lucas 2000; Duinker, Torstensen & Lie 2004; Alkanani, Parrish, Thompson & McKenzie 2007) and even differences between females and males in lipid content and lipid classes have been described (Napolitano & Ackman 1992; Soudant et al. 1996a, b; Pazos, Román, Acosta, Sánchez & Abad 1997; Caers, Coutteau, Cure, Morales, Gajardo & Sorgeloos 1999; Birkely, Grahl-Nielsen & Gulliksen 2003; Duinker et al. 2004). Therefore, the knowledge of the bivalve lipid composition and of the influence of the diet is important to establish the best strategy to develop the culture in hatcheries.
The objectives of this study were (1) to keep adults of D. trunculus in similar gonadal conditions in the hatchery as in the field; (2) to describe the lipid composition, including lipid classes and fatty acid composition of wedge shell adults and its sex-related differences; and (3) to identify possible nutritional deficiencies in the animals kept in the hatchery by studying the influence of phytoplankton lipid characteristics on the lipid composition of D. trunculus.
Material and methods
Sampling
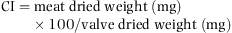
The remaining animals were distributed in triplicates into 300 L plastic tanks containing a 200–400 μm sand bed, in a flow-through circuit at a constant rate of 2 L min−1 with 25 μm filtered seawater. The temperature was kept at 18 °C (water temperature in wild populations was 17.2 ± 0.4 °C). They were fed with a constant mixture of 50% Tetraselmis suecica and 50% Chaetoceros sp. Both algae were added to the sea water. The ration provided was 4% dry weight of algae by meat dry weight per day. The microalgae were cultured in 50 L plastic bags in a temperature-controlled chamber at 20 °C with continuous illumination. The culture medium f2 (Guillard 1975) was used. Three samples of two microalgal species (108 cells) were sampled at exponential phase of culture (7 days) for lipid analyses. They were centrifuged at 1020 g, washed with ammonium formate (3%) and the precipitate was maintained freeze-dried at −20 °C and used for dry weight determination.
Another sampling was carried out after 1 month in hatchery conditions as described above. At the same moment, specimens from the field were also sampled as control.
Histological analysis
For histological analysis, the individuals were fixed in Davidson solution for 48 h then dehydrated in 70% alcohol, embedded in paraffin wax and sectioned with a microtome at 5 μm. Finally, the tissues were stained with haematoxylin and eosin. The histological sections were observed under an optical microscope to identify sex and stage of gametogenic development. The stage assigned to each specimen was according to those established by Gaspar and Monteiro (1998), which included six phases of development: Stage 0, inactive/resting; Stage I, early active; Stage II, late active; Stage III, ripe; Stage IV, partially spawned and Stage V, spent. Whenever two or more stages occurred simultaneously in one single section, the staging criteria decision was based upon the condition of the major proportion of the preparation.
Lipids and fatty acids analysis
The frozen samples for lipids and fatty acids analyses were separated into two subsamples: one was used to obtain the moisture content, by drying them for 24 h in a stove at 110 °C (Horwitz 1980); the other one was used to perform lipid composition analyses. Total lipid was extracted with chloroform: methanol (2:1 v/v) containing 0.01% of butylated hydroxytoluene (BHT) as antioxidant (Christie 1982). The organic solvent was evaporated under a stream of nitrogen and the lipid content determined gravimetrically. Lipid classes were separated by one-dimensional double-development high-performance thin-layer chromatography (HPTLC) using methyl acetate/isopropanol/chloroform/methanol/0.25% (w/v) KCl (25:25:25:10:9 vol.), as the polar solvent system and hexane/diethyl ether/glacial acetic acid (80:20:2 vol.), as the neutral solvent system. Lipid classes were quantified by charring with a copper acetate reagent followed by calibrated scanning densitometry using a CAMAG (Camag Company, Muttenz, Switzerland) TLC Scanner 3 dual wavelength flying spot scanner (Olsen & Henderson 1989). Total lipid extracts were subjected to acid-catalysed transmethylation for 16 h at 50 °C, using 1 mL of toluene and 2 mL of 1% sulphuric acid (v/v) in methanol. The resulting fatty-acid methyl esters (FAME) were purified using thin-layer chromatography (TLC), and visualized with iodine in chloroform:methanol (2:1 v/v) 98% (v/v) containing 0.01% BHT (Christie 1982). Fatty-acid methyl esters were separated and quantified using a Shimadzu GC 2010 gas chromatograph (Shimadzu Corporation, Kyoto, Japan) equipped with a flame ionization detector (280 °C) and a fused silica capillary column Tecnokroma-Suprawax-280™, Tecnokroma Company, Barcelona, Spain (15 m × 0.1 mm I.D.). Helium was used as a carrier gas and the oven initial temperature was 150 °C, followed by an increase at a rate of 90 °C min−1 to a final temperature of 250 °C for 6 min. Individual FAME were identified by reference to authentic standards (C4C24 by Supelco) and to a well-characterized fish oil (Mehaden oil by Supelco, Sigma Aldrich, St. Louis, MO, USA). Dimethylacetals were identified by mass spectrometry in the University of Burgundy. Butylated hydroxytoluene, potassium chloride, potassium bicarbonate and iodine were supplied by Sigma Chemical (St. Louis, MO, USA). Thin-layer chromatography (20 × 20 cm × 0.25 mm) and HPTLC (10 × 10 cm × 0.15 mm) plates, pre-coated with silica gel (without fluorescent indicator) were purchased from Macheren-Nagel (Düren, Germany). All organic solvents for gas chromatography used were of reagent grade and were purchased from Panreac (Barcelona, Spain).
Statistics
One way ANOVA is applied if data complies with normality and homogeneity of variance, if not Kruskal -Wallis is applied. Post hoc Tukey' test is applied in any case after both tests. To all data expressed as percentage, arcsin transformation (Fowler, Cohen & Jarvis 2002) was applied directly. In all statistical tests used, P<0.05 was considered statistically different. A two-factor anova was used to determine the combined effects of diet and sex (Zar 1999).
Results
Condition index and gonadal development
The CI value in the first sampling was 8.72 ± 0.39 and in the second sampling the values were 7.74 ± 0.38 for individuals from the hatchery and 8.42 ± 0.83 for wild individuals. No significant differences were found between CI values either in the first and second sampling or in hatchery and wild groups. The histological study showed that gonadal development was similar in both groups, but it differed in both samplings. In the first sampling, 83.34% of individuals were in stage II and 17.66% in stage III; in separated sexes, in females 87.5% were in stage II and 12.5% in stage III and in males 75% were in stage II and 25% in stage III, whereas in the second sampling all the individuals were ripe (Stage III).
Total lipid and lipid class composition in males and females
The total lipid content (%DW) and the lipid class composition in males and females are presented in Table 1. Total lipid was higher in females than in males. In both sexes, triglycerides (TG) and sterol esters (SE) were the major lipid components in neutral lipids, whereas the principal polar lipids were the phospholipids phosphatidylcholine (PC), phosphatidylethanolamine (PE) and phosphatidylserine (PS) and the free sterols. Significant differences between males and females were detected. The neutral lipids TG and SE doubled male values; they represented, respectively, 25% and 10% of total lipid in females but they both constituted 14% of total lipid in males. Conversely, polar lipids predominated in males and the three major phospholipids were lower in females than in males. The percentages of phosphatidylinositol (PI) and sterol (ST) were similar in both sexes.
| Females | Males | |||||
|---|---|---|---|---|---|---|
| First sampling (n=10) | Hatchery group second sampling (n=10) | Field group second sampling (n=10) | First sampling (n=10) | Hatchery group second sampling (n=10) | Field group second sampling (n=10) | |
| Total lipid | 10.00 ± 1.51 | 11.64 ± 0.81 | 12.44 ± 0.66c | 7.05 ± 0.31a | 10.23 ± 1.36b | 8.27 ± 0.35 |
| LPC | 0.97 ± 0.12 | 0.66 ± 0.13 | 0.56 ± 0.13 | 1.11 ± 0.17 | 1.18 ± 0.23 | 0.98 ± 0.19 |
| PC | 16.36 ± 0.35 | 16.83 ± 0.40 | 18.70 ± 0.46c | 19.25 ± 0.95a | 19.07 ± 0.73 | 17.69 ± 0.53 |
| PS | 8.94 ± 0.38 | 7.98 ± 0.41 | 6.16 ± 0.30c | 14.86 ± 0.94a | 13.21 ± 1.07 | 13.11 ± 0.36 |
| PI | 5.36 ± 0.21 | 5.47 ± 0.32 | 5.28 ± 0.20 | 5.25 ± 0.36 | 5.09 ± 0.43 | 3.83 ± 0.18c |
| PE | 14.91 ± 0.38 | 14.02 ± 0.29 | 14.92 ± 0.29c | 21.25 ± 0.68a | 20.16 ± 0.96 | 21.25 ± 0.76 |
| UKPL | 1.21 ± 0.10 | 2.34 ± 0.32b | 1.27 ± 0.11c | 7.44 ± 0.77a | 7.98 ± 0.65 | 7.52 ± 0.28 |
| ST | 10.55 ± 0.47 | 14.61 ± 1.04b | 10.50 ± 0.32c | 9.47 ± 0.42 | 11.16 ± 0.80 | 11.30 ± 0.48 |
| FFA | 4.46 ± 0.76 | 2.73 ± 0.26b | 2.27 ± 0.16 | 4.56 ± 0.70 | 2.83 ± 0.43b | 2.48 ± 0.85 |
| TG | 25.95 ± 1.05 | 21.88 ± 1.54b | 29.99 ± 0.90c | 9.72 ± 2.10a | 6.60 ± 1.16 | 9.23 ± 0.85 |
| SE | 10.67 ± 0.54 | 12.25 ± 1.07 | 9.95 ± 0.69 | 4.71 ± 1.05a | 10.08 ± 2.25b | 10.83 ± 2.00 |
| UK | 0.16 ± 0.08 | 0.74 ± 0.60 | 0.29 ± 0.12 | 2.40 ± 0.24a | 2.72 ± 0.35 | 1.77 ± 0.19c |
| TPL | 47.74 ± 1.27 | 47.279 ± 1.02 | 46.88 ± 1.13 | 69.15 ± 2.64a | 66.68 ± 2.71 | 64.37 ± 1.79 |
| TNL | 52.10 ± 1.25 | 51.97 ± 1.23 | 52.83 ± 1.08 | 28.45 ± 2.44a | 30.67 ± 2.69 | 33.84 ± 1.71 |
- Values of total lipid are % of total dry weight . Values of lipid classes are mean percentages ± SEM with respect to total lipid. n, number of samples.
- a P<0.05 with respect to females.
- b P<0.05 with respect to first sampling.
- c P<0.05 with respect to hatchery group.
- LPC, lysophosphatidylcholine; PC, phosphatidylcholine; PS, phosphatidylserine; PI, phosphatidylinositol; PE, phosphatidylethanolamine; UKPL, unknown phospholipids; ST, sterol; FFA, free fatty acids; TG, triacylglycerides; SE, sterol ester; UK, unknown; TPL, total polar lipid; TNL, total neutral lipid.
Fatty acid composition in males and females
Donax trunculus male and female gonad fatty acids (% TFA) are shown in Table 2. The most abundant fatty acids in both sexes were the saturated fatty acid 16:0 and the polyunsaturated fatty acid, docosahexaenoic acid (DHA) (22:6n-3); in females, the other dominant fatty acid was the polyunsaturated fatty acid, eicosapentaenoic acid (EPA, 20:5n-3), while in males EPA and the saturated fatty acid 18:0 showed similar values. Total saturated fatty acids (SAFA) showed higher values in females than in males (anova, P<0.001). The three major saturated fatty acids were myristic (14:0), palmitic (16:0) and stearic (18:0), although their levels differed between sexes. Lower percentages of 16:0 and 18:0 (anova, P<0.001) and higher levels of 14:0 (anova, P<0.001) were found in females as compared with males. These differences were more pronounced for 14:0 and 18:0, as the 14:0 level in females tripled the male percentage, whereas the level of 18:0 in males almost doubled that of the females. In addition, two dimethyl acetals DMA 17:0 and DMA 18:0 were detected in both sexes in similar levels. The major monounsaturated fatty acids (MUFA) in both males and females were 16:1n-7, 18:1n-9, 18:1n-7, 20:1n-9 and 20:1n-7. The total MUFA value was markedly higher in females (anova, P<0.001) as the percentages of 16:1n-7, 18:1n-7, 20:1n-9 and 20:1n-7 were almost twice higher than in males.
| Fatty acids (%TFA) | Females (n=10) | Males (n=9) |
|---|---|---|
| 13:0 | 0.53 ± 0.17 | 0.54 ± 0.21 |
| 14:0 | 6.38 ± 0.31 | 2.15 ± 0.28* |
| 15:0 | 0.66 ± 0.03 | 0.76 ± 0.05* |
| 16:0 | 16.31 ± 0.35 | 19.80 ± 0.55* |
| 17:0 DMA | 0.47 ± 0.03 | 0.63 ± 0.05* |
| 17:0 | 0.80 ± 0.02 | 1.10 ± 0.05* |
| 17:0 iso | 0.73 ± 0.02 | 0.70 ± 0.03 |
| 18:0 DMA | 4.93 ± 0.20 | 5.47 ± 0.61 |
| 18:0 | 5.79 ± 0.12 | 8.32 ± 0.31* |
| 20:0 | 0.31 ± 0.02 | 0.18 ± 0.02* |
| 23:0 | 0.80 ± 0.03 | 0.83 ± 0.07 |
| 14:1n-5 | 0.24 ± 0.03 | 0.02 ± 0.01* |
| 14:1 | 0.25 ± 0.03 | 0.44 ± 0.08* |
| 15:1 | 0.62 ± 0.06 | 1.16 ± 0.16* |
| 16:1n-9 | 0.74 ± 0.04 | 0.55 ± 0.04* |
| 16:1n-7 | 5.61 ± 0.22 | 2.61 ± 0.36* |
| 16:1n-5 | 0.64 ± 0.03 | 0.47 ± 0.02* |
| 17:1n-7 | 0.24 ± 0.02 | 0.40 ± 0.17 |
| 18:1n-9 | 2.56 ± 0.14 | 2.67 ± 0.16 |
| 18:1n-7 | 3.97 ± 0.12 | 1.81 ± 0.14* |
| 18:1n-5 | 0.20 ± 0.03 | 0.18 ± 0.06 |
| 20:1n-11 | 0.50 ± 0.02 | 0.37 ± 0.03* |
| 20:1n-9 | 2.04 ± 0.10 | 1.75 ± 0.09* |
| 20:1n-7 | 1.35 ± 0.04 | 0.79 ± 0.02* |
| 18:2n-6 | 1.93 ± 0.40 | 1.91 ± 0.52 |
| 20:2n-6 | 1.74 ± 0.05 | 1.60 ± 0.06 |
| 20:3n-6 | 0.30 ± 0.01 | 0.30 ± 0.03 |
| 20:4n-6 | 2.01 ± 0.08 | 2.63 ± 0.05* |
| 22:4n-6 | 0.64 ± 0.05 | 0.82 ± 0.04* |
| 22:5n-6 | 0.26 ± 0.02 | 0.76 ± 0.25* |
| 16:2n-4 | 0.52 ± 0.06 | 0.42 ± 0.07 |
| 16:3n-4 | 0.39 ± 0.02 | 0.32 ± 0.04 |
| 18:2n-4 | 0.54 ± 0.02 | 0.27 ± 0.04* |
| 18:3n-4 | 0.60 ± 0.06 | 0.27 ± 0.04* |
| 18:3n-3 | 1.17 ± 0.04 | 0.89 ± 0.10* |
| 18:4n-3 | 2.74 ± 0.10 | 1.62 ± 0.16* |
| 20:3n-3 | 0.56 ± 0.02 | 0.25 ± 0.03* |
| 20:4n-3 | 1.56 ± 0.07 | 0.80 ± 0.06* |
| 20:5n-3 | 6.84 ± 0.16 | 7.54 ± 0.19* |
| 21:5n-3 | 0.57 ± 0.03 | 0.80 ± 0.18 |
| 22:5n-3 | 1.27 ± 0.07 | 1.96 ± 0.34 |
| 22:6n-3 | 12.59 ± 0.40 | 16.14 ± 0.53* |
| 22_2i | 1.94 ± 0.06 | 1.31 ± 0.10* |
| 22_2j | 0.73 ± 0.04 | 0.44 ± 0.03* |
| SAT | 32.30 ± 0.41 | 34.37 ± 0.47 |
| MUFA | 18.95 ± 0.36 | 13.21 ± 0.71* |
| PUFA | 38.88 ± 0.60 | 41.06 ± 0.73* |
- Values are mean percentages ± SEM. n, number of samples.
- * P<0.05 with respect to females.
- DMA, dimethylacetal; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SAT, saturated fatty acids.
As shown in Table 2, in both females and males, the three major polyunsaturated fatty acids were EPA, DHA and 20:4n-6 [arachidonic acid (AA)] acids. The percentage of all of them was higher in males than in females, being all these differences significant, whereas the essential fatty acid 18:3n-3 as well as 18:4n-3 and 20:4n3 showed significant higher levels in females. The total polyunsaturated fatty acids (PUFA) was higher in males than in females (anovaP<0.001).
Effect of phytoplankton diet
With the aim of exploring the effect of the controlled diet, the fatty acid composition of males and females was analysed after one month maintained in the hatchery together with diet lipid composition. There was no interaction between sex and diet (two-way anova) and so one-way anova was applied for comparisons.
The changes observed in total lipid and lipid classes in the individuals maintained in the hatchery are shown in Table 1. There were not many significant differences between both samplings. The total lipid increased in both sexes, but it remained higher in females than in males. In both males and females, free fatty acids (FFA) significantly decreased, whereas only in females TG significantly decreased. The lipid classes, which increased after one month in the hatchery were SE in males and ST in females.
When comparing the results from the hatchery with those of the control group, it can be observed that the total lipid, TG and the phospholipids PC and PE in females are higher in the control group, whereas PS and ST are lower. In males, the phospholipid PI is higher in the hatchery.
The fatty acid composition of the phytoplankton species used to feed animals in the hatchery is shown in Table 3. The main fatty acids in T. suecica are 16:0, 18:1n-9 and 18:3n-3 and in Chaetoceros sp. 16:1n-7, EPA and 14:0. In both species, DHA levels are very low; in fact, it does not present in T. suecica and in Chaetoceros sp. it represents only 1.25% of total fatty acid.
| Fatty acids (%TFA) | Tetraselmis suecica (n=3) | Chaetoceros sp. (n=3) |
|---|---|---|
| 14:0 | 0.03 ± 0.50 | 12.33 ± 0.23 |
| 15:0 | 0.38 ± 0.03 | 0.52 ± 0.01 |
| 16:0 | 18.59 ± 0.19 | 9.24 ± 0.11 |
| 17:0 | 2.00 ± 0.04 | 1.30 ± 0.02 |
| 18:0 DMA | 11.36 ± 0.43 | 0.00 ± 0.00 |
| 18:0 | 0.37 ± 0.05 | 0.00 ± 0.00 |
| 20:0 | 0.00 ± 0.00 | 0.15 ± 0.01 |
| 22:0 | 0.00 ± 0.00 | 0.42 ± 0.12 |
| 23:0 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 24:0 | 0.00 ± 0.00 | 0.05 ± 0.09 |
| 14:1 | 0.00 ± 0.00 | 0.19 ± 0.01 |
| 16:1n-9 | 9.07 ± 0.11 | 4.19 ± 0.16 |
| 16:1n-7 | 2.82 ± 0.03 | 25.27 ± 0.29 |
| 16:1n-5 | 0.47 ± 0.31 | 0.64 ± 0.01 |
| 17:1 | 0.81 ± 0.05 | 0.00 ± 0.00 |
| 18:1n-9 | 16.26 ± 0.39 | 0.49 ± 0.02 |
| 18:1n-7 | 3.42 ± 0.15 | 1.15 ± 0.04 |
| 20:1n-11 | 0.17 ± 0.15 | 0.06 ± 0.11 |
| 20:1n-9 | 0.95 ± 0.82 | 0.00 ± 0.00 |
| 22:1n-11 | 0.11 ± 0.12 | 0.00 ± 0.00 |
| 22:1n-9 | 0.62 ± 0.79 | 0.00 ± 0.00 |
| 16:2n-7 | 0.00 ± 0.00 | 6.15 ± 0.19 |
| 18:2n-6 | 4.73 ± 0.09 | 0.37 ± 0.52 |
| 18:3n-6 | 0.24 ± 0.01 | 0.19 ± 0.01 |
| 20:2n-6 | 0.15 ± 0.01 | 0.12 ± 0.01 |
| 20:3n-6 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 20:4n-6 | 0.42 ± 0.03 | 1.18 ± 0.04 |
| 22:2n-6 | 0.15 ± 0.03 | 0.00 ± 0.00 |
| 22:5n-6 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 16:2n-4 | 0.00 ± 0.00 | 4.13 ± 0.08 |
| 16:3n-4 | 0.00 ± 0.00 | 8.66 ± 0.12 |
| 18:3n-4 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 18:3n-3 | 13.26 ± 0.30 | 0.00 ± 0.00 |
| 18:4n-3 | 3.54 ± 0.08 | 0.30 ± 0.00 |
| 20:4n-3 | 0.16 ± 0.01 | 0.00 ± 0.00 |
| 20:5n-3 | 3.28 ± 0.14 | 16.26 ± 0.19 |
| 22:5n-3 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 22:6n-3 | 0.00 ± 0.00 | 1.25 ± 0.06 |
| SAT | 21.64 ± 0.36 | 24.37 ± 0.21 |
| MUFA | 34.69 ± 0.15 | 31.99 ± 0.48 |
| PUFA | 25.92 ± 0.60 | 38.90 ± 0.47 |
- Values are mean percentages ± SEM n, number of samples.
- DMA, dimethylacetal; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SAT, saturated fatty acids.
When fatty acid composition of males and females was compared, it could be observed that the sex-related differences were maintained in the majority of the most important fatty acids, and in both sexes palmitic, EPA and DHA are the main fatty acids. The levels of 14:0, 16:1n-7, 18:1n-7, 20:1n-9, 18:3n-3, 18:4n-3 and 20:4n-3 remained higher in females than in males, whereas the opposite occurred with the values of 16:0, 18:0, 20:4n-6 and 22:6n-3 (one-way anovaP<0.05). In this second sampling, the EPA percentage was higher in females than in males but the difference was not significant. However, some significant differences were found in fatty acid profiles when individuals from different sampling but with the same sex were compared. As shown in 1, 2, the most important changes observed were the lower levels of 16:0, 20:1n-9, 20:2n-6, 18:3n-3, 18:4n-3, 20:4n-3, 22:6n-3 and total PUFA and the higher values of 18:1n-7, 20:4n-6 and 20:5n-3 found in both sexes of the individuals from the second sampling as compared with those from the first one. Furthermore, higher percentages of DMA 18:0 were observed only in males, whereas higher values of MUFA and lower levels of 18:1n-9 and 18:2n-6 were detected in females. The remaining fatty acids showed either no modifications or minor changes. The comparison of fatty acids from hatchery and control group (1, 2) showed that the levels of 16:0, 20:1n-9, 20:2n-6, 18:3n-3, 18:4n-3, 20:4n-3 and 22:6n-3 are lower in the hatchery group and those of DMA 18:0, 18:1n-7, 20:4n-6 and 20:5n-3 are higher, in both sexes (one-way anovaP<0.05). The values of 18:0 and 18:1n-9 were also lower in males and females, respectively, while 16:1n7 showed higher levels in males. The majority of these fatty acids did not change during the experiment in the control group, as it is shown in 1, 2. Hence, the main fatty acids in both sexes are still palmitic and DHA; however in females, EPA is the second most abundant fatty acid while in males it is the stearic. In females, only the values of 20:1n-9, 20:2n-6 and 18:4n-3 were higher in the second sampling, 20:4n-3 diminished in males and the AA and DHA did it in both sexes.
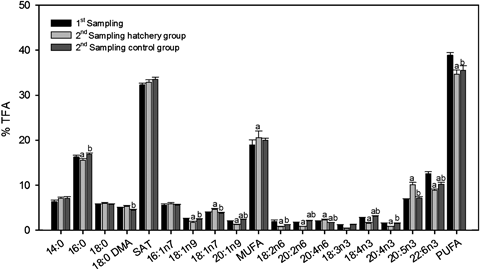
Statistical differences of selected fatty acids composition (% TFA) in Donax trunculus females in the two different samplings: first one, specimens collected from the field and second one specimens from the hatchery 1 month later. Number of samples at the first sampling 9. Number of samples at second sampling 10. Values are means ± SEM. SAT, total saturated fatty acids; MUFA, total monounsaturated fatty acids; PUFA, total polyunsaturated fatty acids. aSignificant differences (P<0.05) with respect to specimens from the first sampling. bSignificant differences (P<0.05) with respect to specimens from the second sampling.
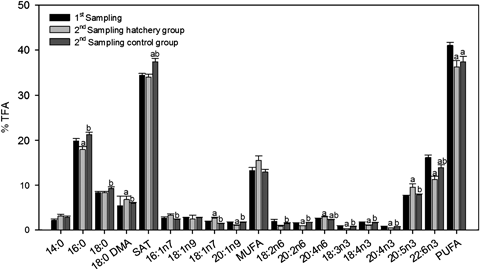
Statistical differences of selected fatty acids composition (% TFA) in Donax trunculus males in the two different samplings: first one, specimens collected from the field and second one specimens from the hatchery 1 month later. Number of samples at the first sampling 10. Number of samples at second sampling: 10. Values are means ± SEM. SAT, total saturated fatty acids; MUFA, total monounsaturated fatty acids; PUFA, total polyunsaturated fatty acids. aSignificant differences (P<0.05) with respect to specimens from the first sampling. bSignificant differences (P<0.05) with respect to specimens from the second sampling.
Discussion
In the current study, we have investigated total lipid, lipid class composition and fatty acid profiles of the species D. trunculus and their possible genre differences. Although during the last years many studies have described the lipid and fatty acid composition of different bivalve species (Gallager et al. 1986; Napolitano, MacDonald, Thompson & Ackman 1992), data about the sex-related differences are scarce.
The lipid content of D. trunculus in the maturation stages was similar to that of the bivalves Mytilus edulis (Zandee, Lluytmans & Zurburg 1980) or Macoma baltica (Jarzebski, Wenne & Habermehl 1986) but were lower than that of the oyster Crassostrea gigas (Soudant et al. 1999) and higher than that of the clam Ruditapes decussatus (Ojea, Pazos, Martínez, Novoa, Sánchez & Abad 2004). In general, although the values differ between species and it is influenced by the reproductive cycle and the environmental conditions, the most abundant lipid classes in bivalve species are the phospholipids PE and PC in the polar fraction and TG in the neutral fraction (Soudant et al. 1999; Freites, Labarta & Fernández-Reiriz 2002; Murphy, Mooney, Nichols & Sinclair 2002; Pazos, Sánchez, Román, Pérez-Perellé & Abad 2003; Duinker et al. 2004; Li, Zhang & Sinclair 2007). In D. trunculus, TG was also the major neutral lipid while in the polar fraction PC was the most abundant lipid. The total lipid content of D. trunculus was higher in females than in males as described for other bivalves as the pectinids Pecten maximus (Soudant et al. 1996a), Argopecten purpuratus (Caers et al. 1999) and Placopecten magellanicus (Napolitano & Ackman 1992). We have also found sex-related differences in the lipid classes of D. trunculus, with the higher level of the three major phospholipids, PC, PS and PE, being the most important in males when compared with females and with the higher levels of TG and SE in females. The high level of TG in females was also described in other bivalves such as the scallops P. magellanicus and P. maximus (Napolitano & Ackman 1992; Soudant et al. 1996b; Pazos et al. 1997; Duinker et al. 2004) and Mya arenaria (Birkely et al. 2003).
Regarding to the fatty acid composition, the predominant fatty acids in D. trunculus were palmitic acid, EPA and DHA, coinciding with other bivalve species (Pazos et al. 1997, 2003; Caers et al. 1999; Soudant et al. 1999; Freites et al. 2002; Murphy et al. 2002; Saito 2004; Taylor & Savage 2006; Alkanani et al. 2007; Li et al. 2007; Narváez, Freites, Guevara, Mendoza, Guderley, Lodeiros & Salazar 2008). In relation to sex-related differences in fatty acid composition of D. trunculus, we have found that males showed higher values of total saturated fatty acids and total polyunsaturated fatty acids, whereas females had higher levels of monounsaturated fatty acids. The spermatozoa of Ruditapes decussatus and male gonads of A. purpuratus also show higher values of SAFA and lower levels of MUFA when compared with oocytes and female gonads (Caers et al. 1999; Ojea et al. 2004). The higher level of SAFA in males is mainly due to the percentages of palmitic and stearic acids that are higher in males compared with females; in fact, stearic acid in D. trucnculus reached major levels than EPA in wild populations. It has been described that the female gonads of the molluscs Patella depressa, Mya truncata, A. purpuratus, Haliotis rubra and Haliotis laevigata have higher levels of 14:0, 16:1n-7, 18:1n-9, 18:2n-6 and 18:3n-3 than male gonads (Caers et al. 1999; Birkely et al. 2003; Morais, Boaventura, Narciso, Re & Hawkins 2003; Grubert, Dunstan & Ritar 2004). In D. trunculus, these differences were maintained except in the polyunsaturated 18:2n-6 whose levels were similar in both sexes. The percentage of the main long-chain polyunsaturated fatty acids (20:4n-6, 20:5n-3 and 22:6n-3) was higher in males than in females of D. trunculus. In other species, the level of 20:4n-6 is usually similar in males and females but the content of 20:5n-3 and 22:6n-3 differs among species. For example, the value of 20:5n-3 is higher in the testes of P. depressa and H. rubra, as we have found for D. trunculus, but lower in those of A. purpuratus and P. maximus (Soudant et al. 1996a; Caers et al. 1999; Morais et al. 2003; Grubert et al. 2004). The value of 22:6n-3 is similar in both sexes of H. rubra but higher in females of P. maximus (Soudant et al. 1996a; Grubert et al. 2004).
As the individuals of D. trunculus in our study were in the latter phases of gametogenesis or ripening, differences in lipid classes and fatty acid composition between males and females might reflect metabolic particularities of gonad tissue related to specific requirements for spermatogenesis and oogenesis. It is well known that during vitellogenesis large amounts of neutral lipid are accumulated in developing eggs to provide energy during embryogenesis (Napolitano & Ackman 1992; Pazos et al. 1997; Utting & Millican 1997; Caers et al. 1999; Duinker et al. 2004; Palacios, Racotta, Kraffe, Marty, Moal & Samain 2005; Li et al. 2007). The elevated TG levels found in the ripe females of D. trunculus probably correspond to the incorporation of neutral lipids in oocytes that usually occurs in the latter phases of oocyte development (Duinker et al. 2004). Furthermore, we have detected an important amount of sterol esters in females, which has been suggested to form a small reserve that would be mobilized when free sterols were required. During gametogenesis, sterols are incorporated into the gametes to be used by the embryo for the biosynthesis of membranes to which they impart rigidity (Soudant et al. 1996a; Pazos et al. 1997, 2003). However, males incorporate lipids mainly as phospholipids and as free sterols that are added into membranes of the spermatozoa (Soudant et al. 1996a), which would explain the higher levels of polar lipids in males of D. trunculus. Differences in fatty acids composition between sexes could be related to differences in the lipid composition as PUFA are mainly located in phospholipids, whereas neutral lipids such as TG and sterol esters accumulate 14:0 and MUFA (Soudant et al. 1996a, 1999; Caers et al. 1999; Pazos et al. 2003; Ojea et al. 2004; Saito 2004).
The diet of suspension feeding bivalves is mainly constituted by phytoplankton, which is also their major fatty acid source; hence, the lipid composition of the diet directly influences that of the bivalves (Napolitano et al. 1992; Parrish, McKenzie , MacDonald & Hatfield 1995; Parrish, Wells, Yang & Dabinett 1998; Berntsson et al. 1997; Alkanani et al. 2007). In D. Trunculus, we have found that a controlled diet constituted by T. suecica and Chaetoceros sp. seems to cause changes in the fatty acid composition as they coincide with that of both phytoplankton species. Furthermore, these changes were not detected in individuals from the field, although both groups showed the same development stage. Specifically, diet seems to produce a decrease of 20:1n-9, 20:2n-6, 18:4n-3, 20:4n-3 and 22:6n-3; their levels in both phytoplankton species are very low or they do not even exist. However, the levels of 18:1n-7, 20:4n-6 and 20:5n-3 increased in both sexes. The higher amounts of 20:5n-3 could be due to their elevated level in Chaetoceros sp., while the increase in 18:1n-7 could come from 16:1n-7 elongation, as this fatty acid is predominant in Chaetoceros sp. We have not found a high percentage of 20:4n-6 in hatchery diet to explain its small increase. Probably, its level is maintained by a specific mechanism as this fatty acid has an important role as a precursor of prostanglandins (Soudant et al. 1996a). The scarce contents of 20:1n-9 and 22:6n-3 detected in Chaetoceros sp. and T. suecica species found in the current study, were reported previously by others authors (Servel, Claire, Derrien, Coiffard & Deroeckhotlzhauer 1994; Utting & Millican 1998; Patil, Källqvist, Olsen, Vogt & Gislerød 2007) as well as the high level of 20:5n-3 (Servel et al. 1994; Soudant et al. 1996a; Patil et al. 2007; Rivero-Rodríguez, Beaumont & Lora-Vilchis 2007). However, despite of the decrease in some unsaturated fatty acids percentages because of diet, the condition index and the reproductive cycle do not seem to be very affected. In fact, the animals reached maturity in the hatchery, although the condition index did not increase.
In conclusion, we report, for the first time, lipid and fatty acid composition of the bivalve D. trunculus and we analyse their sex-related differences. We consider the possibility that these differences were related either to specific requirements of males and females during gametogenesis and/or to differences in the fatty acid composition of the spermatozoa and oocytes present in mature gonads. In addition, we have also found a hatchery influence on gonad fatty acid composition that is probably related to differences in food resource. Furthermore, we have found that a diet constituted by the diatom Chaetoceros sp. and the chlorophyte T. suecica, allow D. trunculus to attain maturation and to maintain the animals in a similar condition as in the field. However, the addition of other phytoplankton species in the diet as a DHA source would probably enhance the condition of the animals as it has been demonstrated that the quantity of DHA affects the egg quality and the larvae survival (Soudant et al. 1996a, b; Berntsson et al. 1997; Utting & Millican 1997, 1998; Caers et al. 1999). With these findings, new culture conditions of this clam are provided, since diet influence on maturation success has been described. Future investigations will include broodstock conditioning with other phytoplankton species as Isochrysis galbana, with different micro-algae mixtures and rations and the effect of adult diet on the quality of larvae.
Acknowledgments
We thank Emma Prieto for helping us in the lipid analyses and Niyazi Acar, PhD Research Scientist from Eye and Nutrition Research Group (University of Burgundy) for identifying dimethylacetals in our samples. This research was supported by the Spanish Government (Plan Nacional de Cultivo de Nuevas Especies en Hatcheries, JACUMAR).




