Digestive dynamics during chyme formation of Octopus maya (Mollusca, Cephalopoda)
Abstract
In the present study, digestive dynamics, measured through the changes in the in situ pH along the digestive tract, was evaluated along with the glycogen concentration in the digestive gland (DG) and the proteolytic enzyme activity of the gastric juice (GJ) (both acid and alkaline proteases) to obtain useful information that will allow for the understanding of the DG's function and the role that it plays as an extracellular digestive enzyme source and for energy storage, providing new information on the digestive physiology of Octopus maya. The results showed that pH along the digestive tract changed according to the postprandial time following the food transit. At the beginning, the pH on crop (St) was 5, changing to 6 when the food arrived. Similar changes were observed on the caecum (Ce) and DG. Glycogen from DG is used as a source of energy during digestion and recovered 8 h after feeding. Maximum activities of digestive gland GJ enzymes were observed 6 h after feeding, indicating that chyme enzymes are still active when they arrive at the DG's lumen. The presence of food in the digestive tract modifies the pH, which, in combination with GJ, favours the activity of the released enzymes of O. maya.
Introduction
All cephalopods are carnivorous and, in the wild, they feed on living preys. They have a very active lifestyle; thus, they have a high metabolic activity and need huge amounts of food (Budelmann, Schipp & Boletzky 1997). The digestive system of cephalopods is highly developed; it consists of several voluminous compartments with a specialized structure and function. The digestion begins in the buccal mass; while the prey is broken mechanically, the posterior salivary glands secrete enzymes and then the food goes through the oesophagus towards the anterior stomach (crop) (Nixon 1979; 1980; Boucaud-Camou & Boucher-Rodoni 1983).
The food is ground thanks to the crop, where enzymes secreted by the digestive gland (DG) form the chyme initiating the extracellular digestion. The chyme goes to the stomach and from there to the caecum (Ce). Later, the chyme obtained moves directly from the Ce towards the DG, where the intracellular digestion is carried out (Boucaud-Camou & Boucher-Rodoni 1983).
Kozlovskaya and Vaskovsky (1970) showed an important enzymatic activity in the DG of cephalopods. Sawano (1935) and Takahashi (1960) also found proteolysis in the ducts of the DG of octopuses and squids. In paralarvae of O. vulgaris, the greatest enzyme source is the DG (Boucaud-Camou & Roper 1995), where most of the enzymatic activity is represented by proteases (Morote, Rodríguez, Mancera, Moyano & Muñoz 2005).
The DG is the largest gland of the cephalopod body. It has many functions: the synthesis and secretion of digestive enzymes, re-absorption and metabolism of nutrients and the synthesis and storage of glycogen. These digestive enzymes are released by apocrine secretion into the lumen of the DG tubules (Budelmann et al. 1997). Most of these enzymes are of a proteolytic nature, like trypsin, chymotrypsin (Boucaud-Camou & Boucher-Rodoni 1983) and cathepsin (Budelmann et al. 1997), and they are present in heterophagosomas, inside the digestive cell.
The number and the size of these heterophagosomes can be related to the stage of digestion (Boucaud-Camou 1973; Boucher-Rodoni 1976; Boucher-Rodoni & Mangold 1977; Boucaud-Camou & Yim 1980; Boucaud-Camou & Boucher-Rodoni 1983; Semmens, Moltschaniwskyj & Alexander 1995). During feeding, the number of heterophagosomes increases slowly, showing slight fluctuations, which could be related to additional enzyme release. Therefore, it is possible to infer that the release and activity of digestive enzymes also respond to the digestive cycle stage.
Previous studies carried out in Sepia officinalis found that the activity of total proteases, acid and alkaline, present in the DG, is directly affected by the time of the feeding cycle. The transition from a predominantly intracellular digestion to an extracellular digestion is accompanied by a greater participation of alkaline enzymes (Perrin 2004).
One of the main factors affecting enzyme activity is the pH, and, depending on the group (acid or alkaline) to which they belong, they will respond to the concentration of hydrogen ions of the medium from which they are released. Enzyme action is affected directly by pH, as activity variations beyond the range produce changes in the molecular conformation and modifications in the electrical charge of the enzyme or in the electrical charge of the rest of the participants in either the substrate connection or the catalysis (Murray, Mayes, Granner & Rodwell 2001). Thus, a change in the pH value of the medium from which the enzymes are secreted is expected, depending on its location and the digestive cycle stage.
The changes in the digestive enzymes activity and glycogen concentration in the DG of Octopus maya in fasting and postprandial states have not yet been studied. The study of the postprandial enzymatic kinetics and of the pH values in different organs of this species will help determine the sequence of events generated by the digestion of foods and allow establishing the secretion rates and enzymatic activity during the digestion process. In addition, this will provide data on the role played by the DG in the glycogen reserve mechanism of this species.
In the present study, digestive dynamics, measured through the changes of the in situ pH along the digestive tract, was evaluated along with the glycogen concentration in the DG of wild and cultivated O. maya individuals. The proteolytic enzyme activity of the gastric juice (GJ) was also evaluated (both acid and alkaline proteases). Here, we aim to obtain useful information that will allow for the understanding of the DG's function and the role that it plays as an extracellular digestive enzyme source and for energy storage, providing new information on the digestive physiology of these animals.
Materials and methods
The present study was performed at the Experimental Cephalopod Production Unit (EPHAPU) at the UMDI-UNAM, Sisal, Yucatan, Mexico.
Animals rearing and diet
Octopus maya specimens were caught in front of Sisal harbour in April 2008. Octopuses were transported to the laboratory and placed in individual flow-through 80-L tanks with aerated seawater for 7 days before the experiment; a PVC tube of 4″ diameter was provided as shelter. Octopuses were fed frozen crabs (Callinectes spp). The juvenile octopuses were obtained from egg-laying females under controlled conditions. The experimental tanks were fed with natural seawater and connected to a recirculation system in which 10% water was renewed daily. Water temperature, salinity and dissolved oxygen were measured daily, whereas pH, nitrates, nitrites and ammonium were evaluated once a week.
Three animal groups were used for the experiments. The first group consisted of 15 wild O. maya specimens (553.23±97.12 g). These animals were used to evaluate the dynamics of in situ pH along the digestive tract before and after feeding, and to obtain the GJ for the electrophoresis study. The second group was formed by 15 wild octopuses (536.01±125.89 g), used to evaluate DG's glycogen concentration and enzymatic activity before and after feeding. The third group was formed by 18 individuals hatched and cultivated during 4 months under controlled conditions (13.98±3.51 g) and used to evaluate the GJ enzymatic activity before and after feeding. The cultured octopuses were fed all time twice a day, 09:00 and 16:00 hours (30% body mass), exclusively with crab paste (95%) and gelatin as an agglutinant (5%) following the feeding process reported previously (Rosas, Tut, Baeza, Sánchez, Sosa, Pascual, Arena, Domingues & Cuzon 2008). This diet was considered to be the maintenance diet. Fresh-frozen crab was used during the feeding experiments.
Sampling and enzymatic preparations
Before sampling, all octopuses (24-h fasted animals) were anaesthetized in a cold seawater bath 5 °C) for 2–5 min depending on the size of the animal. Sampling was performed before (hour 0) and 2, 4, 6 and 8 h after feeding. Culture animals were sampled before and 1, 2, 4 and 6 h after feeding. In each case, three individuals were taken randomly. In all cases, animals were fed with fresh-frozen crab. In situ pH was measured in the digestive tract using a potentiometer (electrode Metrohm) inserted into each digestive cavity, separated from other digestive tract sections with calipers just to avoid sample contamination. The GJ electrophoresis samples were obtained directly from the St and Ce using a sterile 3-cm syringe and stored at −80 °C until analysis. The GJ samples from the DG were obtained directly by dripping after a small cut in the organ's parenchyma. These samples were placed individually in new tubes and preserved at −80 °C until analysis.
Protein, glycogen and enzyme activity measurement
Before the analysis, GJ samples were centrifuged (16 170 g at 4 °C) for 30 min. The total soluble protein was evaluated using the Coomassie blue dye method according to Bradford (1976) using bovine albumin serum as the standard. Glycogen was determined using the method described by Carroll, Longley and Roe (1956). Total proteases were measured using Anson's (1938) method. Acid and alkaline protease activities were assayed using haemoglobin (1%) and casein (1%) as substrates respectively. Both substrates were dissolved using Stauffer's universal buffer (1989) for the corresponding pH: for wild octopuses, enzymes at pH 5.5 (acid) and 7.5 (alkaline), and for cultured octopuses, enzymes at pH 6 and 8 respectively. In a tube, 20 μL of the enzyme extract (dilution 1:10) was mixed with 0.5 mL of buffer and 0.5 mL of a freshly prepared substrate with buffer at the corresponding pH (acid or alkaline) and incubated for 10 min at 38 °C. The reaction was stopped by adding 0.5 mL of 20% (w/v) trichloroacetic acid (TCA) and cooling for 15 min at 4 °C. The precipitated undigested substrate was separated by centrifugation for 15 min at 13 370 g. The absorbance of the supernatants was measured spectrophotometrically at 280 nm against the substrate without an enzyme extract. One unit of enzymatic activity was defined as the change in absorbance per minute per milligram protein of the enzyme used in this assay (ΔAbs min−1 mg protein−1).
SDS-PAGE and SDS-PAGE substrate
Proteolytic enzyme activity present in the GJ of DG, St and Ce from wild octopuses was observed using the 12% SDS-PAGE-gelatin method (Laemmli 1970). The stacking gel was prepared by adding 0.65 mL of 30% polyacrylamide (PAA) to 0.5 M Tris-HCl (pH 6.8) and the resolving gel was prepared by adding 2 mL PAA, 0.5 mL of 1% gelatin to 1.5 M Tris-HCl (pH 8.8). To run the gel, a Tris-glycine-SDS 1X buffer was prepared. The samples were prepared with mercaptoethanol to a concentration of 50 μg protein. A low-molecular-weight standard (4 μL; BIO-RAD, México city, México, 161-0305) was placed on the gel (BIO-RAD, 165-1932, size of the gel 8 × 16 cm) and run with samples at 4 °C and 15 mA. After electrophoresis, the gel was washed with distilled water and incubated in Stauffer's universal buffer, pH 3, with 2% Triton 100 at 38 °C overnight. Subsequently, the gel was washed again with distilled water and stained with 0.25% of Coomassie Brillant Blue in an aqueous solution of 45% methanol and 10% glacial acetic acid for at least 3 h and then distained with the same solution without dye.
Statistical Analysis
For the analysis of the values obtained from the pH in situ, these were transformed into hydrogen ion concentration (M) (Brown, LeMay, Bursten & Burdge 2004). Data were expressed as the mean±standard error (SE). Differences among individuals from different hours after feeding were analysed using a one-way anova, followed by Duncan's multi-comparison test (Montgomery 2004). Before anova, homogeneity of variances was verified by Cochran. Statistical analysis was performed using the statgraphics 5.1 software. Differences are reported as statistically significant when P<0.05, and indicated in graphs by different letters.
Results
Wild octopuses
Changes in the in situ pH values were observed along the digestive tract during chyme formation. A pH value of 5.1 in the crop was observed before feeding; 2 h after feeding, the pH of the crop increased, with values around 6 (5.95±0.05; P=0.0003, Fig. 1). Subsequently, the crop's pH was reduced to be maintained between 5.2 and 5.6 during the rest of the sampling time evaluations. An increment in the pH values in the Ce was observed with time, attaining the highest values at 4 and 6 post prandial hour (PPh) (5.93±0.28 and 6.02±0.07, respectively, P=0.03). The lowest values were observed under the fasting condition (0 PPh, 5.43±0.06 pH, P=0.0021; Fig. 2). The highest pH values of the DG were observed 6 h after feeding (5.63±0.10), being significantly greater than those registered on any other hour evaluated (P=0.0075, Fig. 3). There were no significant differences among the pH values of the DG at 0, 2, 4 and 8 PPh (Fig. 3).
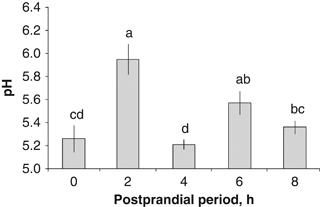
In situ pH values from the crop of wild Octopus maya in the postprandial period.
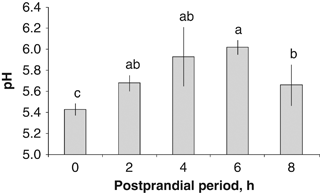
In situ pH values from the caecum of wild Octopus maya in the postprandial period.
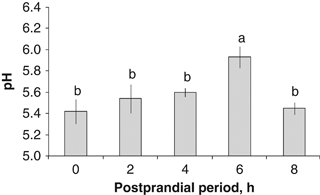
In situ pH values from the digestive gland of wild Octopus maya in the postprandial period.
In the GJ from wild octopuses, the maximum acid protease activity was registered after 6 PPh (21 603.10±1314.33 UI/mg protein, P=0.0151, Fig. 4a). The lowest activities were registered between the fasting state (0 h) and 2, 4 and 8 PPh (Fig. 4a). The highest alkaline protease activity in the GJ was observed at 6 PPh, followed by 8 PPh (84620.50±8788.31 and 75618.50±2670.76 UI/mg protein respectively) (P=0.0013, Fig. 4b). The lowest values were observed at 2 and 4 PPh (41937.00±4617.75 and 45645.80±7491.60 UI/mg protein respectively) (Fig. 4b).
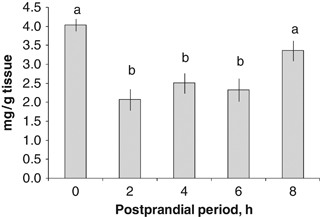
Glycogen concentration in the digestive gland of wild Octopus maya in the postprandial period.
The highest glycogen concentration in the DG was registered in the fasting state, followed by the value observed at 8 PPh (4.039±0.161 and 3.363±0.265 mg/g tissue respectively) (P=0.0016, Fig. 5). There were no differences among the glycogen concentrations of the DG at 2, 4 and 6 PPh (P>0.05; Fig. 5).
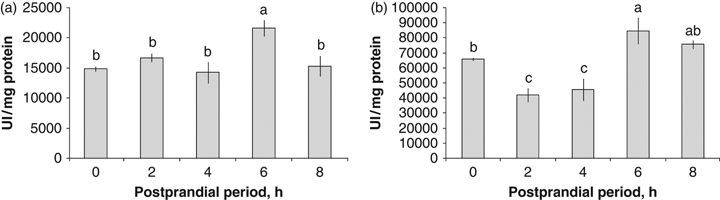
Acid (a) and alkaline (b) proteases activity in the gastric juice of the digestive gland from wild Octopus maya in the postprandial period.
Cultured octopuses
The maximum acid proteases activity in the GJ from cultured octopuses was registered at 6 PPh (5369.74±274.92 UI/mg protein) (P=0.0019, Fig. 6a). Intermediate activity values were registered in the fasting state (0 h), 2 and 4 PPh. The lowest enzymatic activities were registered at 1 PPh (1734.21±261.82 UI/mg protein) (Fig. 6a). The highest alkaline protease activities in the GJ were found at 4 PPh, followed by 6 PPh (8184.08±1736.68 and 7433.29±1661.73 UI/mg protein respectively) (P=0.0100, Fig. 6b). The lowest values were observed in the fasting state (1910.97±783.46 UI/mg protein) (Fig. 6b).
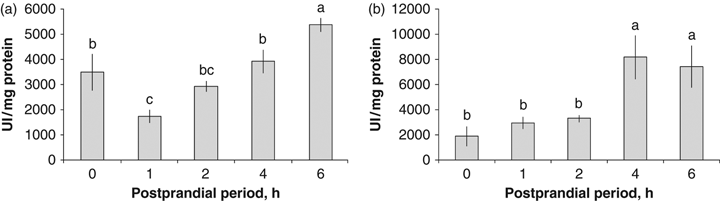
Acid (a) and alkaline (b) proteases activity in the gastric juice of the digestive gland from cultured Octopus maya in the postprandial period.
Zymogram at pH 3
The GJ zymogram of the DG from wild octopuses showed activity bands between 26 and 30, 34, 40 and 48 kDa and the last one between 72 and 100 kDa. The sample from the Ce showed activity bands between 28 and 30, 34, 40 and 50 kDa. The GJ from the St showed activity bands between 25 and 32, 34, 40 and 50 kDa and the last one between 72 and 100 kDa (Fig. 7).
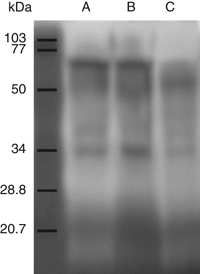
Zymogram of proteases present in the gastric juice from the digestive gland (a), caecum (b) and crop (c) of wild Octopus maya.
Discussion
The effect of food on the pH values of the GJ from the St, Ce and DG of O. maya is shown for the first time in this species. According to our results, changes in the pH of the GJ were observed during chyme formation in the digestive tract from around 5 to 6. Similar results were obtained by Bidder (1950), in freshly dead trawl European squids, where values of 6.2 in the stomach, 5.6–5.8 in the Ce, six in the pancreas and six in DG were observed.
According to our results, the pH level of the St's GJ in the fasting condition was 5.26, which indicates that the first digestion environment is acidic, where cathepsin D could be present (Martínez, Sántos, Álvarez, Mascaró, Pascual & Rosas 2010). After feeding, the pH changed, being less acidic, suggesting that food, on filling the crop, modifies the pH of the digestive environment, favouring the activity of other enzymes besides cathepsins. Once the food passed to the stomach and Ce, the pH of the crop returned to the initial acidic environment, presumably to be prepared for the next digestion (Semmens 2002) or adjusted just to maintain the acidic environment during the transit of chyme along the digestive tract via a new enzyme release (Moguel, Mascaró, Avila-Poveda, Caamal, Sanchez, Pascual & Rosas 2010). Using the pH variations during chyme digestion, it became evident that when the chyme arrives to the Ce, it caused a change in the environment's pH, increasing from 5.4 to 5.9, suggesting again the effects of the digestion process on the digestive environment. Taking into account these data, a pH-regulatory mechanism could be operating to maintain the digestive environment around a pH of 6, just where GJ enzymes depict the maximum activities (Martínez et al. 2010). During digestion, acidic enzyme activity change causes the digestive environment to become more alkaline, due to the release of OH ions during acidic reactions (Alvarez 2003). If such a condition remains for a long time, the digestive environment will change, causing a reduction in the acidic enzyme activity via a change in the pH. To maintain the acidic environment and at the same time the acidic enzyme activity, octopuses could release more GJ during the digestion process just to maintain the acidic enzyme activity, which, in O. maya, is more active than that of alkaline digestive enzymes (Martínez et al. 2010).
The pH of O. maya's crop changed 2 h after feeding, suggesting that food filled this organ during that period of time. The pH change also suggested that digestion started inside the crop with the acidic enzymes as extracellular digestive enzymes. The high activity proteases observed before feeding in the digestive gland's gastric juice (DGGJ) indicate that digestive enzymes are secreted into the digestive gland lumen, presumably to be used during chyme formation in the crop once food has been ingested. The electron-dense luminal contents and secretory granules observed 2 h after feeding in Sepioteuthis lessoniana's digestive gland suggest that the enzymes are released at least 1 h after feeding, in preparation for digestion (Semmens 2002). As food filled the crop, the chyme was slowly transported to the stomach and the Ce, where it arrived around 4 h after feeding. At that time, the chyme could also arrive to DG, where alkaline enzymes showed their maximum activity. It is interesting to note that the maximum activity of alkaline digestive enzymes was only observed in cultured octopuses, suggesting that cultured juveniles could show a faster metabolism than wild pre-adults, implying that size (wet weight) and age could affect the food transit in cephalopods' digestive system. A detailed histological study of O. maya juveniles confirmed these hypotheses (Martinez, López-Ripoll, Avila-Poveda, Santos-Ricalde, Mascaró & Rosas 2011).
A maximum activity of DG enzymes was observed 6 h after feeding, indicating that the chyme is arriving at the DG from the Ce to start the intracellular digestion. This transit took at least more 2 h, during which the Ce had a relatively high pH. It is interesting to note that the maximal protease activity was observed 6 h after feeding, indicating that chyme enzymes are still active when they arrive at the DG's lumen. Histological evidences obtained early demonstrate that during intracellular digestion, an acidic pH could favour observed enzymatic activity (Martinez et al. 2011). A similar process was observed in S. lessoniana 2 h after feeding when the chyme arrives at the DG's lumen (Semmens 2002). Perrin (2004) showed that the intracellular digestion in S. officinalis reaches its maximum activity between 4.5 and 6 h after feeding. Eight hours after feeding, a reduction in the pH suggested that DG's activity was gradually reduced, together with a reduction in enzymatic activity in the DG's lumen. At this time, a new release of digestive enzymes could be responsible for the low pH observed in the crop and the DG in preparation for a new digestive cycle. According to Perrin (2004), in 30-day-old S. officinalis, the decrease in the acid and alkaline proteases activity between 0 and 3 h was followed by an increase in the activity between 3 and 4.5 h. An increment in heterophagosomes was associated with that proteases activity, suggesting an increment in the intracellular digestive activity. Between 4.5 and 8 h, the activity of the acid and alkaline proteases diminished, suggesting a second release of the proteases that alternates with phases of secretion and enzymatic synthesis during the course of digestion. A similar mechanism could operate for O. maya, both maintaining the pool of active extracellular enzymes and the digestive environment in an appropriate pH. Boucaud-Camou and Boucher-Rodoni (1983) reported, for O. vulgaris and Eledone cirhosa, that the enzyme release during the digestion process is alternated with the synthesis and secretion phases, allowing the animals to maintain the digestive process in preparation for the next meal.
Within the digestive process of cephalopods, the DG serves a fundamental physiological function, providing digestive enzymes and storing nutrients (Bustamante 1998), especially those that are used as a source of metabolic energy (Hatfield, Rodhouse & Barber 1992). Glucose is the main energy source for the cells and it is stored as glycogen (Murray et al. 2001). The results obtained in the present study showed that the glycogen stored in the DG was used as an energy source for the digestive process. The recovery of glycogen concentration in the DG 8 h after feeding demonstrates that this nutrient was stored in preparation for the next digestive cycle. As the metabolism of cephalopods is essentially based on proteins (Lee 1994), it is logical to think that the glycogen that is stored in the DG is synthesized via glyconeogenesis from amino acids (Murray et al. 2001; Akagi & Omori 2004).
Enzymes in the GJ from St, Ce and DG of O. maya showed molecular weights between 30 and 50 kDa. Several results demonstrate that digestive enzymes in aquatic organisms have low molecular weights. In the sea cucumber, the existence of at least three proteases, whose molecular weights are 20.6, 39.1 and 114.1 kDa, has been confirmed (Fu, Xue, Miao, Li, Gao & Yang 2005). Furthermore, the 20.6 kDa protease was confirmed to be a metallo-protease and the 39.1 kDa protease a serine protease (Fu et al. 2005). Chymotripsin has been isolated from the viscera of Monterey sardine with an approximate molecular mass of 26 kDa (Castillo, Pacheco, Garcia, Navarrete & Félix 2006); in the blue abalone, enzymes with molecular masses of 28.1, 29.5, 30 and 32 kDa have also been obtained (Hernández, Hernández, Arreguin & Rodríguez 1998). Perera, Moyano, Díaz, Perdomo-Morales, Montero-Alejo, Alonso, Carrillo and Galich (2008), in a study partially characterizing the proteases in the spiny lobster, found bands of activity between 14 and 45 kDa, identifying trypsin isoforms. Cathepsins also have low molecular weights. Cathepsin B is around 37 kDa while cathepsins H and L have a molecular weight around 23 kDa (Aranishi, Hara & Ishihara 1992; Wilson, Good, Panaccio, Wijffels, Sandeman & Spithill 1998; Cardenas-Lopez & Haard 2009). In a previous study in O. maya, the presence of cathepsin D was shown in the GJ of the DG and St (Martínez et al. 2010), which is probably also present in the Ce fluid.
Conclusions
According to the results, we can conclude that the pH of the digestive environment during chyme formation is 5 to 6. As this type of study was not conducted with other types of food, the results obtained are possibly valid only for octopuses fed crab meat or crab paste. This pH favours the activity of proteases present in the DG lumen. The presence of food in the digestive tract modifies the pH, which, in combination with GJ, favours the activity of the released enzymes. The activity of the evaluated proteases present in the DG is rhythmical, demonstrating the gradual release of enzymes into the organ's lumen, corresponding to the digestion and nutrient absorption phases that are carried out in a relatively short time.
Acknowledgments
Thanks are due to CONACYT for scholarship number 48221 to Martinez R. The present study was partially financed by DGAPA-UNAM project IN216006-3 and CONACYT – Básico 24743 to Rosas C.




