Prebiotics in aquaculture: a review
Abstract
A prebiotic is a non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or the activity of one or a limited number of bacteria in the colon. Despite the potential benefits to health and performance as noted in various terrestrial animals, the use of prebiotics in the farming of fish and shellfish has been less investigated. The studies of prebiotics in fish and shellfish have investigated the following parameters: effect on growth, feed conversion, gut microbiota, cell damage/morphology, resistance against pathogenic bacteria and innate immune parameters such as alternative complement activity (ACH50), lysozyme activity, natural haemagglutination activity, respiratory burst, superoxide dismutase activity and phagocytic activity. This review discusses the results from these studies and the methods used. If the use of prebiotics leads to health responses becoming more clearly manifested in fish and shellfish, then prebiotics might have the potential to increase the efficiency and sustainability of aquaculture production. However, large gaps of knowledge exist. To fully conclude on the effects of adding prebiotics in fish diets, more research efforts are needed to provide the aquaculture industry, the scientific community, the regulatory bodies and the general public with the necessary information and tools.
Introduction
Prebiotics are defined as non-digestible components that are metabolized by specific health-promoting bacteria such as Lactobacillus and Bifidobacterium. These bacteria are considered beneficial to the health and growth of the host by decreasing the presence of intestinal pathogens and/or changing the production of health related bacterial metabolites (Roberfroid 1993; Gibson & Roberfroid 1995; Gibson 1998; Manning & Gibson 2004). The latter include for instance short-chain fatty acids (SCFA), which are generally believed to be positive for colonic health. Prebiotics are carbohydrates, which can be classified according to their molecular size or degree of polymerization (number of monosaccharide units), into monosaccharides, oligosaccharides or polysaccharides. According to International Union of Pure and Applied Chemistry nomenclature, oligosaccharides are defined as saccharides containing between three and ten sugar moieties (Mussatto & Mancilha 2007). Other authorities classify saccharides with 3–19 monosaccharide units in this group. However, there is not a rational physiological or chemical reason for setting these definitions (Voragen 1998). Consequently, oligosaccharides can be loosely defined as low molecular weight carbohydrates. Based on the biochemical and physiological properties, the carbohydrates can be classified as digestible or non-digestible. The concept of non-digestible oligosaccharides (NDO) originates from the observation that the anomeric C atom (C1 or C2) of the monosaccharide units of some dietary oligosaccharides has a configuration that makes their glycosidic bonds non-digestible to the hydrolytic activity of the human/animal digestive enzymes (Roberfroid & Slavin 2000). The main categories of NDOs presently available or in development as food additives include carbohydrates in which the monosaccharide unit is fructose, galactose, glucose and/or xylose (Fig. 1).
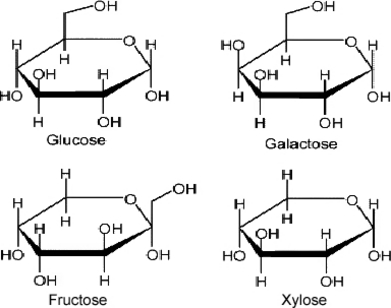
Monosaccharide components of non-digestible oligosaccharides. After Mussatto & Mancilha (2007).
Dietary fibres belong to the broad category of carbohydrates. Burkitt et al. (1972) defined dietary fibre as the sum of polysaccharides and lignin that are not digested by the endogenous digestive enzymes of the human gastrointestinal (GI) tract. They can be classified as water soluble (e.g. inulin and oligofructose), insoluble (e.g. cellulose) or mixed (e.g. bran). Fermentable carbohydrates are considered to be the most promising in terms of a positive influence on the composition and activity of the indigenous microbiota of the GI tract (Gibson & Roberfroid 1995; Williams et al. 2001; Bauer et al. 2006). However, research and application of orally administered prebiotics is in its infancy regarding fish and shellfish production compared to the progress that has been made in the development of prebiotics for terrestrial animals (Patterson & Burkholder 2003).
During the last two decades, traditional use of antibiotics in aquaculture has been criticized because of the potential development of antibiotic-resistant bacteria, the presence of antibiotic residues in seafood, the destruction of microbial populations in the aquacultural environment and the suppression of the aquatic animal’s immune system (Smith et al. 2003; Cabello 2004; Sørum 2006; Sapkota et al. 2008). As an alternative strategy to antibiotics, probiotics have recently attracted extensive attention in aquaculture. Many reports have been published regarding application of probiotics in the aquatic environment (for review see, Ringø & Gatesoupe 1998; Gatesoupe 1999; Verschuere et al. 2000; Irianto & Austin 2002; Ringø 2004; Burr et al. 2005; Gram & Ringø 2005; Balcazar et al. 2006; Farzanfar 2006; Nicolas et al. 2007; Kesarcodi-Watson et al. 2008; Tinh et al. 2008; Wang et al. 2008). However, because of high cost, potential impact on the environment, regulatory issues, and food safety and challenges regarding incorporation into modern extruded feeds, large-scale application of probiotics in the water has been limited. Alternatively, it appears more practical to manipulate the GI tract microbiota in aquatic animals through the use of prebiotics that alter the conditions to favour certain bacterial species which may enhance fish growth efficiency and reduce disease susceptibility of the host organism (Gatlin 2002; Burr et al. 2005; Nicolas et al. 2007). This is supported by other investigations which indicate that the GI tract is a potential port of entry for some pathogenic bacteria (Ringøet al. 2004; Birkbeck & Ringø 2005; Ringøet al. 2007a,b; Salinas et al. 2008; Sugita et al. 2008; Ringøet al. 2009).
However, it has only been during the last decade that there has been an improved understanding of the importance of commensal intestinal microbiota in the fish intestine. Nevertheless, the first study on prebiotics in aquaculture, to the author’s knowledge, was reported in 1995 (Hanley et al. 1995). Since then several studies have been carried out, and an overview of these studies is presented in Tables 1–5. The common prebiotics established in fish to date include inulin, fructooligosaccharides (FOS), short-chain fructooligosaccharides (scFOS), mannanoligosaccharides (MOS), galactooligosaccharides (GOS), xylooligo-saccharides (XOS), arabinoxylooligosaccharides (AXOS), isomaltooligosaccharides (IMO) and GroBiotic®-A. However, no information is available on transgalactooligosaccharides (TOS) used in endothermic animals. Although these are mostly plant-derived additives and fibres are often not naturally present in fish diets, especially not for carnivorous fish, the prebiotic potential of oligosaccharides and other dietary fibres may have interesting applications in aquaculture to stimulate gut health and the presence of beneficial gut bacteria as well as to suppress potentially deleterious bacteria. In addition to the information available on prebiotic effects on the gut microbiota in fish, articles investigating the effect of prebiotics on intestinal morphology are available (Olsen et al. 2001; Sweetman & Davies 2005; Bakke-McKellep et al. 2007; Torrecillas et al. 2007; Yilmaz et al. 2007; Dimitroglou et al. 2008; Salze et al. 2008; Sweetman et al. 2008).
| Prebiotic | Dose and length of administration (oral if not stated otherwise) | Fish species (g) | Results | References |
|---|---|---|---|---|
| Inulin | i.p (10 mg kg−1 body weight – 2 weeks | Grass carp (Ctenopharyngodon idellus) (24.6 ± 3.5 g) | → Susceptibility against Aeromonas hydrophila and Edwardsiella tarda | Wang & Wang (1997) |
| i.p (10 mg kg−1 body weight – 2 weeks | Tilapia (Tilapia aureus) (21.8 ± 3.3 g) | → Susceptibility against A. hydrophila and E. tarda | Wang & Wang (1997) | |
| 150 g kg−1– 4 weeks | Arctic charr (Salvelinus alpinus L.) (218 g) | Intestinal cell damage | Olsen et al. (2001) | |
| 150 g kg−1– 4 weeks | Arctic charr (218 g) | ↓ TVCMicrobiota – control:Pseudomonas, Psychrobacter glacincola, Carnobacterium divergens. Micrococcus, Staphylococcus, StreptococcusMicrobiota – inulin: Bacillus, Carnobacterium maltaromaticum, Staphylococcus, StreptococcusDifferent colonization pattern on enterocytes surface | Ringøet al. (2006) | |
| 75 g kg−1– 3 weeks | Atlantic salmon (Salmo salar L.) (172 g) | → Intestinal cell damage↑ Intestinal growth and relative mass of the gastrointestinal (GI) tract→ Hydrolytic and absorptive capacity | Refstie et al. (2006) | |
| 75 g kg−1– 3 weeks | Atlantic salmon (172 g) | → TVC Control↓Marinilactibacillus psychrotolerans, C. maltaromaticum, Enterococcus faecalisInulin ↓Pseudoalteromonas, Micrococcus→ Intestinal cell damage | Bakke-McKellep et al. (2007) | |
| 5 and 10 g kg−1– 1 week | Gilthead seabream (Sparus aurata L.) (175 g) | Significant inhibition in phagocytosis and respiratory burst in leucocytes | Cerezuela et al. (2008) | |
| 20 g kg−1– 1 month | Siberian sturgeon (Acipenser baerii ) (213 ± 0.7 g) | → Total SCFA and lactate↓ Butyrate↑ Gas production | Mahious et al. (2006a) | |
| 20 g kg−1– 1 month | Turbot (Psetta maxima) larvae | ↑ Growth rateEffects on gut microbiota (Bacillus and Vibrio) | Mahious et al. (2006b) |
- i.p, intraperitoneal injection; TVC, total viable counts; SCFA, short-chain fatty acids.
- Symbols represent an increase (↑), decrease (↓) or no effect (→) on the specified response.
| Prebiotic | Dose and length of administration | Fish species (g) | Results | References |
|---|---|---|---|---|
| FOS | 10 g kg−1– 4 month | Atlantic salmon (200 ± 0.6 g) | → Feed intake, growth or digestibility | Grisdale-Helland et al. (2008) |
| 0, 2 and 6 g kg−1– 58 days | Hybrid tilapia (Oreochromis niloticus ♀ × Oreochromis aureus ♂) (57 g) | → Growth rate↑ Survival↑ Non-specific immunity | He et al. (2003) | |
| 20 g kg−1– 1 month | Turbot larvae | ↑ Growth rateEffects on gut microbiota (Bacillus and Vibrio) | Mahious et al. (2006b) | |
| 0, 1.5 and 2.5 g kg−1– 100 days | Soft-shell turtle (Triortyx sinensis) | ↑ Growth rate at 0.25% inclusion↑ SOD activity at 0.25% inclusion↓ Lysozyme activity | Ji et al. (2004) | |
| scFOS | 0.8 or 1.2 g kg−1– 8 weeks | Hybrid tilapia (5.6 ± 0.02 g) | ↑ Growth rate, feed intake, feed conversion→ Survival and condition factor↑Vibrio parahemolyticus, Aeromonas hydrophila, Lactobacillus spp., Streptococcus faecalis | Hui-Yuan et al. (2007) |
| 1 g kg−1– 56 days | Hybrid tilapia (1.24 ± 0.01 g) | ↑ Uncultured bacterium clones and Thiothrix eikelboomii | Zhou et al. (2009) | |
| 0.25, 0.5, 0.75, 1, 2, 4 and 8 g kg−1– 6 weeks | White shrimp (Litopenaeus vannamet) (75.4 ± 0.8 g) | → Weight gain, feed conversion and survivalscFOS affected gut microbiota | Li et al. (2007) | |
| 0, 0.4, 0.8, 1.2 and 1.6 g kg−1– 8 weeks | White shrimp (0.17 g) | ↑ Growth rate, feed intake, feed conversionscFOS affected gut microbiota | Zhou et al. (2007) |
- SOD, superoxide dismutase.
- Symbols represent an increase (↑), decrease (↓) or no effect (→) on the specified response.
| Prebiotic | Dose and length of administration | Fish species (g) | Results | References |
|---|---|---|---|---|
| MOS | 10 g kg−1– 4 months | Atlantic salmon (200 ± 0.6 g) | ↓ Oxygen consumption↓ Protein and ↑ energy concentration in the whole body | Grisdale-Helland et al. (2008) |
| 2 g kg−1– 4 weeks | Channel catfish (Ictalurus punctatus) (approximately 16 g) | → Growth performance, haematology or immune function→ Survival against Edwardsiella ictaluri | Welker et al. (2007) | |
| 0.2%– 13 dph | Cobia (Rachycentron canadum) larvae | ↑ Larval survival↑ Microvilli alignment↓ Supranuclear vacuoles | Salze et al. (2008) | |
| Artemia nauplii enriched with Bio-MOS® | European lobster (Homarus gammarus) | ↑ Larval survival↓ Early survival and morphological development of early juvenile stages | Daniels et al. (2006) | |
| Artemia nauplii enriched with fluorescently labelled Bio-MOS® | European lobster | Potential breakdown of MOS by Artemia→ Survival and growth | Daniels et al. (2007) | |
| 20 and 40 g kg−1– 67 days | European sea bass (33.7 ± 7.7 g) | ↑ Growth→ Feed conversion↓ Lipid vacuolization↓ Presence of Vibrio alginolyticus on head kidney | Torrecillas et al. (2007) | |
| 2 g kg−1– 90 days | Rainbow trout (30 g) | ↑ Growth and survival↑ Antibody titre and lysozyme activity in one trial→ Bactericidal activity | Staykov et al. (2007) | |
| 0, 1.5, 3 or 4.5 g kg−1– 90 days | Rainbow trout (37.5 ± 1 g) | 1.5 g kg−1↑ growth rate1.5 g and 3 g kg−1↑ Intestinal villi→ Feed conversion, hepatosomic index, intestinal morphology | Yilmaz et al. (2007) | |
| 2 g kg−1– 8 weeks | Rainbow trout (no information given about weight) | ↑ Absorptive surface in the posterior gut region↑ Microvilli density and microvilli length | Dimitroglou et al. (2008) | |
| 0 and 4 g kg−1– 12 weeks | Rainbow trout (13.2 g) | ↑ Growth↑ Haemolytic – and phagocytic activity↑ Mucus weight↑ Survival against Vibrio anguillarum | Rodrigues-Estrada et al. (2008) | |
| 10 g kg−1– 3 weeks | Red drum (Sciaenops ocellatus L.) (500 g) | ↑ Protein and organic ADC values↓ Lipid ADC | Burr et al. (2008) | |
| 0 and 3 g kg−1– 5 weeks | Gulf sturgeon (Acipenser oxyrinchus desotoi) (130 g) | → Growth performance, feed conversion and gross gastrointestinal morphology | Pryor et al. (2003) | |
| 0, 2 and 6 g kg−1– 58 days | Hybrid tilapia (8.1 g) | → Growth rate↑ Survival↑ Non-specific immunity | He et al. (2003) | |
| 0, 1.5, 3 and 4.5 g kg−1– 80 days | Hybrid tilapia (9.8 g) | → Growth parameters and body indicesDry matter and protein contents of fillets increased with increasing rates of MOS | Genc et al. (2007a) | |
| 0, 2, 4, 6, 8 and 10 g kg−1– 45 days | Nile tilapia (13.6 ± 0.7 g) | → Haematological parameters↓ Daily feed consumption with increasing level | Sado et al. (2008) | |
| 0, 2, 4, and 6 g kg−1– 3 weeks | Nile tilapia (0.82 g) | ↑ Weight, length and average daily growth of fish fed 4, and 6 g↑ Survival against Streptococcus agalactiae | Samrongpan et al. (2008) | |
| 0, 1.5, 3 and 4.5 g kg−1– 48 days | Tiger shrimp (Penaeus semisulcatus) (0.34 g) | 3 g kg−1↑ growth, feed conversion and survivalNo detrimental effect was noted on hepatopancreas tissue | Genc et al. (2007b) |
- dph, days posthatching; ADC, apparent digestibility coefficient.
- Symbols represent an increase (↑), decrease (↓) or no effect (→) on the specified response.
| Prebiotic | Dose and length of administration | Fish species (g) | Results | References |
|---|---|---|---|---|
| GOS | 10 g kg−1– 4 month | Atlantic salmon (200 ± 0.6 g) | ↑ Nitrogenous and energy losses in the non-faecal nitrogen excretion | Grisdale-Helland et al. (2008) |
| 10 g kg−1– 3 weeks | Red drum (500 g) | ↑ Protein and organic ADC values↓ Lipid ADC | Burr et al. (2008) | |
| XOS | 0, 0.15, 2.1 and 3.2 g kg−1– 45 days | Crucian carp (Carassius auratus gibelio) (16.8–17.6 g) | ↑ Growth→ Survival↑ Enzymatic activity | Xu et al. (2009) |
| AXOS | 10 and 20 g kg−1– 10 weeks | African catfish (Clarias gariepinus) (approximately 20 g) | → Growth↑ Acetate, propionate and total SCFA production→ Butyrate production | Rurangwa et al. (2008) |
| 10 g kg−1– 18 weeks | Siberian sturgeon (31.1 ± 0.8 g) | Microbial community in the hindgut was affected | Delaedt et al. (2008) | |
| 10 and 20 g kg−1– 10 weeks | Siberian sturgeon (approximately 20 g) | → Growth↑ Acetate, propionate and total SCFA production→ Butyrate production | Rurangwa et al. (2008) | |
| IMO | 2 g kg−1– 28 days | Pacific white shrimp | → Microbial population, immune responses and resistance to white spot syndrome virus | Li et al. (2009b) |
- ADC, apparent digestibility coefficient; SCFA, short-chain fatty acids.
- Symbols represent an increase (↑), decrease (↓) or no effect (→) on the specified response.
| Prebiotic | Dose and length of administration | Fish species (g) | Results | References |
|---|---|---|---|---|
| GroBiotic®-AE | 10 and 20 g kg−1– 2 (trial 1) and 4 (trial 2) weeks | Hybrid striped bass (Morone chrysops × Morone saxatilis) (trial 1–91.4 g; trial 2–19.7 g) | ↑ Feed efficiency↑ Respiratory bursts↑ Resistance against Streptococcus iniae | Li & Gatlin (2004) |
| 20 g kg−1– 16 weeksWeek 16–21 in situ infection of Mycobacterium marinum | Hybrid striped bass (64.5 g) | ↑ Growth performance↑ Resistance against M. marinum | Li & Gatlin (2005) | |
| 20 g kg−1– 16 weeks and thereafter exposed to Flavobacterium columnare | Golden shiners (Notemigonus crysoleucas) (1.06 g) | ↑ Resistance against F. columnare | Sink et al. (2007) | |
| 20 g kg−1– 10 weeks and thereafter exposed to F. columnare for 10 days | Golden shiners (0.46 g) | → Survival↑ Resistance against F. columnare | Sink & Lochmann (2008) | |
| 10 g kg−1– 3 weeks | Red drum (500 g) | ↑ Protein, lipid and organic ADC values | Burr et al. (2008) |
- ADC, apparent digestibility coefficient.
- Symbols represent an increase (↑), decrease (↓) or no effect (→) on the specified response.
The results cited in the present review include works on prebiotics in aquaculture published in peer-reviewed, scientific journals as well as minimally circulated investigations available as short communications and abstracts presented in books from international conferences. The latter is performed to indicate that there are numerous interesting investigations ongoing albeit not yet been published in scientific journals.
Inulin
Some of the more commonly used prebiotics in animal feeds include inulin, FOS and TOS (Vulevic et al. 2004). Inulin-type fructans are composed of β-d-fructofuranoses attached by β-2-1 linkages. The first monomer of the chain is either a β-d-glucopyranosyl or β-d-fructopyranosyl residue. They constitute a group of oligosaccharides derived from sucrose that are isolated from natural vegetable sources. Inulin is found in a variety of edible grains, fruits and vegetables such as wheat, onions, leeks, garlic, asparagus, artichokes and bananas (Roberfroid 1993). Inulin appears to have a beneficial effect on the gut microbiota, particularly in the colon of endothermic animals (Havenaar et al. 1999; Possemiers et al. 2009). Although inulin is not a natural fibre in fish diets, inulin may have interesting applications in aquaculture to stimulate the ‘good’ gut bacteria, suppress pathogens and enhance immune response. An overview of the studies carried out using inulin as a prebiotics is presented in Table 1. Furthermore, insoluble inulin (γ-inulin) has been suggested to possess adjuvant activity because it activates the alternative complement pathway (Silva et al. 2004). During complement activation, several complement fragments are released during the activation cascade. Some of the fragments have distinct effects (anaphylatoxic and chemotactic) on leucocytes harbouring specific receptors. It is known that long-chained inulin stimulates the human immune system by binding to specific lectin-like receptors on leucocytes and inducing macrophage proliferation (Causey et al. 1998; Seifert & Watzl 2007; Meyer 2008). As such, both the innate and adaptive arms of the immune system are modulated by γ-inulin. Whether γ-inulin is absorbed in the fish intestine and thus availability for complement activation is not yet known. Soluble forms of inulin (α and β) are not believed to possess complement activation properties.
To our knowledge, the first preliminary study carried out using inulin was conducted by Wang & Wang (1997). In this 14-day study, inulin was administered via intraperitoneal injection into grass carp (Ctenopharyngodon idellus) (24.6 ± 3.5 g) and tilapia (Tilapia aureus) (21.8 ± 3.5 g). Although survival rates against Aeromonas hydrophila and Edwardsiella tarda were higher, the values were not significantly different from that of the control fish.
Arctic charr and Atlantic salmon
In a study with Arctic charr (Salvelinus alpinus L.; average body weight 218 g), Olsen et al. (2001) showed that 15% inclusion of inulin resulted in intestinal damage. The authors speculated that the reason for this effect may be linked to accumulation of lamellar structures and large vacuoles, but no clear evidence was presented. The effect of 15% dietary inulin and dextrin on aerobic and facultative aerobic bacteria associated with the hindgut (distal intestine) of Arctic charr was investigated by Ringøet al. (2006). Traditional culture-based analysis of the microbiota indicated a notable reduction in the culturable bacterial population level in inulin fed fish. This observation was confirmed by electron microscopical analysis which indicated that fewer bacterial cells were associated with the surfaces of enterocytes of fish fed inulin compared to that of dextrin fed fish.
Refstie et al. (2006) and Bakke-McKellep et al. (2007) reported results from an experiment evaluating the effects of 7.5% dietary inulin, extracted soybean meal and fishmeal on digestive parameters and gut microbiota in Atlantic salmon (Salmo salar L.) reared in seawater. At this inclusion level, dietary inulin did not damage the distal intestine, and it stimulated intestinal growth (higher relative mass of the GI tract) but did not affect the nutrient hydrolytic and absorptive capacity of the salmon GI tract (Refstie et al. 2006). Bakke-McKellep et al. (2007) showed that inclusion of inulin in the diet reduced the diversity of gut microbiota. They lacked Pseudoalteromonas and Micrococcus spp. in the intestine, as well as several species of other genera identified in the other dietary groups. The numbers of isolated lactic acid bacteria species such as Marinilactibacillus psychrotolerans and Carnobacterium maltaromaticum were higher in the digesta than adherent to the mucosa and were higher in the fish fed fishmeal than in soybean meal or inulin fed fish. Enterococcus spp., mostly Enterococcus faecalis, was not detected in the fishmeal fed fish, and their numbers were higher in the gut of fish fed soybean meal than inulin fed fish. When oxytetracycline was added to the inulin diet, less than detection level (102 bacteria g−1) of bacteria was detected in the mid and distal intestinal digesta. The results observed by Bakke-McKellep et al. (2007) from feeding Atlantic salmon dietary inulin are in accordance with those reported for Arctic charr (Ringøet al. 2006) but contradict findings in mammalian studies (Gibson & Roberfroid 1995; Pool-Zobel et al. 2002; Xu et al. 2002). The reason for this has not been elucidated, but Bakke-McKellep et al. (2007) suggested that inulin has a selective effect on Atlantic salmon and Arctic charr GI microbiota. Whether this effect is valid for other fish species and whether it has any effect on fish health remain to be clarified.
Gilthead seabream
Cerezuela et al. (2008) showed in their in vivo study with seabream (Sparus aurata L.) (175 g) fed 5 or 10 g inulin kg−1 a significant inhibition in phagocytosis and respiratory burst by leucocytes. The authors suggested that seabream leucocytes do not seem to have a receptor for inulin and that inulin does not seem to be an optimal prebiotic for seabream.
Sturgeon
In their study evaluating the impact of 2% inulin on microbial fermentation in the spiral valve of Siberian sturgeon (Acipenser baeri), Mahious et al. (2006a) reported no significant differences in total SCFA and lactate content in the spiral valve content as a result of feeding fish on inulin supplemented diet. However, the production of butyrate was significantly higher in the control group fed a diet supplemented with 2% cellulose. The concentrations of SCFAs in the intestinal content could, however, also be affected by their rate of uptake from the intestine, metabolism by enterocytes and/or the rate of SCFA transport across the gut epithelium (Clements & Choat 1995). This can partially explain the lower concentration of butyrate in the inulin fed fish. Inclusion of inulin in the diet significantly increased gas production. Based on their results, the authors suggested that the SCFA production was probably because of the fact that the gut microbiota was able to use inulin as substrate (Mahious et al. 2006a). However, no information was presented demonstrating an effect of inulin on the gut microbiota. Furthermore, end products of inulin fermentation by gut microbiota, such as SCFA, may be used as energy sources by intestinal cells. This hypothesis could explain the higher growth rate of sturgeon fed inulin. Enhanced SCFA production after prebiotic supplementation may increase SCFA supply to immune cells located along the gut-associated lymphoid tissue as reported in animals (Bach Knudsen et al. 2003) and activate these cells via so-called SCFA-receptors.
Turbot
Mahious et al. (2006b) evaluated the effect of dietary Raftiline ST (chicory inulin), at 2% inclusion, on growth and gut microbiota of turbot (Psetta maxima) larvae. Growth was not stimulated, and no clear effect on the gut bacterial community was observed. However, the study focused mainly on Vibrio spp. and Bacillus spp. and did not provide any information about other gut bacteria. The intestinal microbiota in both the control group and larvae fed a diet enriched with inulin was dominated by Vibrio spp. (approximately 65%) in others it was estimated to be 36%, while Bacillus spp. was surprisingly not detected.
Effects on intestinal histomorphology
By means of light- and electron microscopies, Olsen et al. (2001) demonstrated in their early study on Arctic charr that dietary inulin at 15% supplementation level had a destructive effect on microvillous organization in the hindgut compared to fish fed control diet (15% dextrin). The microvilli were often in disarray, lacking in some areas and were less straight than in the control animals. The presence of inulin-induced lamellar bodies (2.3% of cellular volume) dominated much of the cell interior, and inulin also caused an increase in vacuoles from 14.3% of cell volume in control fish to 22.1% of cell volume in inulin fed fish (Fig. 2). This study clearly showed the potentially harmful effects of feeding high levels of inulin to Arctic charr. The damage to the enterocytes appeared to be linked to the accumulation of lamellar structures that may have been absorbed inulin. Based on their results, the authors stated that inulin that cannot be degraded by the cells would accumulate to an extent that cell function became impaired, and that the increase in lysosome-like structures in pyloric caeca probably reflects a cellular response to the accumulation of this indigestible component.

The epithelium in the hindgut of Arctic charr (Salvelinus alpinus L.) fed an inulin diet for 4 weeks. The cells are highly vacuolated, and many of the vacuoles have a lamellar content (small arrows) that may be inulin. The apical surface of these cells shows signs of damage including loss of membrane and microvilli (large arrows). After Olsen et al. (2001).
In the study of Bakke-McKellep et al. (2007), statistical analysis did not reveal significant effects of inulin inclusion (7.5% of diet) in the diet on histological scores (widening and cellular infiltration of the lamina propria, degree of enterocyte vacuolisation and mucosal fold height). However, the mid intestine of six out of 12 inulin fed fish showed moderate leucocyte infiltration in the muscular layers. Eleven of 12 inulin fed fish showed normal morphology of the distal intestine, characterized by the presence of well-differentiated enterocytes with many absorptive vacuoles. However, there appeared to be increased vacuolization in fish fed inulin. The contrasting findings in Arctic charr may be because of differing dietary levels of inulin, 15% for Arctic charr versus 7.5% for salmon, or differing analytic methods. With the aid of transmission electron microscopy, Olsen et al. (2001) reported changes in the organization of microvilli and the presence of intracellular lamellar bodies in distal intestine enterocytes. Light microscopy does not allow sufficient resolution to evaluate these structures. However, Olsen et al. (2001) also reported increased vacuolization of distal intestinal enterocytes (measured as percentage of cell volume). Therefore, the effect of inulin, including possible beneficial effects, in Atlantic salmon diets merits further study. The increased distal intestinal somatic index in inulin fed fish (Refstie et al. 2006) appeared to be the result of hypertrophy of the muscularis externa. Greger (1999) reported similar hypertrophy of the caecal wall in rats fed inulin, possibly caused by increased peristaltic activity.
Further research is needed to determine any potential health benefits of dietary inulin, including effects on fish gut bacteria and their ability to inhibit colonization of pathogenic bacteria, which might have some relevance to aquaculture. Furthermore, it is thus far not possible to conclude whether the indigenous gut microbiota of fish play a role in providing natural resistance to pathogenic microorganisms.
Fructooligosaccharides (FOS)
One of the most common prebiotics studied in humans and terrestrial animals is FOS, a general term that includes all NDOs composed of fructose and glucose units (Swanson et al. 2002a). FOS refers to short and medium chains of β-d-fructans in which fructosyl units are bound by β-(2-1) glycosidic linkages and attached to a terminal glucose unit. Because of a lack of β-fructosidases, mammalian digestive systems cannot hydrolyse the β-(2-1) glycosidic bonds (Teitelbaum & Walker 2002). FOS can be fermented by certain bacteria expressing this enzyme, such as lactobacilli and bifidobacteria (Sghir et al. 1998; Manning & Gibson 2004). Dietary inclusions of FOS will thus selectively support the growth and survival of such bacteria in the GI tract of animals. It is assumed that there are no specific cellular FOS receptors in vertebrates. As such it is rather speculative to assert any immunological effects by direct action of FOS on host cells.
Despite occasional inconsistent results in terrestrial species, some studies have shown that FOS influenced protein digestion and intestinal morphology (Teitelbaum & Walker 2002; Swanson et al. 2002b). These modifications might contribute to improved growth, feed efficiency and disease susceptibility. Dietary supplementation of FOS has been shown to enhance growth rate of some aquatic animals such as Atlantic salmon, hybrid tilapia (Oreochromis niloticus♀ × Oreochromis aureus ♂), turbot larvae and soft-shell turtle (Triortyx sinensis) (Table 2).
Atlantic salmon
Feeding 10 g FOS kg−1 to Atlantic salmon in a 4-month trial, Grisdale-Helland et al. (2008) showed 5% and 6% higher feed efficiency and energy retention, respectively, compared to fish fed the basal diet. However, no significant effects on feed intake, growth, nutrient digestibilities, blood neutrophil oxidative radical production (NBT) or serum lysozyme were observed.
Tilapia
In hybrid tilapia, FOS did not affect growth but increased survival and enhanced activity of innate defence mechanisms [lysozyme and alternative complement activity (ACH50)] (He et al. 2003).
Turbot
Mahious et al. (2006b) investigated the effect of a weaning diet containing 2% Raftilose P95 on growth, gut microbiota and the ability of gut bacteria to utilize Raftilose and Raftiline of turbot larvae. Raftilose had a positive effect on growth, and some effect was also reported on the gut microbiota. Bacillus spp. were only isolated from larvae fed Raftilose, but not from rearing groups fed cellulose powder, Raftiline or lactosucrose. Of the selected gut bacteria isolated from the larvae weaned with Raftilose, Bacillus spp. were the only bacteria able to use Raftilose as a single carbon source, but they could not utilize Raftiline.
Soft-shell turtle
Ji et al. (2004) investigated the effect of FOS (1.5 and 2.5 g kg−1) on growth rate, survival, and activities of superoxide dismutase (SOD) and lysozyme on soft-shell turtle. Stimulated growth, increased survival and higher SOD activity were reported. However, a decreased lysozyme activity was noted at 0.25% inclusion level, but no further information was given.
Short-chain fructooligosaccharides (scFOS)
Supplementation of scFOS has been shown to confer benefits on nutrient utilization, growth and disease susceptibility of various endothermic animal species through improved GI microbiota (Biggs et al. 2006; Respondek et al. 2008). From an aquaculture point of view this is important as during the last decade, there has been an improved understanding of the importance of intestinal microbiota in fish. In fish and shrimp, Chinese scientists have published some information on the effect of scFOS on the intestinal microbiota of hybrid tilapia and white shrimp (Litopenaeus vannamei) (Table 2). However, no investigations appear to have been carried out on scFOS addition to salmonid diets.
Tilapia
In the study by Hui-Yuan et al. (2007), an 8-week feeding trial with hybrid tilapia showed that specific growth rate, daily feed intake and feed conversion ratio were significantly improved with increasing level of scFOS, while hepatopancreas somatic index decreased. Survival rate and condition factor were however not affected. Gut microbiota was also investigated but only culturable counts of Vibrio parahemolyticus, A. hydrophila, Lactobacillus sp. and Streptococcus faecalis were conducted. The results showed a trend of increasing population levels of the investigated bacteria with increasing level of dietary scFOS, but no significant differences were observed.
In a more recent study, Zhou et al. (2009) evaluated the autochthonous gut microbiota by denaturing gradient gel electrophoresis (DGGE) in hybrid tilapia fed either yeast culture or scFOS. There were clear differences in the bacterial community by feeding the fish yeast culture and scFOS, dissimilarity coefficients were 0.22 and 0.26, respectively. When the fish were fed scFOS some unique bands were present in different groups, and these belonged to uncultured bacterium clones and Thiothrix eikelboomii. Whether these bacterial strains are beneficial to the host remains uncertain and needs to be investigated in future studies.
White shrimp
Two studies have investigated the effect of dietary supplementation of scFOS on intestinal microbiota of white shrimp (Li et al. 2007; Zhou et al. 2007). Li and co-authors conducted a 6-week trial in a recirculating system and showed that different inclusion levels of scFOS (0.25, 0.5, 0.75, 1, 2, 4 and 8 g kg−1) did not improve weight gain, feed conversion or survival of shrimp. However, DGGE analysis suggested that the gut microbiota was affected by scFOS compared to shrimp fed a basal diet and that the gut microbial community from shrimp fed the scFOS supplemented diets (1–8 g kg−1) were very similar (similarity coefficient = 92.3%). In this study, most of the gut bacteria were identified as either uncultured or unidentified to genus and species level, and the bacteria showed high similarity to bacteria associated with marine sediments and biofilms and to intestinal bacteria previously reported in humans and rats.
In the report of Zhou et al. (2007), it was shown that dietary scFOS supplementation at concentrations from 0.04% to 0.16% improved specific growth rate and feed conversion of white shrimp cultured in a recirculation system, although survival was relatively low (42–61%) for all treatments. Significant differences were observed in the counts of V. parahemolyticus, A. hydrophila, Lactobacillus sp. and S. faecalis. The counts of V. parahemolyticus were the highest in the gut of shrimp fed 0.04% and 0.08% scFOS, while the population level of S. faecalis was the highest when the shrimps were fed 0.12% and 0.16% scFOS. Whether the microbial shift had any positive effect on the fish health, contribution to inhibit colonization of pathogenic bacteria in the gut or to improve innate immunity remains to be elucidated.
Mannanoligosaccharides (MOS)
MOS are glucomannoprotein complexes derived from the cell wall of yeast (Saccharomyces cerevisiae) (Sohn et al. 2000), and its use in terrestrial animals is well documented (Benites et al. 2008; Klebaniuk et al. 2008; Yang et al. 2008, 2009). The effects of MOS on aquatic animals have been investigated in several recent studies (Table 3). The mannose receptor (MR) is an endocytic receptor of macrophages and endothelial cell subsets whose natural ligands include both ‘self’ glycoproteins and microbial glycans. It is also expressed by immature cultured dendritic cells (DC), where it mediates high efficiency uptake of glycosylated antigens (Linehan et al. 2000). Atlantic cod (Gadus morhua L.) have been shown to possess receptors akin to mammalian MRs (Sørensen et al. 2001). Furthermore, a C-type lectin possessing MR features has been found in shrimp (Zhao et al. 2009). Mannose-containing ligands may also bind to other receptors such as DC-SIGN and dectin-2 resulting in leucocyte activation (Gazi & Martinez-Pomares 2009). Because mannose-containing molecules induce intracellular signalling that may increase production of proinflammatory cytokines, MOS may possess beneficial features as feed additives to fish and shellfish.
Atlantic salmon
Grisdale-Helland et al. (2008) showed that Atlantic salmon fed 10 g MOS kg−1 for 4 months had 11% lower oxygen consumption, 5% lower protein and 3% higher energy concentration in the whole body and 7% greater energy retention than in fish fed the basal diet containing cellulose. Based on their results from the FOS and MOS studies, the authors suggested that these prebiotics have potential in salmon production. However, as this study did not cover the innate immune mechanisms of protection against pathogens and possible beneficial effect on the gut microbiota, these topics should be given high priority in future studies.
Channel catfish
Dietary supplementation of 2 g MOS kg−1 was evaluated in channel catfish (Ictalurus punctatus) (Welker et al. 2007). Inclusion of MOS did not affect growth performance, haematology or immune function. Although, survival from Edwardsiella ictaluri infection was higher (87.5 ± 2.5%) in fish fed MOS than in the control group (75.0 ± 7.5%), but the increase was not significant. Some improvements in plasma cortisol, glucose, lactate and nitrite were observed in MOS fed fish after exposure to low water condition for 30 min.
Cobia
Live feed [rotifers (Brachionus plicatilis) and Artemia] enriched with MOS (0.2% dry weight basis) and fed to cobia (Rachycentron canadum) larvae resulted in greater larval survival following stress challenge at 6 and 7 days posthatching (dph) following exposure to hyposaline water and at 13 dph when challenged with hypersaline (55 g L−1) water compared to control-fed larvae (Salze et al. 2008). Based on their results, the authors suggested the apparent protective nature of MOS to hypo-osmotic challenge can have practical applications for the emerging inland, tank-based cobia production. MOS supplementation appeared to improve microvilli alignment (more uniform, densely packed, and longer and narrower microvilli) compared to larvae fed the control diet, which may enhance the function of the protective mucin barrier and influence ion regulation. Furthermore, the number and size of supranuclear vacuoles (SNVs) in the apical region of enterocytes noted in MOS-treated cobia were fewer and smaller than those observed in control fish. SNV may act as a supplementary mechanism for the absorption and transport of lipids and intact proteins and polypeptides in anterior and posterior intestine, respectively (Watanabe 1984; McLean et al. 1999). The implication of fewer, smaller SNVs in the MOS-treated larvae is not known.
European lobster
The use of MOS in European lobster (Homarus gammarus) diets has been investigated in two studies (Daniels et al. 2006, 2007). In the first study, Daniels et al. (2006) showed that low addition (20ppt) of MOS improved survival and growth of lobster larvae to their first juvenile stage (IV) as well as during later juvenile stages. However, the authors stated that negative effects were revealed with the inclusion of MOS at higher concentration (200ppt), with increased mortality in early juvenile stages and decreased morphological development to stage VIII. Meanwhile, as no clear evidence was presented on decreased morphological development, further studies have to be carried out. In a later study (Daniels et al. 2007), fluorescently labelled MOS particles were made, and the results indicated potential breakdown of MOS by Artemia and fluorescence in the lobster gut. However, to validate these results, further research is needed.
European sea bass
Torrecillas et al. (2007) investigated the effects of two inclusion levels of MOS (2% and 4%) on growth, feed utilization, immune status and disease susceptibility against Vibrio alginolyticus in European sea bass (Dicentrarchus labrax). In fish fed the MOS-supplemented diets growth was significantly improved at both inclusion levels. The authors suggested that the increased growth may be related with enhanced amino acid absorption as demonstrated in chickens (Iji et al. 2001). The study also showed that dietary MOS incorporation at 4% significantly improved head kidney macrophages phagocytic activity, but this effect was significantly reduced at 2% inclusion level. After a 21-day challenge test, the number of fish infected by V. alginolyticus was significantly reduced by the dietary MOS supplementation.
Rainbow trout
Staykov et al. (2007) used MOS (2 g kg−1) derived from the outer cell wall of Saccharomyces cerevisiae strain 1026 to evaluate the effect on growth performance and immune status of rainbow trout (Oncorhynchus mykiss). In general, fish fed the MOS supplemented diet had significantly improved growth performance. In the first trial, improved antibody titre and lysozyme activity were reported, but in the second trial, the effect was less pronounced. Bactericidal activity was not affected by the MOS treatment. Even though some positive results were reported, further research is needed to understand the immune status and possible effect of MOS on disease susceptibility. Also, the purity of MOS with respect to possible β-glucan impurities was not addressed in this study.
Yilmaz et al. (2007) investigated the effect of dietary MOS on growth, body composition and liver and foregut histology of rainbow trout (37.5 ± 1 g). The fish were fed four experimental diets supplemented with 0 (control), 1.5, 3.0 or 4.5 g MOS kg−1. Improved growth performance was generally observed in fish fed the diet supplemented with 1.5 g MOS kg−1. Intestinal villous folds of fish fed diets supplemented with 1.5 or 3.0 g MOS kg−1 MOS were longer than those of fish fed 4.5 g kg−1 or no dietary MOS (P < 0.05). MOS at any level had no detrimental effects on the intestinal morphology. There were no significant differences in feed conversion ratio, protein efficiency ratio or hepatosomatic index (P > 0.05).
Dimitroglou et al. (2008) reported increased absorptive surface area and increased microvilli density and length in rainbow trout fed 0.2% MOS for 8 weeks. In their study with rainbow trout, Rodrigues-Estrada et al. (2008) investigated the effect of 4 g MOS kg−1 on growth, immunological parameters and susceptibility against Vibrio anguillarum. Inclusion of MOS stimulated growth, haemolytic activity and phagocytic activity, mucosa weight and improved survival when the fish were challenge with V. anguillarum.
Red drum
Burr et al. (2008) reported that 1% inclusion of MOS to a basal soybean-meal-based diet fed for red drum (Sciaenops ocellatus L.) increased protein, organic matter, carbohydrate and energy apparent digestibility coefficient (ADC) values. However, the lipid ADC values were significantly lower for fish fed the MOS diet compared to fish basal diet. The authors suggested that the decrease in lipid uptake may have been because of energy needs already being met by catabolism of carbohydrates and protein or that MOS interfered with the uptake of dietary lipids by down-regulating enzymes involved in lipid digestion/absorption. As no general conclusion could be given, the authors suggested that there is a further need to determine the mode of action responsible for the reduction in lipid digestibility.
Sturgeon
Pryor et al. (2003) fed 0 and 3 g MOS kg−1 to Gulf sturgeon (Acipenser oxyrinchus desotoi) but reported no difference in growth performance (specific growth rates, feed conversion ration and condition factor), gross gastrointestinal morphology or spiral valve villi structure (width and length) between the experimental groups. The authors speculated that because villi structure, gastrointestinal morphology and growth are dependent on the gut microbiota, any differences in bacterial community could affect the parameters measured. However, as analysis of the gut microbiota was not included in the present study, no conclusion can be drawn. Pryor and co-authors also suggested that the lack of effect observed in their study could be related to that MOS would leach from the diet in the environment before being consumed, but such an effect was not evaluated.
Tilapia
He et al. (2003) reported improved survival, enhanced lysozyme and ACH50 activities in hybrid tilapia fed 0.6% MOS compared to fish fed the control diet. However, growth was not affected. Genc et al. (2007a) showed no significant differences between the treatment groups (0, 1.5, 3.0 or 4.5 g MOS kg−1) on growth parameters (live weight gain, specific growth rate, feed conversion ratio, protein efficiency ratio) or organosomatic indices (hepoatosomatic and viscerosomatic) of hybrid tilapia. However, dry matter and protein contents of fish fillets increased with increasing levels of dietary MOS. Villi length in the intestine was investigated, but the only significant difference observed was between fish fed 1.5 g MOS kg−1 and fish fed 4.5 g MOS, respectively. As no suggestion regarding mode of action was put forward, this has to be elucidated in future studies.
Sado et al. (2008) showed in their study with juvenile Nile tilapia (13.6 ± 0.7 g) that different inclusion levels of MOS (0, 2, 4, 6, 8 and 10 g kg−1) did not significantly affect haematological parameters (red blood cell, white blood cell, haemoglobin, haematocrit, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration and total plasmatic protein). However, daily feed consumption decreased with increasing levels of MOS. The authors speculated that prebiotic dose, administration duration and population status (age, sex and gonad maturation) could affect the results. To confirm this hypothesis, it is necessary to carry out further studies.
Based on their results that 4 and 6 g inclusion levels of MOS significantly improved weight, 15.4% increase and resistance against Streptococcus agalactiae, 43.3% for the control group and 0% for fish were fed 4 and 6 g MOS, Samrongpan et al. (2008) suggested that MOS is a beneficial feed supplement for Nile tilapia fry.
Tiger shrimp
Genc et al. (2007b) evaluated the effect of three different inclusion levels (1.5, 3 and 4.5 g kg−1) of MOS on growth, body composition and hepatopancreas histology of tiger shrimp (Penaeus semisulcatus) at postlarval stage. At the end of this 48-day study, enhanced growth performance, feed conversion and survival were observed in shrimp fed on a diet containing 3 g MOS kg−1. However, protein content in the whole body decreased with increasing levels of dietary MOS. No detrimental effect was noted on the histomorphology of the hepatopancreas (digestive gland). Genc et al. (2007b) put forward the hypothesis that the lower body protein concentration in shrimp fed the MOS diets may have been the result of lower amino acid utilization and diet digestibility. Based on their results, the authors suggested that inclusion of 3 g MOS kg−1 could be used as a growth promoter in tiger shrimp diets. As no result was presented on disease susceptibility against common pathogens in tiger shrimp culture, this topic should be investigated in future studies.
As the MOS studies on Atlantic salmon, cobia, common carp, European catfish, European sea bass, rainbow trout, red drum, sturgeon, tilapia, European lobster and tiger shrimp did not include bacteriological investigations and the ability of the gut microbiota to inhibit colonisation of pathogenic agents, these topics should be included in future studies, as well as the effect on innate immunity and disease susceptibility to well known fish pathogenic bacteria.
When using light microscopy, Torrecillas et al. (2007) found that MOS included in diets for European sea bass resulted in lower lipid vacuolisation and regular-shaped morphology of hepatocytes around the sinusoidal spaces in the liver, indicating a better utilisation of dietary nutrients. No differences on gut morphology by adding MOS to the diet were observed. However, better microvilli alignment upon MOS feeding has been observed in sole (Solea senegalensis) Sweetman & Davies (2005), rainbow trout (Yilmaz et al. 2007; Dimitroglou et al. 2008) and larval cobia (Salze et al. 2008). Sweetman & Davies (2005) showed that dietary MOS improved anterior gut morphology of sole (Solea senegalensis). Electron microscopy showed more densely packed complex microvilli structure as well as more regular and longer intestinal villi in the MOS-treated fish. In addition, scanning electron microscopy revealed fewer mucosal lesions in MOS fed fish. Sweetman et al. (2008) showed significant effect of MOS on microvilli structure of salmon (Fig. 3), where the microvilli density of MOS fed fish was 4.6 times greater than that of control fish. Microvilli length was increased by 21% in the MOS fed group. Furthermore, the authors stated that scanning electron microscopy micrographs also indicate considerably fewer mucosal lesions in MOS fed fish. However, this was not clearly demonstrated and has to be further investigated in future studies.
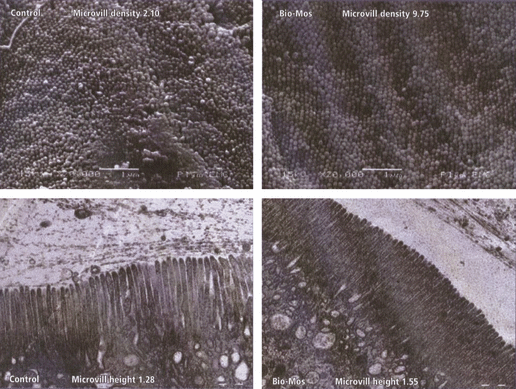
Microvilli structures in the gastrointestinal tract of Atlantic salmon (Salmo salar L.) fed a control diet (without MOS) or a MOS supplemented diet. After Sweetman et al. (2008). MOS, mannanoligosaccharides.
Galactooligosaccharides (GOS)
GOS consisting of 2–20 molecules of galactose and glucose and can be produced through enzymatic treatments of lactose (Yang & Silva 1995), and the prebiotic has been widely been used in endothermic animals (Sako et al. 1999; Vos et al. 2007). However, to our knowledge, only two studies have been carried out using GOS in fish (Table 4). According to Burr et al. (2008), 1% GOS significantly increased protein and organic matter ADC values, while lipid ADC values were significantly decreased compared with red drum fed the basal diet. In contrast to these results, Grisdale-Helland et al. (2008) reported that GOS had no effect on digestibility in Atlantic salmon or on feed intake or growth. However, protein concentration in the whole body and protein retention were reduced by 6% and 9%, respectively, possibly as a result of increased nitrogenous losses in non-faecal excretions.
Xylooligosaccharides (XOS)
XOS are xylose-based oligomers and have some specific characteristics that are driving research efforts to develop applications in fields related to the food and feed industries. XOS appear naturally in bamboo shoots, fruits, vegetables, milk and honey (Vazquez et al. 2000). Like other oligosaccharides, XOS are non-digestible and act as prebiotics promoting the growth of beneficial bifidobacteria in the colon of mammals (Crittenden & Playne 1996; Hsu et al. 2004). To the authors knowledge, only one study has been carried out on fish allogynogenetic crucian carp (Carassius auratus gibelio) (Table 4). XOS was added to fish basal semi-purified diets at three concentrations by dry feed weight: 50, 100 and 200 mg kg−1 (Xu et al. 2009). After 45 days, survival was not affected by any diet as no mortalities were observed. There were significantly higher (P < 0.05) relative gain rate and daily weight gain (DWG) of fish fed the XOS-containing diets, highest in the fish fed 100 mg XOS kg−1, compared with the control. Likewise, the protease and amylase activities in the intestinal content and hepatopancreas generally increased dose-dependently with maximum levels in fish fed the diet containing 100 mg XOS kg−1. Growth performance as well as enzymatic activity levels was generally lower in fish fed the diet containing 200 mg compared to 100 mg XOS kg−1. The authors speculated that the beneficial influence of XOS on growth and the better enzyme activities might be associated to an alteration of the gut microbiota. However, as no such information was presented, the hypothesis needed to be tested in future studies.
Arabinoxylooligosaccharides (AXOS)
Arabinoxylans (AX) are the main non-starch polysaccharides found in many cereal grains and are part of dietary fibre (for review see, Swennen et al. 2006; Grootaert et al. 2007). They consist of β-(1,4)-linked d-xylopyranosyl residues to which arabinofuranosyl moieties are attached. They are degraded in the colon of mammals by specific intestinal bacteria possessing AX-degrading enzymes. Although some health effects of AX are documented the effects of their hydrolysis products, the AXOS, are less studied (Grootaert et al. 2007; Courtin et al. 2008). AXOS can be produced by enzymatic depolymerisation of lignocellulose such as wheat bran (Yamada et al. 1994; Swennen et al. 2006). To date, only two studies have been carried out on AXOS in fish (Table 4).
Siberian sturgeon and African catfish
Delaedt et al. (2008) investigated the impact of 1% AXOS on the distal gut microbiota of Siberian sturgeon in an 18-week study. The microbial community was investigated by DGGE using eubacterial primers as described by Muyzer et al. (1993). While the DGGE fingerprints of gut samples from control fish seem to be dominated by a limited number of microorganisms, the bacterial community in AXOS-fed fish was more diverse, indicating that the gut microbiota in Siberian sturgeon is influenced by AXOS administration. Whether this change in gut microbiota has any beneficial health effects needs evaluation in future studies.
Rurangwa et al. (2008) investigated the effect of 1% and 2% AXOS on growth and SCFA production in the hindgut of Siberian sturgeon and African catfish (Clarias gariepinus). Inclusion of AXOS had no effect on growth of either species, but an effect was reported on acetate, propionate and total SCFA production with higher levels observed in the AXOS-fed fish. As this effect was less pronounced in catfish, the authors suggested that the hindgut microbiota is different in the two species and that this would be investigated in future research.
Isomaltooligosaccharides (IMO)
IMO are a mixture of isomaltose, isomaltotriose, panose, isomaltotetraose, etc. (Kaneko et al. 1995). Information is available on IMO as prebiotics in poultry (Zhang et al. 2003; Chung & Day 2004; Thitaram et al. 2005) and pig (Li et al. 2009a), and IMO has also been named Bifidus-factor because of its powerful growth stimulation of bifidobacteria. To our knowledge, only one study has been carried out on aquatic animals (Li et al. 2009b). In this study, no clear effect was noted on microbial population, immune responses and resistance to white spot syndrome virus in Pacific white shrimp (L. vannamei) (Table 4).
GroBiotic®-A
The commercial product GroBiotic®-A is a mixture of partially autolysed brewers yeast, dairy ingredient components and dried fermentation products. The yeast membrane is composed of many different polysaccharides, one of them is insoluble β-glucans. It is widely acknowledged that yeast β-glucans as well as β-glucans from other sources may induce immunological responses in fish (Dalmo & Bøgwald 2008). It is highly likely that β-glucan components in barley and yeast are capable of inducing some immune responses because of β-glucan receptors on leucocytes. The fact that there may be β-glucan receptors which may induce intracellular signalling events indicates that yeast and barley supplements as not prebiotics per se but rather immunostimmulatory in their effects. An overview of the studies carried out using GroBiotic®-A in fish is presented in Table 5.
Hybrid stripped bass
Li & Gatlin (2004) evaluated GroBiotic®-A in diets for juvenile hybrid striped bass (Morone chrysops × Morone saxatilis) and observed significantly enhanced feed efficiency. The response of the intestinal microbiota was not defined in this study. However, the study indicated that supplementation with GroBiotic®-A enhanced respiratory burst of head kidney leucocytes and resistance against Streptococcus iniae infection. However, the interpretation of these beneficial influences was complicated by the presence of brewer’s yeast, which is generally considered to be an immunostimulant for fishes (Siwicki et al. 1994; Ortuño et al. 2002; Li & Gatlin 2003; Rodríguez et al. 2003). Later, Li & Gatlin (2005) reported enhanced growth performance in sub-adult hybrid striped bass fed both a diet supplemented with GroBiotic®-A and a diet supplemented with brewer’s yeast compared to fish fed the basal diet throughout the feeding trial, with significantly (P < 0.05) enhanced weight gain observed after 16 weeks of feeding. At the end of the feeding trial, fish fed 2% brewers yeast showed significantly higher feed efficiency than those maintained on the other diets. The in situ mycobacterial challenge employed in this experiment resulted in overall cumulative mortality of approximately 25%. Fish fed 2% GroBiotic®-A had a significantly (P < 0.05) enhanced survival (80%) compared to the other treatments (72–73%) at the end of 21 weeks. This study indicates that the prebiotics in concert with immunostimmulatory compounds in GroBiotic®-A can have an enhanced effect on disease susceptibility compared to brewers yeast alone.
Golden shiners
The objective of the study by Sink et al. (2007) was to determine whether supplementation of GroBiotic®-A in high-fat diets could be used to increase survival of golden shiners (Notemigonus crysoleucas) subjected to Flavobacterium columnare challenges. As the survival for the group fed the dairy yeast prebiotic was significantly higher than those of fish fed control diets (4% and 10% poultry fat), the authors suggested that the prebiotic has promising applications in golden shiners exposed to F. columnare. Later, Sink & Lochmann (2008) conducted an investigation with golden shiners that were fed either a control diet or a 2% dairy yeast prebiotic for 10 weeks in outdoor pools before challenge with F. columnare. This preliminary study suggested that prebiotic supplementation in golden shiners prior to a stressful event (captured and held in nets within the tanks fro 30 min) could significantly reduce the mortality caused by F. columnare.
Red drum
Burr et al. (2008) reported that GroBiotic®-A increased protein, organic matter, energy and carbohydrate ADC values, but the lipid values were similar compared to fish fed a basal diet containing 9 g cellulose kg−1. In their study on the effects of prebiotics (inulin, MOS, GOS and GroBiotic®-A) on nutrient digestibility of red drum, Burr et al. (2008) speculated that increased nutrient digestibility might to some extent be because of the microbial community producing enzymes that either occur only at low levels or are lacking in the host. However, as no such evaluation was performed in this study, further studies are needed to confirm this hypothesis.
Use of prebiotic to enhance health-promoting bacteria
It has been documented in a number of farmed animals that their GI tract microbiota plays important roles in affecting the nutrition and health of the host organism (Pineiro et al. 2008; Salminen & Isolauri 2008). Thus, various means of altering the intestinal microbiota to achieve favourable effects such as enhancing growth, digestion, immunity and disease resistance of the host organism have been investigated in various terrestrial livestock as well as in fish and humans (Zoetendal et al. 2004; Fortun-Lamothe & Boullier 2007; Gomez & Balcazar 2008; Metzler & Mosenthin 2008; Reid 2008). Dietary supplementation of prebiotics that beneficially affects the host by stimulating growth and/or activity of a limited number of health-promoting bacteria such as Lactobacillus and Bifidobacterium in the intestine, while limiting potentially pathogenic bacteria such as Salmonella, Listeria and Escherichia coli, has been reported to favourably affect various terrestrial species (Lebeer et al. 2008; Fung et al. 2009; Schroeter & Klaenhammer 2009). However, such information is completely absent to date for aquatic organisms. In general, the GI tract microbiota of fish, including those produced in aquaculture, has been poorly characterized, especially the anaerobic microbiota. Therefore, more detailed studies of the microbial community of cultured fish are needed to assess the effectiveness of prebiotic supplementation.
Probiotics and synbiotics
Microbial products that are considered alternatives to the prophylactic use of chemicals are good candidates (Gatesoupe 2008). Probiotics are live microorganisms added to feed or rearing water that when administered to fish in adequate amounts confer increase in viability, enhance immune and digestive systems, promote growth and general welfare.
Synbiotics, as defined by Gibson & Roberfroid (1995) are a mixture of prebiotics and probiotics that beneficially affects the host by improving the survival and implantation of live microbial dietary supplements in the GI tract by selectively stimulating the growth and/or by activating the metabolism of one or a limited number of health-promiting bacteria, and thus improving the host ‘welfare’. As less information per se is available about synbiotics in aquatic animals (Li et al. 2009b), this topic should be given high priority in future studies.
Concluding remarks and future perspectives
Despite the potential benefits to health and performance as noted in various terrestrial species, less information is available about the effect of prebiotics in fish. In fish the effect of prebiotics on growth, feed conversion, gut microbiota, mucosal barriers, cell damage/morphology, disease susceptibility and on innate immune parameters have been investigated to a limited extent and variably in different species. It is likely that many factors, some influenced by the microbiota, are involved in disease resistance. Per se one particular topic that is missing is if the prebiotics supplementation effect can varied within a normal seasonal cycle and during growth. Furthermore, the surrounding environment (water temperature, oxygen availability, etc.) might have greater influences than the diet on fish health or potentially confound interpretations of the prebiotic findings. We therefore recommend that these topics are included in future prebiotic studies.
It has been suggested that oligosaccharides and polysaccharides other than prebiotics also present in plants such as legumes and grains may actually induce an inflammatory response. For example, oligosaccharides in soybeans, such as raffinose, stachyose and verbascose (a variable mix of α-galactosyl homologues of sucrose), have been suggested to be involved in soybean-meal-induced enteropathy in the distal intestine of salmonid fishes, possibly causing the diarrhoea by their osmotic activity (Refstie et al. 2000, 2005). It may be speculated that these oligosaccharides, besides inducing proinflammatory responses, may also modulate the action of immune regulatory cytokines that may be beneficial during an intestinal inflammatory response. Further research is needed in this area to differentiate the health-promoting effects from potentially deleterious responses and know what are the modes of action of the different chemical classes of oligosaccharides.
Following the numerous genome sequencing tools that are currently used, future research on prebiotic effects should involve transcriptome and proteome analysis using high throughput assays. In addition, transcriptome and proteome profiling of gut microbiota should be achievable. It is also important to differentiate pure prebiotics from non-prebiotic substances because the former molecules do not bind to cell receptors. In the case of, e.g. mannosylated substances where MRs, dectin-2 and DC-SIGN, may be involved one may expect different effects compared to the microbiota and physiological effects caused by prebiotics. From a bacteriological point of view, it is of great importance to evaluate whether the microbial shift has any positive effect on fish and shellfish health, contribution to inhibit colonization of pathogenic bacteria in the gut and improving innate immunity. Studies on prebiotics should, therefore, be given high priority in the future, and challenge studies should be included as a golden standard to assess their effects on fish health.
Acknowledgements
This review is a revision of the section prebiotics in the ad hoc report ‘A risk-assessment on the use of plant ingredients in diets for carnivorous fish’ financed by the Norwegian Scientific Committee of Food Safety. The report is available at: http://www.vkm.no.




