Growth performance and body composition of pacu Piaractus mesopotamicus (Holmberg 1887) in response to dietary protein and energy levels
Abstract
Improper dietary protein and energy levels and their ratio will lead to increased fish production cost. This work evaluated effects of dietary protein : energy ratio on growth and body composition of pacu, Piaractus mesopotamicus. Fingerling pacu (15.5 ± 0.4 g) were fed twice a day for 10 weeks until apparent satiation with diets containing 220, 260, 300, 340 or 380 g kg−1 crude protein (CP) and 10.9, 11.7, 12.6, 13.4 or 14.2 MJ kg−1 digestible energy (DE) in a totally randomized experimental design, 5 × 5 factorial scheme (n = 3). Weight gain, specific growth rate increased and feed conversion ratio (FCR) decreased significantly (P < 0.05) when CP increased from 220 to 271, 268 and 281 g kg−1 respectively. Pacu was able to adjust feed consumption in a wide range of dietary DE concentration. Fish fed 260 CP diets showed best (P < 0.05) protein efficiency ratio and FCR with 11.7–12.6 MJ kg−1; but for the 380 CP-diets group, significant differences were observed only at 14.2 MJ kg−1 dietary energy level, suggesting that pacu favours protein as energy source. DE was the chief influence on whole body chemical composition. Minimum dietary protein requirement of pacu is 270 g kg−1, with an optimum CP : DE of 22.2 g MJ−1.
Introduction
The pacu, Piaractus mesopotamicus, is a Characin native of rivers, floodplains, lakes and flooded forests of Parana, Paraguay and Uruguay basins, central South America. Because of its omnivorous feeding habit, high growth rates, good meat quality, and good consumer acceptance, pacu has become an important aquaculture species (Urbinati & Gonçalves 2005).
Dietary protein requirements for best growth of pacu are reported to vary from 260 to 360 g kg−1 depending on fish size, dietary protein source or dietary energy level (Brenner 1988; Fernandes et al. 2000); however, the wide range of variation turns this information rather controversial. Protein utilization for growth may be improved by partially replacing dietary protein with non-protein energy sources (lipid and carbohydrate) (Seenappa & Devaraj 1995; Satpathy et al. 2003; Kim & Lee 2005; Wang et al. 2005; Ozório et al. 2006; Mohanta et al. 2007).
However, knowing dietary protein requirements alone do not suffice. Improper dietary protein to energy ratio leads to increased fish production costs and deterioration of water quality resulting from wasted feed. It is thus important adjusting dietary protein to energy levels to formulate and produce commercial feeds (Lee & Kim 2005). Scarce information is available on optimum dietary protein to energy ratios or utilization of dietary non-protein energy sources for pacu (Carneiro et al. 1994; Abimorad & Carneiro 2007). Consolidating bibliographic information, Fracalossi (2002) suggested that the optimal dietary protein : energy (P : E) ratio for optimum growth of pacu ranges on 72–109 mg kcal−1. This exceedingly wide range brings too much uncertainty to fish nutritionists, researchers and fish farmers alike. Therefore, the present experiment was designed to determine optimum dietary protein and energy levels and their ratio for nutrition and feeding of pacu.
Materials and methods
Trials were set up in a closed-loop, indoor system, under continuous aeration and emergency oxygenation systems, 12 h light : 12 h dark photoperiod. Water quality parameters: pH (7.7 ± 0.3), dissolved oxygen (6.1 ± 0.1 mg L−1), ammonia (≤0.5 mg L−1) and temperature (28.7 ± 1.8 °C) were monitored daily and remained within acceptable values for pacu (Urbinati & Gonçalves 2005). Pacu fingerlings were obtained from commercial hatchery and acclimatized to the laboratory conditions for 1 week, feeding on a 400 g kg−1 crude protein (CP) commercial diet.
Twenty-five diets were formulated to contain 220, 260, 300, 340 and 380 g CP kg−1 diet, with five levels of digestible energy (DE) per level of CP: 10.9, 11.7, 12.6, 13.4 and 14.2 MJ DE kg−1 diet (Table 1). To minimize differences in nutrient utilization among dietary treatments, the ratio between CP from fish meal to dietary CP was fixed in 100 g kg−1 for all diets, and all diets had a minimum of 7.5 g kg−1 available phosphorus (Pezzato et al. 2006). DE of soybean meal, fish meal, wheat bran and corn were calculated, considering results of gross energy previously determined, from data of apparent digestibility coefficient for pacu reported by Abimorad & Carneiro (2004); DE for corn gluten meal and broken rice were estimated from data of Pezzato et al. (2002) and Oliveira Filho & Fracalossi (2006).
| DE (MJ kg−1) | 10.9 | 11.7 | 12.6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP (g kg−1) | 220 | 260 | 300 | 340 | 380 | 220 | 260 | 300 | 340 | 380 | 220 | 260 | 300 | 340 | 380 |
| Ingredient (g kg−1) | |||||||||||||||
| Soybean meal | 228.5 | 325.7 | 414.2 | 497.9 | 593.6 | 234.9 | 333 | 378.4 | 425.7 | 503.2 | 190.6 | 334.8 | 274.6 | 313.1 | 355.3 |
| Fish meal | 30 | 40 | 45 | 50 | 55 | 30 | 40 | 45 | 50 | 55 | 30 | 40 | 45 | 50 | 55 |
| Corn gluten meal | – | 10 | 10 | 10 | 10 | – | 10 | 40.5 | 69.7 | 54.7 | 34.6 | 10 | 115.3 | 150.1 | 155.4 |
| Wheat bran | 158 | 8.7 | 10 | 67.1 | 66.4 | 174.5 | 10 | 10 | 10 | 154.6 | 168.7 | 10 | 10 | 10 | 200 |
| Corn | 10 | 85.1 | 50.3 | 25 | 62.7 | 241.4 | 348.5 | 296.5 | 251 | 10 | 370.6 | 404.7 | 349.9 | 274.5 | 53 |
| Rice meal | 456.9 | 410 | 360 | 240 | 100 | 210 | 155 | 130.9 | 100 | 149 | 100 | 100 | 100 | 100 | 100 |
| Soybean oil | 11.6 | 15.6 | 15.5 | 15 | 15 | 19.2 | 18.7 | 15 | 11 | 15 | 27.9 | 17.3 | 22.8 | 20.2 | 15.8 |
| Cellulose | 59.8 | 59.8 | 54.8 | 54.8 | 57.1 | 49.8 | 44.6 | 43.5 | 42.4 | 18.4 | 37.3 | 42.9 | 42.2 | 41.9 | 25.4 |
| Vitamin–mineral mix1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| l-Lysine HCL | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Dicalcium phosphate | 35 | 35 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| BHT | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Diet cost (US$ kg−1)2 | 0.31 | 0.33 | 0.34 | 0.34 | 0.35 | 0.29 | 0.31 | 0.34 | 0.36 | 0.37 | 0.31 | 0.31 | 0.38 | 0.41 | 0.42 |
| Chemical composition (g kg−1)3 | |||||||||||||||
| Crude protein | 214 | 251 | 300 | 347 | 377 | 230 | 265 | 304 | 347 | 383 | 224 | 263 | 293 | 351 | 384 |
| Nitrogen free-extract | 572 | 520 | 457 | 424 | 363 | 538 | 515 | 471 | 434 | 409 | 538 | 534 | 509 | 439 | 387 |
| Crude fibre | 45 | 41 | 40 | 42 | 51 | 49 | 32 | 35 | 36 | 29 | 42 | 39 | 31 | 34 | 34 |
| Fat | 45 | 68 | 81 | 61 | 71 | 75 | 73 | 73 | 60 | 54 | 71 | 55 | 57 | 76 | 64 |
| Ash | 62 | 63 | 63 | 73 | 78 | 63 | 63 | 65 | 69 | 79 | 60 | 62 | 59 | 43 | 72 |
| Dry matter | 938 | 943 | 941 | 947 | 940 | 955 | 948 | 948 | 946 | 953 | 935 | 953 | 949 | 942 | 941 |
| Gross energy (MJ kg−1) | 17.3 | 18.2 | 18.7 | 18.5 | 18.7 | 18.4 | 18.5 | 18.7 | 18.6 | 18.7 | 18.0 | 18.2 | 18.4 | 19.3 | 18.7 |
| CP : DE ratio (g MJ−1) | 19.7 | 23.1 | 27.6 | 31.3 | 34.7 | 19.6 | 22.6 | 25.9 | 29.6 | 32.7 | 17.8 | 21.0 | 23.4 | 28.0 | 30.6 |
| DE (MJ kg−1) | 13.4 | 14.2 | ||||||||
| CP (g kg−1) | 220 | 260 | 300 | 340 | 380 | 220 | 260 | 300 | 340 | 380 |
| Ingredient (g kg−1) | ||||||||||
| Soybean meal | 121.5 | 127.8 | 162 | 185.5 | 231.6 | 10 | 10 | 72.1 | 100 | 155.8 |
| Fish meal | 35 | 45 | 45 | 50 | 55 | 35 | 45 | 45 | 50 | 55 |
| Corn gluten meal | 56 | 118.2 | 195.6 | 210 | 255 | 134.1 | 195.2 | 246.5 | 270 | 300 |
| Wheat bran | 320 | 250 | 10 | 242.8 | 126.1 | 298.8 | 273.1 | 100 | 263 | 215.5 |
| Corn | 269.1 | 267 | 373.4 | 108.8 | 128.3 | 310.6 | 270.8 | 306.1 | 97.5 | 109.2 |
| Rice meal | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 50 |
| Soybean oil | 42.1 | 31.9 | 32.1 | 29.2 | 26.7 | 51.4 | 41.6 | 49.3 | 46.5 | 41.7 |
| Cellulose | 15.1 | 19.9 | 41.8 | 33.5 | 37.2 | 15.9 | 21.2 | 38.8 | 32.8 | 32.7 |
| Vitamin–mineral mix1 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| l-Lysine HCL | 6 | 5 | 5 | 5 | 5 | 9 | 8 | 7 | 5 | 5 |
| Dicalcium phosphate | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| BHT | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Diet cost (US$ kg−1)2 | 0.34 | 0.38 | 0.42 | 0.44 | 0.48 | 0.39 | 0.43 | 0.47 | 0.48 | 0.51 |
| Chemical composition (g kg−1)3 | ||||||||||
| Crude protein | 218 | 261 | 301 | 347 | 386 | 224 | 266 | 309 | 350 | 385 |
| Nitrogen free-extract | 576 | 531 | 485 | 428 | 410 | 546 | 508 | 467 | 422 | 396 |
| Crude fibre | 31 | 30 | 31 | 34 | 33 | 33 | 36 | 33 | 38 | 31 |
| Fat | 54 | 63 | 67 | 61 | 57 | 89 | 80 | 88 | 83 | 79 |
| Ash | 62 | 61 | 53 | 63 | 61 | 56 | 58 | 53 | 61 | 62 |
| Dry matter | 941 | 946 | 938 | 933 | 946 | 947 | 948 | 949 | 953 | 953 |
| Gross energy (MJ kg−1) | 17.6 | 18.2 | 18.6 | 18.4 | 18.9 | 18.6 | 18.7 | 19.3 | 19.3 | 19.5 |
| CP : DE ratio (g MJ−1) | 16.3 | 19.5 | 22.5 | 25.9 | 28.8 | 15.7 | 18.7 | 21.7 | 24.6 | 27.1 |
- BHT, butylated hydroxytoluene.
- 1 Vitamin and mineral mix (Supre Mais®, Valinhos, SP, Brazil), ingredient kg−1: vitamins: A = 1 200 000 UI; D3 = 200 000 UI; E = 12 000 mg; K3 = 2400 mg; B1 = 4800 mg; B2 = 4800 mg; B6 = 4000 mg; B12 = 4800 mg; folic acid = 1200 mg; calcium pantothenate = 12 000 mg; C = 48 000 mg; biotin = 48 mg; choline = 65 000 mg; niacin = 24 000 mg; minerals: Fe = 10 000 mg; Cu = 600 mg; Mn = 4000 mg; Zn = 6000 mg; I = 20 mg; Co = 2 mg; Se = 20 mg.
- 2 Considering exchange rate at 07/02/2007 of US$ 1.00 = R$ 1.91140.
- 3 Original matter basis.
Feedstuffs were homogenized through a 1.0-mm sieve, mixed, moistened with distilled water (30% v : w) and pelleted (2.0 mm) in a mincer. Diets were then dried in a forced ventilation oven (50 °C; 24 h) and dried pellets were hermetically packed in black plastic bags and stored at −4 °C until use.
Experimental procedures
Fish were randomly stocked into 60-L cages (12 fish per cage; 15.5 ± 0.4 g) housed in 1-m3, circular plastic tanks (three cages per tank) in a totally randomized experimental design, 5 × 5 factorial scheme, and fed with the experimental diets until apparent satiation twice a day (07:00 and 16:00 hours) for 60 days. Feed consumption was recorded weekly for each cage.
where Wi is initial weight, Wf is final weight, FI is total feed intake, ln is natural logarithm, FBP is final body protein, IBP is initial body protein, FBE is final body gross energy, IBE is initial body gross energy and TEI is total gross energy intake.
Economical evaluation of diets was made as proposed by Martínez-Llorens et al. (2007), as follows:
- •
Economic efficient ratio (US$ kg−1) – ECR = feed offered (kg) × feed cost (kg−1) ÷ weight gain (kg).
- •
Economic profit index (US$ fish−1) – EPI = final weight (kg fish−1) × fish sale price (kg−1) − ECR (kg fish−1) × weight increase (kg).
Prices of diets and fish were calculated using the exchange rate US$ 1 = R$ 1.91, as of 7 July 2007. Pacu sale price was estimated at US$ 2.8 kg−1.
Three fish were randomly sampled from each replicate, killed by anaesthetic overdoses (benzocaine; 500 mg L−1), individually measured and weighed; livers from each fish were excised and weighed to calculate HSI. Whole body composition was determined in a pooled sample of 10 fish from the original population, and in pools of three fish per cage at the end of growth trial. Specimens for body analysis were ground, frozen, and kept under freezing temperature (−20 °C) prior to chemical analyses.
The analysis of diets and fish samples for moisture, CP, lipids and ash contents followed standard Association of Official Analytical Chemist (AOAC 2000) methods. Gross energy was determined in a calorimetric bomb and nitrogen-free extract was calculated by difference (100 − CP − crude lipid − crude ash − crude fibre).
Statistical analysis
A two-way anova for completely randomized design, 5 × 5 factorial scheme was performed; a one-way anova was also performed to evaluate effects of CP : DE ratio. Means of statistically different parameters and factors were compared by Tukey’s test (P < 0.05). Pearson’s correlation coefficient (r) values were utilized to evaluate possible interactions among parameters. Data were analysed with the aid of software statistica version 7.0 (Statsoft, Inc., Tulsa, OK, USA).
Results
Survival rate during the experimental period was 100% and no refusal of feed pellets was registered. Growth performance data are shown in Table 2.
| Diets CP : DE (g CP kg−1 : MJ DE kg−1) | WG (%) | FCR | DFI (g 100 g−1 fish) | SGR (% day−1) | PER | TPI (g) | PPV (%) | EPV (%) | ECR (US$ kg−1) | EPI (US$ fish−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| CP : 10.91 | 254 | 1.16b | 2.1a | 2.1 | 3.0b | 161ab | 51.0 | 12.6ab | 0.38cd | 1.64 |
| CP : 11.7 | 268 | 1.07a | 2.0ab | 2.2 | 3.2a | 171ab | 51.4 | 12.8ab | 0.36d | 1.75 |
| CP : 12.6 | 276 | 1.09ab | 2.1a | 2.2 | 3.2ab | 183a | 49.2 | 12.5b | 0.40c | 1.76 |
| CP : 13.4 | 284 | 1.07a | 2.1a | 2.2 | 3.2ab | 179a | 53.2 | 13.1a | 0.44b | 1.74 |
| CP : 14.2 | 236 | 1.10ab | 1.9b | 2.0 | 3.1ab | 154b | 48.1 | 13.1a | 0.50a | 1.53 |
| 220 : DE2 | 216b | 1.21a | 2.0 | 1.9b | 3.8a | 113c | 61.5a | 12.9ab | 0.40c | 1.49b |
| 260 : DE | 269ab | 1.11b | 2.1 | 2.2ab | 3.5b | 149b | 56.2a | 12.6ab | 0.39c | 1.71ab |
| 300 : DE | 261ab | 1.06b | 2.0 | 2.1ab | 3.2c | 162b | 47.9b | 13.2a | 0.41bc | 1.68ab |
| 340 : DE | 286a | 1.05b | 2.0 | 2.2a | 2.8d | 204a | 45.7bc | 12.9ab | 0.43ab | 1.77a |
| 380 : DE | 287a | 1.06b | 2.0 | 2.2a | 2.5e | 224a | 41.7c | 12.5b | 0.45a | 1.77a |
| anova | ||||||||||
| DE | 0.1592 | 0.0426 | 0.0030 | 0.1026 | 0.0485 | 0.0264 | 0.2878 | 0.0375 | <0.0001 | 0.0759 |
| CP | 0.0057 | <0.0001 | 0.1733 | 0.0049 | <0.0001 | <0.0001 | <0.0001 | 0.0329 | <0.0001 | 0.0213 |
| DE × CP | 0.4819 | 0.0289 | 0.1564 | 0.4877 | 0.0225 | 0.2290 | 0.9660 | 0.0019 | 0.0005 | 0.4397 |
| SME | 7.00 | 0.01 | 0.02 | 0.03 | 0.06 | 5.75 | 1.10 | 0.12 | 0.01 | 0.06 |
- Values are means (n = 15) in the same column with different superscript differ (Tukey’s test; P < 0.05).
- WG, weight gain; FCR, feed conversion ratio; DFI, daily feed intake; SGR, specific growth rate; PER, protein efficiency ratio; TPI, total protein intake; PPV, protein productive value; EPV, energy productive value; ECR, economic efficiency ratio; EPI, economic profit index; SME, standard mean error.
- 1 Pooled means without differentiation of dietary DE.
- 2 Pooled means without differentiation of dietary CP.
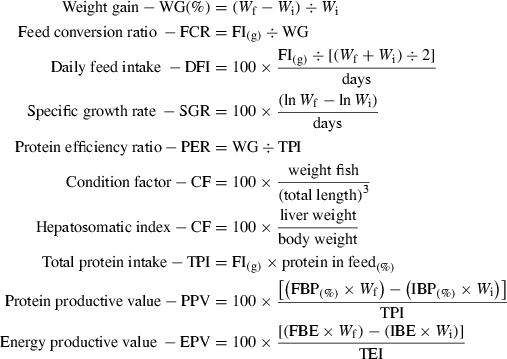
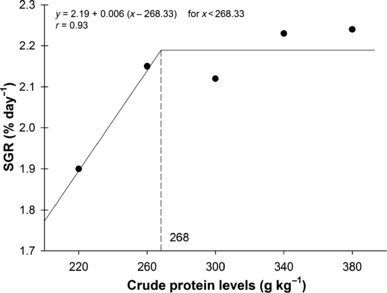
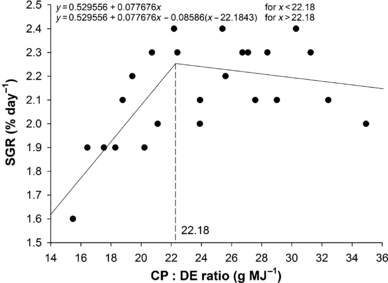
The relationship between WG and SGR (Fig. 1) with CP levels was best expressed by the broken line regression model, and the breakpoint in the growth response curve was estimated at 271 g kg−1 and 268 g kg−1 diet respectively. When data were analysed regarding CP : DE ratio, the best CP : DE ratio for both, WG and SGR (Fig. 2), was 22.2 mg kcal−1. DFI ranged from 19 to 21 g kg −1 fish, and was affected by dietary DE levels (y =75.9747 − 18.0077x + 1.4582x2 − 0.0393x3; r = 0.99). Fish fed lowest DE (10.9 MJ kg−1) levels consumed approximately 10% more than fish fed highest (14.2 MJ kg−1) level. There was no effect (P > 0.05) of CP or interaction between CP and DE on DFI. FCR was significantly influenced by dietary CP and DE. Increasing dietary CP level from 220 to 281 g kg−1 increased FCR, but further elevation to 380 g CP kg−1 did not significantly increase feed conversion (Fig. 3). The same trend was observed when DE was increased from 10.9 to 11.7 MJ kg−1, but increasing DE levels to 14.2 MJ kg−1 did not significantly increase feed efficiency.
Crude protein requirement of juvenile pacu (Piaractus mesopotamicus) by broken line model as a function of specific growth rate (SGR). Each point is the mean of 15 replicates.
Optimal crude protein : digestible energy ratio (CP : DE) of juvenile pacu (Piaractus mesopotamicus) by two-slope broken line model as a function of specific growth rate (SGR).
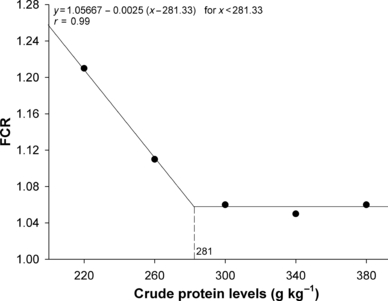
Crude protein requirement of juvenile pacu (Piaractus mesopotamicus) by broken line model as a function of feed conversion ratio (FCR). Each point is the mean of 15 replicates.
Total protein intake was affected (P < 0.05) by dietary levels of CP and DE, and increased with increasing CP level. Considering DE, the highest TPI value was observed in diets with 12.6 MJ kg−1, and was significantly different in fish fed diets with 14.2 MJ kg−1. PER decreased (P < 0.05) with increasing CP (Table 2), although different trends were observed depending of DE level (Table 3). There was a modest increase (P < 0.05) in TEP when DE was increased from 10.9 to 11.7 MJ kg−1, but further elevation to 13.39 MJ kg−1 did not significantly increase PER, and the highest value of DE (14.22 MJ) led to decreased PER (Table 2). PPV was also affected (P < 0.05) by CP levels, decreasing with increasing CP, although significant difference was observed for 300 g kg−1 CP only (Table 2); there was no significant effect of DE or CP : DE interaction on PPV. EPV was significantly affected by both dietary CP and DE levels and interaction CP : DE.
| Crude protein (g kg−1) | Digestible energy (MJ kg−1) | ||||
|---|---|---|---|---|---|
| 10.9 | 11.7 | 12.6 | 13.4 | 14.2 | |
| Feed conversion ratio | |||||
| 220 | 1.25b | 1.18b | 1.16 | 1.22b | 1.21b |
| 260 | 1.21abB | 1.00aA | 1.00A | 1.12abAB | 1.21bB |
| 300 | 1.05a | 1.05ab | 1.14 | 1.01a | 1.03ab |
| 340 | 1.09ab | 1.04ab | 1.04 | 0.99a | 1.08ab |
| 380 | 1.18abB | 1.09abAB | 1.09AB | 1.00aAB | 0.94aA |
| Protein efficiency ratio | |||||
| 220 | 3.6a | 3.9a | 3.9a | 3.7a | 3.8a |
| 260 | 3.2abB | 3.8aA | 3.8aA | 3.4abAB | 3.2abB |
| 300 | 3.2ab | 3.8b | 2.9b | 3.3ab | 3.2ab |
| 340 | 2.7bc | 2.8bc | 2.8b | 3.0bc | 2.7b |
| 380 | 2.2cB | 2.4cAB | 2.4bAB | 2.6cAB | 2.8bA |
| Energy productive value (%) | |||||
| 220 | 13.1 | 12.7 | 12.2ab | 13.6 | 13.1 |
| 260 | 12.1 | 12.6 | 13.4a | 12.7 | 12.2 |
| 300 | 13.2 | 12.7 | 13.5a | 13.0 | 13.8 |
| 340 | 12.7 | 13.3 | 12.8a | 12.6 | 12.9 |
| 380 | 12.3AB | 12.5AB | 9.6bB | 13.7A | 13.5A |
| Economic efficiency ratio (US$ kg−1) | |||||
| 220 | 0.39AB | 0.34bcB | 0.36bcB | 0.41AB | 0.48A |
| 260 | 0.40B | 0.31cC | 0.31cC | 0.43B | 0.52A |
| 300 | 0.36B | 0.36bcB | 0.43abAB | 0.43AB | 0.48A |
| 340 | 0.37B | 0.38abB | 0.43abB | 0.44B | 0.52A |
| 380 | 0.41B | 0.40aB | 0.46aAB | 0.48A | 0.48A |
| Whole body gross energy (MJ kg−1) | |||||
| 220 | 25.1AB | 24.0AB | 24.6aB | 26.2aA | 24.0AB |
| 260 | 23.2 | 23.2 | 23.3ab | 25.0ab | 25.0 |
| 300 | 23.1BC | 22.6C | 25.5aA | 24.1abABC | 24.8AB |
| 340 | 23.6 | 23.5 | 25.1a | 22.4b | 24.6 |
| 380 | 23.9AB | 22.9AB | 20.1bB | 25.5abA | 23.9AB |
- Means of three replicates.
- Values in a column followed by different lowercase superscripts differ (P < 0.05) regarding dietary protein within energy levels.
- Values in a row followed by different capital superscripts differ (P < 0.05) regarding dietary energy within protein levels.
Two-way interaction showed that FCR and PER in 260 CP diets were better at 11.7 and 12.6 MJ kg−1. However, in 380 CP diets, these parameters were significantly different, the lower value recorded for 14.2 MJ diet only (Table 3).
Costs of experimental diets (US$ kg−1) increased proportionally to increasing dietary DE and CP levels, and decrease with increasing CP : DE ratio (Fig. 4). Increasing DE increased ECR linearly (P < 0.05); similar trend was observed for CP, but the highest value (0.45) registered for 380 CP level was not significantly different of 340 CP level. When splitting interactions were considered (P < 0.05), it was observed that DE was the major factor influencing ECR at all CP levels (Table 3). On the other hand, dietary CP influenced significantly efficiency of diets containing 11.7 and 12.6 MJ DE kg−1. The EPI was affected (P < 0.05) by CP only; a similar trend was observed regarding WG.
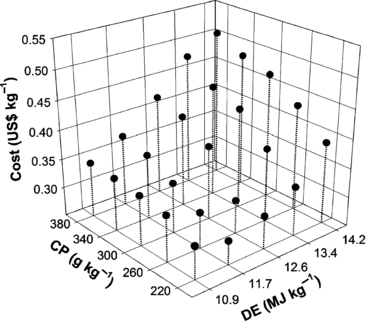
Cost of experimental diets as a function of crude protein (CP) and digestible energy (DE) levels.
Body composition and morphological parameters of pacu at the beginning and end of the trials are shown in Table 4. Increasing dietary DE resulted in increased body lipid contents (P < 0.05), and as a consequence, dry matter (P < 0.05) incorporation; carcass gross energy contents thus also increased (P < 0.05) with increasing dietary DE contents. In addition, gross energy contents decreased significantly with increasing CP and there was effect of the interaction (P < 0.05) between CP and DE levels. There was significant increase of whole body protein with increasing dietary CP, but no difference (P > 0.05) was found in fish fed with 340 and 380 CP. Body protein decreased significantly with increasing DE levels, although no significant difference was observed when DE increased from 12.6 to 14.2 MJ kg−1. Ash showed a decreasing trend (P < 0.05) with increasing DE levels, but inverse trend (P < 0.05) was observed with increasing CP level. The HSI was not influenced by dietary (P > 0.05) CP and DE levels. However, CF of fish increased (P < 0.05) with increasing dietary DE.
| Diets CP : DE (g CP kg−1 : MJ DE kg−1) | Moisture (g kg−1) | Protein (g kg−1) | Fat (g kg−1) | Ash (g kg−1) | Gross energy (MJ kg−1) | HSI (g kg−1) | CF |
|---|---|---|---|---|---|---|---|
| Initial | 775 | 142 | 36 | 34 | 20.7 | 2.3 | 2.04 |
| CP : 10.91 | 678a | 161a | 105bc | 41a | 23.8ab | 1.5 | 3.56b |
| CP : 11.7 | 675a | 158ab | 103c | 40ab | 23.2b | 1.5 | 3.62ab |
| CP : 12.6 | 666ab | 151bc | 122abc | 37ab | 23.9ab | 1.3 | 3.70ab |
| CP : 13.4 | 654b | 160abc | 130a | 38b | 24.8a | 1.5 | 3.69ab |
| CP : 14.2 | 668ab | 151c | 124ab | 38ab | 24.4ab | 1.3 | 3.78a |
| 220 : DE2 | 665 | 157a | 125 | 39ab | 24.8a | 1.4 | 3.67 |
| 260 : DE | 669 | 155a | 115 | 36b | 23.9ab | 1.5 | 3.67 |
| 300 : DE | 668 | 149a | 119 | 39ab | 24.0ab | 1.4 | 3.62 |
| 340 : DE | 669 | 160ab | 113 | 38ab | 23.9ab | 1.4 | 3.67 |
| 380 : DE | 671 | 161b | 110 | 41a | 23.5b | 1.4 | 3.71 |
| anova | |||||||
| DE | 0.0031 | 0.0032 | 0.0028 | 0.0178 | 0.0089 | 0.0723 | 0.0246 |
| CP | 0.8790 | 0.0434 | 0.1267 | 0.0318 | 0.0178 | 0.4555 | 0.7777 |
| DE × CP | 0.0924 | 0.5126 | 0.3816 | 0.5503 | 0.0008 | 0.4916 | 0.2297 |
| SME | 0.23 | 0.50 | 0.56 | 0.18 | 41.91 | 0.02 | 0.03 |
- Values means (n = 15) in the same column with different superscript differ (Tukey’s test; P < 0.05).
- HSI, hepatosomatic index; CF, condition factor; SME, mean square error.
- 1 Pooled means without differentiation of dietary DE.
- 2 Pooled means without differentiation of dietary CP.
Discussion
Dietary protein contents markedly influence cost of practical diets. Consequently, to minimize feed-related costs, dietary protein level and protein retention by fish shall be optimized. Dietary protein level was a main factor affecting pacu growth (Table 2); estimated protein requirement, broken line method, ranged on 270–280 g kg−1. This result is similar to those reported for pacu (260 g kg−1) by Fernandes et al. (2000), and for other omnivorous Brazilian Characins, such as curimbatáProchilodus scrofa (260 g kg−1) by Bomfim et al. (2005), piauçu Leporinus macrocephalus (280 g kg−1) by Pezzato et al. (2000), piracanjuba Brycon orbignyanus (290 g kg−1) by Sá & Fracalossi (2002), and tambaqui Colossoma macropomum (250 g kg−1) by Vidal Júnior et al. (1998). However, dietary protein requirements reported for the neotropical catfish jundiáRhamdia quelen by Meyer & Fracalossi (2004) are a little higher − 326–373 g kg−1.
Specific growth rate ranged on 1.9–2.2, which is a relatively high score compared to other research reports. For instance, Abimorad & Carneiro (2007) reported SGR of 0.75–0.81% day−1 for juvenile pacu (11.5 g) fed diets with varying protein, carbohydrate and lipid contents. However, Tesser et al. (2005) registered SGR of 3.15 and 3.53 for pacu fingerlings (4.3 g) fed semi-purified diets (casein–wheat gluten protein) with varying arginine levels and sources.
All tested diets were well accepted by pacu; feed intake values ranged on 19 and 21 g kg−1 fish. These values are similar to those reported by Tesser et al. (2005) for the same specie. When fish fed on diets containing the highest levels of energy, voluntary feed intake decreased; same trend was observed and reported by Portz et al. (2001) for largemouth bass Micropterus salmoides, Borba et al. (2003) for piracanjuba B. orbignyanus, and Salhi et al. (2004) for the black catfish, Rhamdia quelen. However, differences on DFI between the lowest and highest dietary energy level were small. This suggests that pacu can adjust voluntary feed intake in a wide range of dietary energy concentration (10.9–13.4 MJ kg−1). Actually, according to Santinha et al. (1999), when fish are fed to satiation, growth might not depend on diet composition but on the ability of fish to regulate food consumption.
Pacu fed low-protein diets used dietary protein more efficiently, as reflected by the higher PER and PPV values recorded, than fish fed the high protein diets. In general, protein retention efficiency increases with low protein intake, as reported, for instance, by Vásquez-Torres et al. (2002), Salhi et al. (2004), Cotan et al. (2006) and Sáet al. (2007). Catacutan et al. (2001) actually suggest the existence of a compensatory mechanism enabling higher PER and PPV for fish fed low-protein diets, which explains the largest protein retention values recorded for fish fed the 220 g kg−1 CP diet in this study. However, PER increased in diets 260 CP when DE increased from 10.9 for 11.7 MJ kg−1, but no further increase of PER with increasing DE to the maximum level was observed; however, in diet 380 CP, the improvement of PER was significant only when DE reached 14.2 MJ kg−1. Similar trend was observed to FCR. The increase of the PER with increasing ED up to 11.7 MJ kg−1 may suggest low use of dietary protein as energy source (De Silva et al. 1991; Salhi et al. 2004). However, De Silva & Anderson (1995) do not consider PER the best method for this evaluation, as it does not measure how much protein is used for maintenance, and furthermore, consider lipid deposition as protein. These authors recommend that, in such cases, the PPV or nitrogen retention shall be used for evaluation of growth. Therefore, results registered for PER and FCR suggest that pacu favours dietary protein as energy source and in low dietary CP concentrations, an adjusted CP : DE ratio is critical for best performance of juvenile pacu.
If increasing dietary DE levels did not improve protein retention, no protein sparing effect occurred. Protein sparing effect has not also been reported for other neotropical, omnivorous fish, such as piauçu (Pezzato et al. 2000), piracanjuba (Borba et al. 2003), curimbata (Bomfim et al. 2005) and lambari Astyanax bimaculatus (Cotan et al. 2006). A discreet protein sparing effect was shown for the neotropical Characin tambaqui Colossoma macropomum; increasing dietary lipid contents from 5% to 20% resulted in increased net protein utilization from 2% to 2.3% (Van der Meer et al. 1997). A noteworthy protein sparing effect recorded for a neotropical species concerns the jundiá; the species decreased its protein requirements from 373 to 326 g kg−1 CP when metabolizable energy (ME) was increased from 13.4 to 15.3 MJ kg−1 (Meyer & Fracalossi 2004), probably because the jundiá is an omnivorous species with carnivorous tendencies (Oliveira Filho & Fracalossi 2006).
Pacu has good production performance and elicits positive economic return in intensive systems (Jomori et al. 2005; Urbinati & Gonçalves 2005). Given that feeding practices usually represent up to 60% of the production costs in intensive fish farming (De Silva & Anderson 1995), probing the economics of the experimental diets seems opportune. ECR and EPI increased with increasing dietary protein contents. As protein is the most expensive macronutrient in aquafeeds, improvement of EPI and ECR resulted from increased weight gain brought out by increasing dietary CP. Both EPI and ECR are intrinsically related to weight gain; both EPI and weight gain trend lines either peak or trough, respectively, with increasing or decreasing dietary CP contents. The influence of dietary DE and its interaction with dietary CP on ECR values does not reflect results recorded for both WG and FCR. Vidal et al. (2008) did not register influence of varying protein to energy ratio on ECR and the EPI estimated for the Mediterranean yellowtail Seriola dumerili.Sweilum et al. (2005) evaluated the economic return of Nile tilapia of different weight classes (23 and 40 g) fed with different levels of ME (10.5, 12.6 and 14.7 kJ g−1) and CP (200, 250 and 300 g kg−1), and reported that profit increased significantly with increasing dietary CP and was reduced with increasing DE in smaller fish; however, maximum profit was obtained when fish fed intermediate CP and ME levels. The use of the EPI was a good tool for evaluating profitability of production of juvenile pacu with the tested diets, given that fish of this size are sold live and per unit price. However, if results recorded for EPI refer to adult, food fish, caution is advisable. Factors such as carcass and/or fillet yield, which are directly related to feeding practices, can represent prize selling price but are not taken into account by this index. Migratory Characins may build up large amounts of visceral fat which, in nature, are used as energy source for migration and spawning (Suárez et al. 1995; Resende 2003), but are a rather undesirable trait for farming purposes. In this case, using economic indices that consider the yield of edible fishery products are mandatory for accurate evaluation.
Dietary energy affected more significantly body composition of pacu (Table 4). Appropriate CP : DE ratio is essential for faster growth and better fishery product quality (Duan et al. 2001). The best CP : DE for WG was 22.2 g CP MJ−1 DE. However, this is to be regarded with caution; the ultimate goal of pacu farming is producing marketable fish protein, usually in the form of the fillet or steak flesh. Therefore, the best CP : DE observed in this study for performance parameters, can be not appropriate when considering final product quality.
Contents of different components of fish body will usually increase with increasing body mass; fish were thus leaner (high body protein: fat ratio) at the beginning of the trials in comparison to final body composition status (Table 4). Although in a narrow range, fish fed high-energy diets had lower moisture contents than fish fed low-energy diets, that is, there was a inverse relationship between body moisture and body lipid (r = −0.55; P < 0.0001) and between body protein and body lipid (r = −0.53; P < 0.0001). Other studies also reported increased body fat proportionally to dietary energy levels (Van der Meer et al. 1997; Borba et al. 2003; Salhi et al. 2004).
Increased HSI in direct relationship to dietary energy level was observed in several studies (Nematipour et al. 1992; Jover et al. 1999; Gummadi & Reigh 2003; Kim et al. 2004); such an effect was not observed in this study. This result matches results reported for piracanjuba by Borba et al. (2003), and white seabream Diplodus sargus by Sáet al. (2007). Changes in condition are accompanied by changes in the composition of body tissues, the most noticeable changes being in the relative per cent contents of lipids and moisture (Jobling 2001).
This study showed that minimum dietary requirements of pacu fingerlings are 270 g kg−1 CP, with an optimum CP : DE of 22.2 g CP MJ−1 DE. Even though increasing dietary DE levels did not result in significant protein sparing effect, the reduced protein requirement of pacu at such young age – 320–400 g kg−1 CP, way below levels recommended in the literature – encourages reviewing feeding practices and continued research efforts in the aim to produce minimum cost–maximum profit diets for intensive farming of the species.
Acknowledgements
A.J.A. Bicudo (05/51968-9) and R.Y. Sado (05/51967-2) are recipients of Doctoral scholarships from ‘Fundação de Amparo à Pesquisa do Estado de São Paulo’ (Sao Paulo State Research Foundation – FAPESP), which also granted partial research funds. J.E.P. Cyrino is research scholar of Brazil’s ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Council for Scientific and Technological Development – CNPq)’ which also partially funded the research project.




