Influence of dietary protein levels on growth performance and body composition of African bonytongue fingerlings, Heterotis niloticus (Cuvier, 1829)
Abstract
Two experiments were conducted to examine the influence of dietary protein levels on growth and carcass proximate composition of Heterotis fingerlings. Four isoenergetic practical diets were formulated to contain dietary protein levels from 250 to 400 g kg−1 diet. Replicate groups of young Heterotis (initial live weight 3.96 and 26.40 g in experiments 1 and 2 respectively) were handfed twice daily to apparent satiation for a period of 42 and 28 days respectively. Statistical analysis revealed that growth rate was significantly affected by dietary protein level (P < 0.01). The highest weight gain was observed in fingerlings fed with 300 and 350 g protein kg−1 diet for fish size ranging between 3–15 and 26–62 g respectively. There was no significant difference between groups fed with 300, 350 and 400 g protein kg−1 diet for Heterotis fingerlings (3–15 g) in the one hand; in the other hand, significant differences were found between fish (26–62 g) fed with 350 g protein kg−1 diet and those receiving 300 and 400 g protein kg−1 diet, with no significant difference between each other. The specific growth rate varied from 2.4% to 3.1% day−1. The whole-body protein, lipid, moisture and ash contents were not significantly affected by dietary protein levels (P > 0.05). The relationships between percentage weight gain and dietary protein levels suggested very similar dietary protein requirement (about 310 g crude protein kg−1 diet) for Heterotis ranging from 3 to 62 g. The maximum growth occurred at about 345 g protein kg−1 diet.
Introduction
In west and central Africa, fishing remains the main source for indigenous fish supply to the population. Obviously, these fishing grounds have attained maximum sustainable levels and for the last 15 years, there is even a decline trend. To meet up with the increasing demand for animal protein from the growing human population, without increased fish importation, there is an imperative need to develop aquaculture in the African continent. Apart from Nigeria, aquaculture sector contributes to <5% of local fish demand in most African countries. Despite its aquaculture potential, Sub-Saharan Africa lags far behind. It is therefore indispensable to stimulate the development of this sector through diversification including the culture of indigenous species such as African bonytongue (Heterotis niloticus). This species has a relatively high commercial value and strong aquaculture potential, but very few data are available on its farming.
Heterotis niloticus originated from tropical Africa (Greenwood 1973; Li & Wilson 1996). It is found in big rivers (Senegal, Gambia, Niger and Nile) and in some tropical African lakes like Lake Chad (Aubenton 1955; Levêque et al. 1990). Moreover, it was successfully introduced in the south of Cameroon (Depierre & Vivien 1977), in Ivory Coast (Moreau 1974; Lazard 1980), in the Democratic Republic of Congo, in Gabon (Mbega 2004) and in Madagascar.
Heterotis niloticus is a warm water bony fish well appreciated for human consumption in Sub Saharan Africa. It possesses an important potential market, with a commercial value twice higher than that of tilapia. The rapid growth (3–4 kg in a 12-month cycle), late sexual maturity, short food chain and natural reproduction of this species in small and large ponds make it a good candidate for aquaculture production. Initial research on this species began in the 1950s. Unfortunately, the number of studies diminished considerably just after independence in 1960, before the recent regain of interest. These studies were conducted to better understand the biology (Aubenton 1955; Daget 1957; Omorinkoba et al. 1991; Okoye & Abubakar 1996; Fagbenro 2001; Achionye-Nzeh & Omoniyi 2002; Adite et al. 2006), the ecology (Moreau 1974; Moreau & Moreau 1982; Adite et al. 2005) and the culture (Tillon 1957; Lemasson 1957; Tillon 1959; Olanyan & Zwilling 1963; Reizer 1964) of this species. Heterotis was classified in the omnivorous fish category (Micha 1976; Mbega 2004; Adite et al. 2005).
Protein is a main component in fish feed. Increasing protein level in feed can lead to improved fish production, but excessive dietary protein level is not economical for fish culture. Valid information on the protein requirement of fish is essential for any new aquaculture attempt. It is therefore not surprising that protein requirement studies are usually among the first fish nutrition experiments to be conducted when a new fish species is being considered for aquaculture. Dietary protein requirements has been investigated in a number of fish species (NRC 1993), but no research has been conducted so far on H. niloticus.
This study was designed to investigate the effects of dietary protein levels on growth and carcass proximate composition of Heterotis fingerlings, and to estimate their specific dietary protein requirement. As a consequence, an appropriate supply of dietary protein would allow supporting high growth rate and reducing nitrogen wastes.
Materials and methods
Rearing conditions
The trial was conducted in hapas that were constructed with nets of fine texture. These hapas with rectangular dimensions (1 × 0.5 × 1.1 m; vol. = 400 L) were placed in a rectangular fish pond (300 m2, 1.2 m deep) located at the Government Aquaculture Station in Melen (Yaounde, Cameroon). The pond was free from aquatic vegetation, completely independent, well exposed to sunlight and had a well-designed system of inlet and outlet to maintain the water level in the hapas at 0.8 m for the duration of the experiment. During the feeding trial, fingerlings were exposed to natural photoperiod (6.15 am–6.45 pm daylight followed by night). Water temperature ranged from 25 to 31 °C while pH ranged from 6.5 to 7.0. The experimental durations were 42 days (from December 2006 to January 2007) and 28 days (from September to October 2006) for experiments 1 and 2 respectively.
Experimental fish
Heterotis fingerlings weighing <1 g were caught in river Nyong near the town of Akonolinga (Cameroon), and transported to the Melen aquaculture station where they were stored for several weeks in a fertilized fish pond. After this phase, 50 fish were randomly distributed into each hapa and fed diet containing 300 g protein kg−1 diet for 2 weeks (during a pre-experimental period). After this conditioning period, each diet was tested on 25 fish per hapa, in triplicate for experiment 1 and duplicate for experiment 2. Before the fish allotment, 50 fish were randomly sampled and individually weighed (initial mean weight: 3.96 ± 0.13 g for experiment 1 and 26.40 ± 0.64 g for experiment 2). At the end of the experiment, all fish were individually weighed and total length measured.
Diet formulation, preparation and feeding
Diet formulations are shown in Table 1. Four isocaloric experimental diets for each trial were formulated to contain graded levels of protein (250, 300, 350 and 400 g kg−1 diet) (Tables 2 and 3 for experiment 1 and 2 respectively). These protein contents were chosen based on the results of the protein requirements of other omnivorous fish species such as tilapia (Shiau 2002) and channel catfish (Robinson et al. 2000). Fish meal was obtained from Coppens International BV (Helmond, the Netherlands). Apart from fish oil, other ingredients were obtained from local markets. Fish and soybean meals were used as the main protein source. Maize meal, wheat bran and fish oil levels were adjusted accordingly to make the diets iso-energetic. Gross energy levels of the diets were calculated based on 23.7, 39.5 and 17.2 kJ g−1 for protein, lipid and nitrogen-free extract respectively (Guillaume et al. 1999).
| Ingredients | Diets (g protein kg−1 diet) | |||
|---|---|---|---|---|
| 250 | 300 | 350 | 400 | |
| Fish meal | 130 | 180 | 235 | 285 |
| Soybean meal | 130 | 180 | 235 | 285 |
| Whole maize meal | 320 | 275 | 220 | 170 |
| Wheat bran | 320 | 275 | 220 | 170 |
| Menhaden fish oil1 | 50 | 40 | 40 | 40 |
| Vitamin premix2 | 20 | 20 | 20 | 20 |
| Mineral premix3 | 20 | 20 | 20 | 20 |
| Carboxymethylcellulose4 | 10 | 10 | 10 | 10 |
- 1,4 Sigma-Aldrich products (Bornem, Belgium).
- 2 Vitamin Mix Fish 0.5% INVE Aquaculture, Belgium (composition per kg: vitamin A, 2 500 000 IU; vitamin D3, 500 000 IU; vitamin E, 30 000 mg; vitamin K3, 2000 mg; vitamin B1, 2000 mg; vitamin B2, 5000 mg; panthotenic acid, 10 000 mg; niacin, 5000 mg; vitamin B6, 4000 mg; folic acid, 2000 mg; vitamin B12, 4 mg; vitamin C, 20 000 mg; biotin, 200 mg; and inositol, 80 000 mg).
- 3 Mineral Mix MLNP 763, INRA Belgium (composition per kg: dibasic calcium phosphate, 500 g; calcium carbonate, 215 g; sodium chloride, 40 g; potassium chloride, 90 g; magnesium hydroxide, 124 g; iron sulphate, 20 g; zinc sulphate, 4 g; manganese sulphate, 3 g; cobalt sulphate, 0.02 g; potassium iodide, 0.04 g; sodium selenite, 0.03 g; and sodium fluoride, 1 g).
| Constituents1 (‰) | Diets (g protein kg−1 diet) | |||
|---|---|---|---|---|
| 250 | 300 | 350 | 400 | |
| Moisture | 89 | 84 | 83 | 83 |
| Crude protein | 253 | 295 | 347 | 393 |
| Total lipid | 62 | 54 | 58 | 62 |
| Ash | 59 | 70 | 76 | 93 |
| NFE2 | 538 | 497 | 436 | 370 |
| Gross energy (kJ g−1) | 18 | 18 | 18 | 18 |
- 1 Values are the mean of three replicate analyses.
- 2 Nitrogen-free extract (NFE) calculated as: 1000 − (‰ moisture + ‰ protein + ‰ lipid + ‰ ash).
| Constituents1 (‰) | Diets (g protein kg−1 diet) | |||
|---|---|---|---|---|
| 250 | 300 | 350 | 400 | |
| Moisture | 100 | 105 | 101 | 103 |
| Crude protein | 259 | 301 | 357 | 396 |
| Total lipid | 61 | 53 | 59 | 60 |
| Ash | 64 | 71 | 80 | 89 |
| NFE2 | 517 | 471 | 404 | 352 |
| Gross energy (kJ g−1) | 17.4 | 17.3 | 17.7 | 17.8 |
- 1 Values are the mean of three replicate analyses.
- 2 Nitrogen-free extract (NFE) calculated as: 1000 − (‰ moisture + ‰ protein + ‰ lipid + ‰ ash).
Soybean meal was boiled in a pressure cooker for 2 h followed by sun drying. The different experimental diets were made by adding appropriate volumes of water to ingredients. The resulting paste was transformed into spaghetti (2 mm for experiment 1 and 3 mm for experiment 2) with the aid of food blender (Kenwood KM 800, Havant, UK). After sun drying at a temperature of 28–35 °C for about 3 days, the spaghetti were manually broken and converted into pellets. The pellets were stored at −20 °C until use.
Fish were fed by hand twice a day (09:30–10:00 and 14:30–15:00 hours) to apparent satiation. Pellets were distributed slowly, allowing all fish to eat. The daily feed supply was recorded.
Sample collection and methods for chemical analysis
Initially, 10 fish were sampled for initial whole-body proximate composition. At the end of the experiment, seven fish from each treatment were randomly selected for final analysis of body composition. All samples were stored at −20 °C prior to analysis.
Proximate composition of feed ingredients, experimental diets and fish were analysed following Association of Official Analytical Chemists methods (AOAC 1999). Crude protein (total nitrogen × 6.25) was measured using the Kjeldahl method after acid digestion. Total lipid content was estimated using the Soxhlet apparatus method according to Folch et al. (1957). Moisture was determined by drying the sample at 105 °C for 24 h to a constant weight. Ash was determined by incinerating the dried sample in a muffle furnace at 550 °C for 12 h. Because of problems of conservation, proximate composition of final fish (experiment 2) was not analysed.
Data processing and statistical analysis
From these data, final mean weight, specific growth rate (SGR; % day−1) [100 × (ln final body weight − ln initial body weight)/duration of experiment (days)], weight gain (WG) [(final body weight − initial body weight) (g) × 100/initial body weight (g)], feed efficiency (FE) [(final body weight − initial body weight) (g)/total feed intake (g)], protein efficiency ratio (PER) [(final body weight − initial body weight) (g)/total protein intake (g)], protein deposition (PD) [100 × (final body weight × final body protein − initial body weight × initial body protein)/total feed intake × dietary protein] were determined.
Data were analysed for comparison among different dietary treatments using one-way analysis of variance (anova) after verifying the homogeneity of variance using Hartley’s test (Dagnelie 1975). Differences among mean values were tested by least significance difference. The significance level was 5%. Percentage data were transformed to arcsine values before analysis.
To determine the levels of dietary protein for maximum growth, the relationship between dietary protein and WG was fitted using a second-order polynomial equation, where WG was a function of dietary protein, using the formula 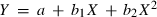 (Espinós et al. 2003). An estimation of the dietary protein requirement, based on percentage WG, was performed by the broken line model (Robbins et al. 1979).
(Espinós et al. 2003). An estimation of the dietary protein requirement, based on percentage WG, was performed by the broken line model (Robbins et al. 1979).
Results
Experiment 1
Growth, survival and feed utilization Weight gain, SGR, FE and survival rate of Heterotis fingerlings during the feeding trial are shown in Table 4. WG and SGR were significantly affected by dietary protein level (P < 0.01). Heterotis fingerlings fed with 300 g protein kg−1 diet displayed the highest growth rate, but values did not differ significantly from those of fish fed with 350 and 400 g protein kg−1 diet (P > 0.05). However, the WG and SGR of this group were significantly (P < 0.01) higher than those of fingerlings fed with 250 g protein kg−1 diet, which displayed the poorest growth.
| Diets (g protein kg−1 diet) | ||||
|---|---|---|---|---|
| 250 | 300 | 350 | 400 | |
| Final weight (g) | 11.2 ± 0.5a | 14.8 ± 1.1b | 14.6 ± 1.0b | 14.1 ± 0.9b |
| Weight gain (%) | 183 ± 2a | 272 ± 14b | 269 ± 20b | 254 ± 10b |
| SGR (% day−1) | 2.5 ± 0.0a | 3.1 ± 0.1b | 3.1 ± 0.1b | 3.0 ± 0.1b |
| Feed intake1 | 50 ± 3a | 46 ± 1a | 34 ± 2b | 33 ± 2b |
| FE | 0.4 ± 0.0a | 0.6 ± 0.1a | 0.6 ± 0.1a | 0.8 ± 0.0b |
| PER | 1.52 ± 0.16a | 1.86 ± 0.34a | 1.61 ± 0.18a | 2.12 ± 0.11a |
| PD | 22.4 ± 2.7a | 28.2 ± 4.8a | 24.0 ± 2.1a | 30.1 ± 1.6a |
| Survival (%) | 76 ± 2a | 68 ± 6ab | 59 ± 3b | 76 ± 2a |
| Moisture2 | 80.5 ± 0.1 | 79.6 ± 0.3 | 80.3 ± 0.5 | 80.5 ± 0.5 |
| Protein2 | 13.6 ± 0.2 | 14.0 ± 0.2 | 13.6 ± 0.2 | 13.5 ± 0.2 |
| Total lipid2 | 1.1 ± 0.1 | 1.3 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 |
| Ash2 | 4.0 ± 0.1 | 4.1 ± 0.3 | 3.5 ± 0.1 | 3.8 ± 0.1 |
- SGR, specific growth rate; FE, feed efficiency; PER, protein efficiency ratio; PD, protein deposition.
- Values are mean ± SE of three replicates. Mean values in a row with different superscript letters are significantly different (P < 0.05).
- 1 Feed intake (g week−1).
- 2 Values are mean ± SE of three replicates and expressed in (%). Initial whole body composition was 79.5% moisture, 12.2% protein, 0.87% lipid and 5.1% ash.
Survival in all treatments ranged from 59% to 76% and was affected by dietary protein level. The survival rate of fish fed with 300 and 350 g protein kg−1 diet did not differ from each other, but survival of fingerlings fed with 350 g protein kg−1 diet was significantly (P < 0.05) lower than that of the fish fed with 250–400 g protein kg−1 diet. Feed intake decreased progressively with graded dietary protein level and was found to differ significantly (P < 0.001) between groups fed with 250–300 g kg−1 and those receiving 350–400 g protein kg−1 diet. FE increased progressively with graded dietary protein level and was found to differ significantly (P < 0.05) between fingerlings fed with 250–350 and 400 g protein kg−1diet. On the contrary, PER and PD did not differ significantly between treatments (P > 0.05) (Table 4).
Body composition The whole body composition of Heterotis fingerlings is presented in Table 4. Moisture, ash content, whole-body protein and lipid content were not significantly affected by dietary protein level (P > 0.05). Initial whole body composition of fish contained less moisture, protein and lipid than final body composition of fish, regardless of experimental diets.
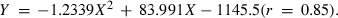
The maximum WG was observed when the dietary protein level was 340 g kg−1 diet. Using the broken line model of Robbins et al. (1979), the dietary protein requirement for the Heterotis fingerlings (3–15 g) based on percentage WG was estimated to be 306 g protein kg−1 diet (Fig. 1).
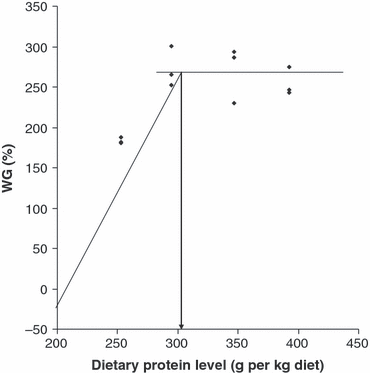
Estimation of the dietary protein requirement of Heterotis fingerling according to the broken line model (initial average weight 3.96 g).
Experiment 2
Growth, survival and feed utilization Weight gain and SGR were significantly affected by dietary protein level (P < 0.05) (Table 5). Fish fed with 250 g protein kg−1 diet displayed the lowest growth rate. The WG and SGR of fingerlings fed with 300 and 400 g protein kg−1 diet did not differ from each other, but were significantly (P < 0.05) lower than those of fish receiving 350 g protein kg−1 diet.
| Diets (g protein kg−1 diet) | ||||
|---|---|---|---|---|
| 250 | 300 | 350 | 400 | |
| Final weight (g) | 51.7 ± 1.8a | 58.6 ± 2.6b | 61.6 ± 2.2b | 58.9 ± 1.6b |
| Weight gain (%) | 96 ± 0a | 122 ± 4b | 133 ± 1c | 123 ± 0b |
| SGR (% day−1) | 2.4 ± 0.1a | 2.9 ± 0.1b | 3.0 ± 0.0c | 2.9 ± 0.0b |
| Feed intake1 | 230 ± 7 | 208 ± 18 | 152 ± 16 | 97 ± 1 |
| FE | 0.7 ± 0.1a | 0.7 ± 0.1a | 0.8 ± 0.0b | 1.0 ± 0.0c |
| PER | 2.53 ± 0.03a | 2.26 ± 0.03b | 2.18 ± 0.05c | 2.43 ± 0.02a |
| Survival (%) | 88 ± 2a | 84 ± 4ab | 76 ± 4bd | 72 ± 2cd |
- SGR, specific growth rate; FE, feed efficiency; PER, protein efficiency ratio.
- Values are mean ± SE of two replicates. Mean values in a row with different superscript letters are significantly different (P < 0.05).
- 1 Feed intake (g week−1).
Similarly, survival was affected by dietary protein level. The survival rate generally showed a decreasing trend with increasing dietary protein level and was significantly higher (P < 0.05) in fingerlings fed with 250–300 g protein kg−1 diet than in fish receiving 350–400 g protein kg−1 diet (Table 5). FE increased progressively with graded dietary protein level and was found to differ significantly (P < 0.01) between fingerlings fed 250–300 and 350–400 g protein kg−1 diet. There was no significant (P > 0.05) difference between fish receiving 250 and 300 g protein kg−1 diet, but fingerlings fed with 350 g protein kg−1 diet had significantly (P < 0.01) lower FE than those of fish receiving 400 g protein kg−1 diet. The highest PER was obtained with diets containing 250 and 400 g protein kg−1 diet whereas the lowest PER was obtained with diet containing 350 g protein kg−1 diet.
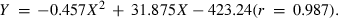
The maximum WG was observed when the dietary protein level was 349 g kg−1 diet. Using the broken line model of Robbins et al. (1979), the dietary protein requirement for the Heterotis fingerlings (26–62 g) based on percentage WG was estimated to be 311 g protein kg−1 diet (Fig. 2).
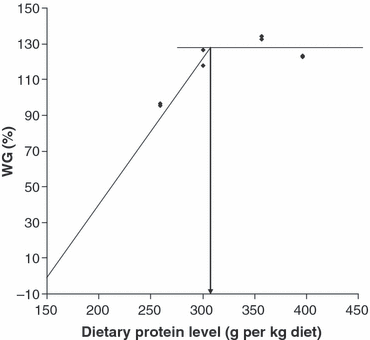
Estimation of the dietary protein requirement of Heterotis fingerling by broken line model (initial average weight 26.40 g).
Discussion
In this study, an estimation of the dietary protein requirements of H. niloticus fingerlings was performed by formulating diets incorporating readily available and cheap local ingredients. In a majority of similar studies, researchers employed various purified and semi purified diets with high-quality protein sources such as casein, gelatin or synthetic amino acids that yielded more precise values. These protein sources being relatively expensive and not readily accessible for an average fish nutritionist in developing countries, our choice was guided by the amino acid composition of proteins (experimental ingredients) and their digestibilities in the one hand; in the other hand by similar estimations of dietary protein requirements of tropical bagrid catfish, Mystus nemurus fingerlings demonstrated by Khan et al. (1993) and by Ng et al. (2001). These authors reported the requirements of 420 and 440 g kg−1 diet in this species when using practical and semi purified diets respectively. Identical results were obtained in other studies involving tilapia juveniles (Jauncey 1982; El-Sayed & Teshima 1992; Gunasekera et al. 1995; Al Hafedh 1999). Despite the slight difference, the similarity observed permits the use of practical diets in the determination of fish protein requirements. Moreover, in our study, experimental diets were formulated by adjusting levels of fish and soybean meals with maize meal and wheat bran; the part of protein which comes from fish meal was highly digestible and its amino acid composition was close to requirements; the digestibility of other protein which comes from practical ingredients depends on digestibility of dietary amino acids which varies among feed ingredients. So, it is crucial to underline that the amount of non-protein energy in the feed and the quality of the protein influence the growth response of fish fed with diet containing different levels of protein.
In this study, the relationship between dietary protein levels and WG was fitted using a second-order polynomial equation (Espinós et al. 2003). The maximum WG was observed when the dietary protein level was 340 g kg−1 diet (initial average weight 3.96 g) and 349 g kg−1 diet (initial average weight 26.40 g). The dietary protein requirement of H. niloticus fingerlings between 3 and 15 g was estimated to be 306 and 311 g kg−1 diet for fish between 26 and 62 g when fish meal and soybean were the major protein sources. These values are slightly lower than those obtained for other omnivorous fish species (Oreochromis niloticus and Cyprinus carpio) of similar body weight (350–420 g protein kg−1 diet), as recommended by Tacon (1987). These low dietary protein requirements of Heterotis fingerlings can be correlated to the natural feeding of this species. In fact, juveniles of this species naturally ingest zooplankton, phytoplankton, seeds, aquatic insect and other small benthic organism (Daget 1957; Lauzanne 1976; Micha 1976; Adite et al. 2005).
Protein requirements between fish species are complicated by the difference in species, size and age of fish, diet formulation, stocking density, protein quality, hygiene and experimental conditions between studies (NRC 1993). However, the dietary protein requirement of young Heterotis (3–62 g), as estimated in this study, was slightly lower or very close to the range determined for fingerlings of other omnivorous species, such as cyprinid fish Spinibarbus hollandi (327 g protein kg−1 diet; Yang et al. 2003), common carp Cyprinus carpio (310 g protein kg−1 diet; Takeuchi et al. 1979), jundia Rhamdia quelen (326 g protein kg−1 diet; Meyer & Fracalossi 2004), channel catfish Ictalurus punctatus (280–320 g protein kg−1 diet; Robinson et al. 2000) and Nile tilapia (280–300 g protein kg−1 diet; De Silva et al. 1989; 300–360 g protein kg−1 diet; Shiau 2002).
Dietary energy has a major impact on the utilization of dietary protein in fish, and energy affects quantitative requirements for protein (Wilson 2002). In this study, isoenergetic experimental diets were formulated by adjusting levels of fish and soybean meals with maize meal, wheat bran and fish oil. The protein to energy ratio also may influence the dietary protein requirements. Therefore, further study is needed to evaluate the response of Heterotis fingerlings to diets containing various protein and lipid levels, to establish the optimal dietary protein to energy ratio and to determine the maximum inclusion level of dietary lipid to spare protein for growth. Numerous investigations were conducted to elucidate the protein-sparing effect of many fish species (Hillestad & Johnsen 1994; Thoman et al. 1999; De Silva et al. 2002; Lee et al. 2002; Espinós et al. 2003; Meyer & Fracalossi 2004; Kim & Lee 2005).
From 250 to 300–350 g protein kg−1 diet, statistical analysis on juveniles of 3–15 g indicated a significant increase in WG and SGR with increasing levels of dietary protein. Above this value, we observed a plateau in the growth rate. A similar trend was revealed in juveniles of 26–62 g, except that growth decreased above diet containing 350 g protein kg−1 diet. Investigations with other fish species have reported the same kind of growth profile, fish growth either attaining a maximum output (Shiau & Huang 1989; Al Hafedh 1999; Lee et al. 2001; Kim et al. 2001; Yang et al. 2002; Giri et al. 2003; Islam & Tanaka 2004; Meyer & Fracalossi 2004) or decreasing (Henken et al. 1986; Yang et al. 2003; Ng et al. 2001) above a plateau varying from species to species.
In juveniles with mean body weight varying from 9 to 44 g, SGR values obtained in this study are 3.13. These growth rates are higher than those reported in other young omnivorous species such as Nile tilapia [0.49–0.75 according to Al Hafedh (1999) and 1.90–2.20 according to Abou et al. (2007)] and jundia (1.84–2.61) according to Meyer & Fracalossi (2004). However, these values are in the same order of magnitude as those reported for juveniles of African catfish Clarias gariepinus (2.17–3.10) according to Nyina-wamwiza et al. (2007). The SGR values obtained in this study are very much higher than those reported on the same species by different authors. Actually, SGR values of between 1.36 and 2.77 were reported for juveniles of Heterotis (30–50 g) fed with groundnut cake and brewer’s grain and 0.87 according to Bard (1960) under unfavourable feeding conditions; values of 0.47 (Okoye & Abubakar 1996) and 1.27 (Omorinkoba et al. 1991) have been reported for Heterotis fingerlings of 5 g reared in fertilized ponds and in polyculture with Nile tilapia and African catfish irregularly fed with pellets of 250 g protein kg−1 diet. The good result recorded in this study is explained by the use of fish meal and soybean meal as major protein sources. Nevertheless, Tillon (1959) recorded an SGR value of 3.48 for Heterotis fingerlings (3–6 g) fed with cotton seed at 100 kg ha−1 day−1.
Feed efficiency values obtained in this study are 0.96, which are higher than those reported for young tilapia of similar sizes (Al Hafedh 1999). However, these values are in the same order of magnitude as those reported for juveniles of jundia R. quelen (0.47–0.87) according to Meyer & Fracalossi (2004). On the other hand, FE values obtained in this study are lower than those reported for fingerlings of African catfish C. gariepinus of similar sizes (0.85–1.29) according to Nyina-wamwiza et al. (2007). From the two experiments, FE values obtained with Heterotis fingerlings (3–15 g) were slightly lower than those obtained for juveniles (26–62 g). These data suggest that FE increased with the size of fingerlings in H. niloticus. Nonetheless, this hypothesis has to be further studied at other ontogenetic stages of this species.
From 250 to 300–350 g protein kg−1 diet, statistical analysis on juveniles of 26–62 g indicated a significant increase in WG and FE with increasing levels of dietary protein. Above this value the WG does not significantly decrease, while FE still increases significantly. A similar trend was observed in fingerlings of 3–15 g. This kind of growth and FE profile obtained in this study can be explained by the decreasing feed intake in fish receiving dietary protein (from 250 to 400 g kg−1 diet). Moreover, energy-balanced experimental diets were obtained by adjusting levels of fish and soybean meals with maize meal and wheat bran. These data suggest that the amount of non-protein energy in the feed probably affects intake in Heterotis fingerlings; and then dietary carbohydrate affects diet digestibility and growth in Heterotis fingerlings (3–62 g). In omnivorous fish such as common carp (Cyprinus carpio), Nile tilapia (Oreochromis niloticus) and channel catfish (Ictalurus punctatus), dietary carbohydrate utilization is more important (Wilson 1994). Therefore, more research is needed to study carbohydrate utilization in Heterotis fingerlings, to estimate the maximum dietary starch content acceptable without significant reduction of growth.
The whole-body protein, lipid, moisture and ash contents were not significantly affected by dietary protein levels. Similar results were observed in the carcass composition of Nile tilapia (El-Saidy & Gaber 2005). Other studies in which fish meal was used as main protein source for investigating the dietary protein requirement of fish showed that body ash content was not influenced by the dietary protein level (Shiau & Huang 1989; Yang et al. 2002, 2003).
Conclusion
This study is the first controlled nutritional research in H. niloticus, a promising species for Sub Saharan Africa aquaculture exploitation. The results of this study indicate that the maximum growth of Heterotis fingerlings (3–62 g) was achieved at about 345 g protein kg−1 diet when fish and soybean meals were used as the major sources of protein. Using the broken line model, the dietary protein requirement for H. niloticus fingerlings (3–62 g) was estimated to be 310 g kg−1 diet.
Acknowledgements
This research was financially supported by the CUD–CIUF (Commission Universitaire pour le Développement – Conseil Interuniversitaire de la Communauté Française de Belgique), by the Ministry of Livestock, Fisheries and Animal Industries (Cameroon), the Institute of Agricultural Research for Development (Cameroon) and the University of Namur, Unit of Research in Organismal Biology (Belgium). The staff of Melen Aquaculture Station and fishermen from Akonolinga are highly acknowledged for their help in fingerling collection and fish rearing respectively. Thanks are also due to B. Nkengfac, J. Kouam, D. Mbassi, P. Elang Endende and D. Chuba for their helpful discussions.




