Seven novel KIT mutations in horses with white coat colour phenotypes
Summary
White coat colour in horses is inherited as a monogenic autosomal dominant trait showing a variable expression of coat depigmentation. Mutations in the KIT gene have previously been shown to cause white coat colour phenotypes in pigs, mice and humans. We recently also demonstrated that four independent mutations in the equine KIT gene are responsible for the dominant white coat colour phenotype in various horse breeds. We have now analysed additional horse families segregating for white coat colour phenotypes and report seven new KIT mutations in independent Thoroughbred, Icelandic Horse, German Holstein, Quarter Horse and South German Draft Horse families. In four of the seven families, only one single white horse, presumably representing the founder for each of the four respective mutations, was available for genotyping. The newly reported mutations comprise two frameshift mutations (c.1126_1129delGAAC; c.2193delG), two missense mutations (c.856G>A; c.1789G>A) and three splice site mutations (c.338-1G>C; c.2222-1G>A; c.2684+1G>A). White phenotypes in horses show a remarkable allelic heterogeneity. In fact, a higher number of alleles are molecularly characterized at the equine KIT gene than for any other known gene in livestock species.
Introduction
Pigment cells in developing vertebrates are derived from a transient and pluripotent population of cells located in the neural crest. In vertebrates, the melanocyte precursor cells migrate along the dorso-lateral pathway to the distal sites of the body, proliferate and subsequently differentiate into pigment-producing melanocytes (Erickson 1993). Different signalling pathways influencing the development, migration and proliferation of melanocytes have been identified. Key factors include the endothelin B receptor (EDNRB) and its ligand endothelin-3 (EDN3) (Baynash et al. 1994; Hosoda et al. 1994; Yamada et al. 2006), and the transcription factors PAX3 (Potterf et al. 2000), SOX10 (Pingault et al. 1998) and MITF (Hodgkinson et al. 1993). In addition to these factors, the KIT receptor, a type III receptor protein tyrosine kinase also referred to as steel factor, plays an essential role in melanocyte development (Chabot et al. 1988; Geissler et al. 1988). KIT signalling is crucial for the development of haematopoietic, gonadal and pigment stem cells and acts as an essential survival factor for migrating and proliferating melanoblasts (Blume-Jensen et al. 1991; Steel et al. 1992). The influence on various cell lines explains the frequently observed pleiotropic effects of KIT mutations.
In humans, mice, pigs and horses, various KIT gene mutations have been determined. Besides pigmentation disorders, KIT mutations often cause pleiotropic effects. In mice, KIT mutations lead to modification of coat colour, anaemia and male sterility (Geissler et al. 1981, 1988; Reith et al. 1990; Guerif et al. 2002). In humans, KIT mutations have been identified as the cause of piebaldism, an autosomal dominant disorder of pigmentation (Giebel & Spritz 1991). Gain-of-function KIT mutations have been implicated in gastrointestinal stromal tumours, acute myelogenous leukaemia and systemic mastocytosis (Hirota et al. 1998; Isozaki & Hirota 2006). The white coat colour of hundreds of millions of commercially produced pigs and the belted coat colour phenotype of the Hampshire pig breed are also caused by mutations at the KIT locus (Marklund et al. 1998; Giuffra et al. 1999).
KIT gene mutations in the horse show a broad range of phenotypic expression. Sabino-1, tobiano, roan and dominant white are depigmentation phenotypes caused by KIT gene mutations. The sabino-1 spotting pattern is caused by an intronic mutation causing partial skipping of exon 17 (Brooks & Bailey 2005). Horses heterozygous for the sabino-1 mutation are characterized by intense white patches on the legs and face usually combined with white body spots and roan areas. Horses homozygous for the sabino-1 mutation are almost completely white. The tobiano spotting pattern is caused by a large chromosomal inversion disrupting a regulatory element of the KIT gene (Brooks et al. 2007; Haase et al. 2008). Horses homozygous or heterozygous for the tobiano mutation are phenotypically indistinguishable. Tobiano horses generally have white lower legs, and the white body patches cross the dorsal midline. The roan phenotype in horses is characterized by intermingled white and pigmented hair on the body. Roan is caused by an as yet unknown KIT mutation (Marklund et al. 1999).
The dominant white coat colour is phenotypically very similar to the coat colour in homozygous sabino horses. The skin and hair of dominant white horses are completely or almost completely unpigmented (Pulos & Hutt 1969). Dominant white is inherited as a monogenic autosomal dominant trait. It is hypothesized to be embryonic lethal in the homozygous state. To date, no homozygous horse could be identified. We previously reported four different mutations in the coding sequence of the KIT gene in white horses of different horse breeds (Haase et al. 2007). Currently, there is little known about possible pleiotropic effects of KIT mutations in horses. Haematological parameters in horses carrying the p.Tyr717X mutation were no different from those in horses with wildtype KIT alleles (Haase et al. 2009). In the present study, we report the genetics and coat colour phenotypes of new segregating white coat colour phenotypes in independent horse families.
Materials and methods
Horses
A total of 80 horses from nine horse families representing five different horse breeds were analysed in this study [36 Thoroughbreds (TB), 27 Quarter Horses, one German Holstein Horse (Warmblood), seven Icelandic Horses and nine South German Draft Horses, Fig. S1]. We used 112 solid-coloured Franches-Montages Horses as controls. All horses in the study were tested for the absence of the sabino-1 mutation. All white horses were tested for the absence of the chromosomal inversion on equine chromosome 3 associated with the tobiano spotting pattern. The sabino-1 mutation was genotyped by direct sequencing of a PCR product containing exon 17 of the KIT gene and testing for tobiano was conducted as described (Brooks et al. 2007).
DNA extraction and mutation analysis
Genomic DNA was isolated from peripheral blood using the Nucleon BACC 2 genomic DNA extraction kit (GE Healthcare), and DNA from 15 to 20 hair bulbs was extracted using the QIAamp DNA Mini kit (Qiagen). PCR and sequencing was performed as described previously (Haase et al. 2007). Sequences were assembled with sequencher 4.8 (GeneCodes).
Results
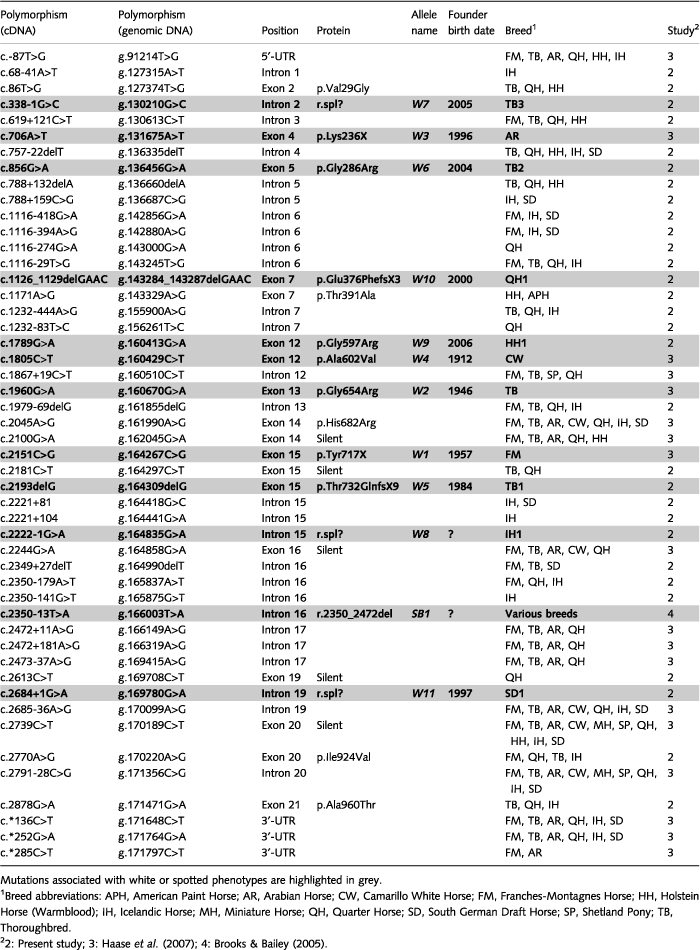
We recently identified four equine KIT mutations in dominant white horses (Haase et al. 2007) and propose to designate these as W1–W4. We now analysed the KIT gene in nine new horse families from five different breeds segregating for a white or partially white coat colour phenotype. We sequenced all 21 KIT exons and their flanking sequences in 192 horses. Among these, 35 horses had a white or partially white coat colour phenotype (Fig. 1). We tested these 35 horses for the absence of known KIT mutations including sabino-1 and tobiano. Comparative sequencing revealed a total of 30 new polymorphisms in the equine KIT gene (Table 1). Among these, we identified seven new mutations affecting the KIT coding sequence in the white horses of seven different families and designated these as W5–W11 (Table 1, Fig. S2).

Coat colour phenotypes of horses with different W mutations. Note the striking variability in the phenotypic expression of the W5 and W10 alleles. For most of the other W alleles, only very few animals exist, therefore it is not clear whether these mutations result in consistent phenotypes.
Thoroughbred family 1 –W5 allele
From this family, 22 horses in total were available, comprising six white horses, two nearly white horses, four horses with a sabino-like phenotype and 10 solid-coloured horses (Fig. 1a–d). Sequence analysis revealed a 1-bp deletion in exon 15 (c.2193delG) that produces a frameshift and a premature stop codon (p.Thr732GlnfsX9). The c.2193delG mutation is predicted to truncate the KIT protein in the middle of the kinase insert domain. All white and nearly white horses were heterozygous for the 1-bp deletion. The deletion was also present in three of four horses with a sabino-like coat colour phenotype. The deletion was absent in all 10 solid-coloured and one sabino-like horse from this family. However, this sabino-like horse had substantially more pigmentation than the three phenotypically distinct sabino-like horses with the c.2193delG mutation (Fig. 1b,d).
Thoroughbred family 2 –W6 allele
A single white horse with some residual pigmentation from this family was available for mutation analysis (Fig. 1e). This horse had a missense mutation located in exon 5 of the KIT gene (c.856G>A). The mutation affects the extracellular ligand-binding domain of the KIT protein and leads to a non-conservative exchange of glycine with arginine (p.Gly286Arg). As only one single white horse was available, it was not possible to analyse solid-coloured horses from the same family.
Thoroughbred family 3 –W7 allele
A solid-coloured dam and her partially white filly were available from this family. The filly had some residual pigmentation around the ears and along the neck and back (Fig. 1f). The dam was recorded to have nine more non-white foals. The white filly was heterozygous for a mutation at the 3′-splice site of intron 2 (c.338-1G>C). The mutation was absent in the solid-coloured mother.
Icelandic Horse family 1 –W8 allele
An Icelandic Horse family consisting of a partially white horse, its solid-coloured parents, and four solid-coloured maternal half-sibs was analysed. The solid-coloured horses had a black or chestnut coat colour without or with just minimal white facial markings. The partially white horse had a mottled phenotype with substantial residual pigmentation (Fig. 1g). The partially white horse was heterozygous for a splice-site mutation at the end of intron 15 (c.2222-1G>A). None of the solid-coloured horses in this family had the mutation.
Holstein Horse family 1 –W9 allele
A single completely white Holstein Horse (Fig. 1h) born out of solid-coloured parents was heterozygous for a missense mutation in exon 12 (c.1789G>A). This mutation affects the intracellular tyrosine kinase domain of the KIT protein and leads to a non-conservative exchange of a glycine with an arginine (p.Gly597Arg). No solid-coloured animals from this family were available for analysis.
Quarter Horse family 1 –W10 allele
A family of 27 Quarter Horses was available for analysis. Of these 27 horses, five were registered with a white coat colour phenotype, five as spotted and the remaining 17 as solid-coloured. Sequence analysis revealed a 4-bp deletion located in exon 7 (c.1126_1129delGAAC). The deletion causes a frameshift and introduces a premature stop codon into the open reading frame of the KIT gene, which is predicted to truncate the protein in the extracellular domain (p.Glu376PhefsX3). All white and all spotted horses were heterozygous for the 4-bp deletion. None of the solid-coloured horses in this family had the mutation (Fig. 1i–k).
South German Draft Horse family 1 –W11 allele
A completely white South German Draft Horse stallion and eight of his offspring were analysed (Fig. 1l). The offspring consisted of three white horses, four solid-coloured horses and a single leopard-spotted horse, which inherited the leopard-spotting phenotype from its dam. All white South German Draft Horses had a mutation affecting the 5′-splice site of intron 20 (c.2684+1G>A). The mutation showed perfect co-segregation with the white phenotype in the family.
In addition to these seven families, we sequenced two more independent TB families with white horses. However, we could not detect any KIT coding mutations in these horses that could explain the white coat colour phenotype. None of the seven newly described candidate causative mutations was present in 112 solid-coloured Franches-Montagnes Horses.
Discussion
This study indicates that white phenotypes occur in many different horse breeds because of independent mutations in the KIT gene. Although we do not have functional data on the different KIT alleles, the knowledge of KIT mutations in other species and the data on the previously published equine KIT mutations (Brooks & Bailey 2005; Brooks et al. 2007; Haase et al. 2007) strongly suggest that the seven newly discovered mutations affecting the KIT coding sequence are indeed causative for the observed white or partially white phenotypes. These seven candidate causative mutations were absent from 112 solid-coloured Franches-Montagnes Horses. One can argue that these mutations might represent breed-specific polymorphisms, which would not be expected to occur in the Franches-Montagnes breed. However, of the other 37 presumably neutral polymorphisms in the KIT gene, 22 (59%) also segregate in the Franches-Montagnes breed. Therefore, the absence of all seven candidate causative mutations from solid-coloured Franches-Montagnes Horses provides additional suggestive support for the causality of these mutations.
Two of the seven candidate causative mutations are frameshift mutations (c.2193delG; c.1126_1129delGAAC) and three represent splice site mutations (c.338-1G>C; c.2222-1G>A; c.2684+1G>A). It seems highly likely that such mutations affect the KIT protein function. The remaining two candidate causative mutations are missense mutations (c.856G>A corresponding to p.Gly286Arg and c.1789G>A corresponding to p.Gly597Arg). For the two missense mutations, it is more difficult to predict whether they indeed affect the KIT function. The p.Gly286Arg mutation affects the extracellular ligand-binding domain and the p.Gly597Arg mutation the first intracellular tyrosine kinase domain. The wild-type glycine residues at both positions are conserved in the human and murine KIT proteins. Both mutations cause an exchange of glycine to arginine, which are extremely dissimilar amino acids. Therefore, it is conceivable that these mutations also affect the KIT function.
As the genotype–phenotype correlations of the different KIT alleles in horses are complex, it is not possible to predict the exact genotype of a white or partially white horse from its phenotype. We therefore propose to use the allele designations W1–W11 for the characterized KIT mutations to distinguish these horses. We observed a remarkable phenotypic variability for some of the equine W alleles. The alleles W1, W5 and W10 occur in completely white horses but also in horses with substantial residual pigmentation (Haase et al. 2007; Fig. 1). All horses carrying the W2, W3, W4, W6, W7, W9 and W11 alleles were completely white. However, only very few horses with these alleles were available. Therefore, it cannot be excluded that these alleles may also result in partially white horses. The reason for the variability in the coat colour phenotype of horses with the W1, W5 and W10 alleles is not known. The lack of melanocytes in these horses might be because of haploinsufficiency of the KIT gene, and subtle variations in the amount of residual KIT protein might already cause the observed phenotypic variability. On the other hand, it is also possible that one or more trans-acting modifier genes determine the amount of residual pigmentation in these horses.
The pedigree records allow a precise dating of the individual mutation events for this dominant trait. Whenever a white foal is born out of solid-coloured parents, the most likely explanation is a KIT mutation in the germline of one of its parents or alternatively a mutation in the early developing embryo itself, which might lead to mosaic foals. Many of the described W alleles arose during the last 10 years; therefore, our study included several founder animals where mosaicism cannot be excluded. One example for such a scenario is the W8 allele observed in a single ‘mottled’ Icelandic horse, which represents the founder animal for this mutation (Fig. 1g). This horse might be a mosaic, and it remains to be determined whether it will consistently produce offspring with the mottled phenotype.
The equine KIT gene shows a remarkable allelic diversity. Obviously, the striking coat colour phenotypes of the horses and the dominant inheritance increase the chances that spontaneous mutations in this gene are recognized in the first place. Therefore, the observed allelic diversity does not necessarily implicate a particularly high mutation rate. To date, 11 different W mutations, the sabino-1 mutation and the tobiano mutation are characterized as functional mutations of the equine KIT locus (Brooks & Bailey 2005; Brooks et al. 2007; Haase et al. 2007). In addition, the as yet uncharacterized roan mutation is also linked to the KIT locus (Marklund et al. 1999). Finally, in the two families where we did not find any coding mutations, further non-coding regulatory mutations at the KIT locus or mutations in other coat colour genes might be responsible for the observed white phenotypes. Thus, there are at least 14 functionally relevant KIT alleles in horses with white or white spotted coat colour. According to our knowledge, this is the largest observed allelic spectrum for any gene in domestic animals, although other examples of more limited allelic heterogeneity are also well known in domestic animals (Schmutz et al. 2002; Drögemüller et al. 2007).
In conclusion, we confirmed the significant role of KIT mutations in white horses and identified seven new candidate causative mutations. This will allow an improved classification of white horses based on their genotypes. The identified KIT mutations alone do not explain the variable phenotypic expression in some horse families.
Acknowledgements
The authors would like to thank Brigitta Colomb for expert technical assistance and Carmen Schwippert, Henriette Smit-Arriens and Hartwig Tewes for providing samples and pictures of white horses. The authors would like to thank Toshio Matsuda and Yanagi Sports Co., Ltd. for providing samples and pictures of white horses in Japan, and also appreciate the help of Kei-ichi Hirota and Mitsuo Fukunaga in the Laboratory of Racing Chemistry (LRC), and owners, trainers and staff of the Japan Racing Association (JRA) and National Association of Racing (NAR) for collecting samples in Japan. The authors would also like to thank all other involved horse owners and breeding organizations for donating samples and pictures and for sharing pedigree information of their horses. This study was supported by a grant from the Swiss National Science Foundation.