Common promoter deletion is associated with 3.9-fold differential transcription of ovine CCR5 and reduced proviral level of ovine progressive pneumonia virus
Nucleotide sequence data have been deposited in the DDBJ/EMBL/GenBank databases under the accession number FJ008056.1. Nucleotide sequence variant data have been deposited in dbSNP accessions ss105325111–ss105325167.
Summary
Chemokine (C-C motif) Receptor 5 (CCR5) is a chemokine receptor that regulates immune cell recruitment in inflammation and serves as a coreceptor for human immunodeficiency virus (HIV). A human CCR5 coding deletion (termed delta-32) results in strong resistance to HIV infection, and sequence variants in CCR5 regulatory regions have been implicated in delayed progression to acquired immune deficiency syndrome. Both ovine progressive pneumonia virus (OPPV), also known as maedi-visna, and HIV are macrophage-tropic lentiviruses, have similar genomic structures, and cause lifelong persistent host infection, suggesting CCR5 may have a role in regulating OPPV provirus levels. Therefore, the ovine CCR5 genomic sequence was determined, and sequence variants were obtained from the open reading frame and surrounding regulatory sites. One CCR5 variant contained a 4-base deletion within a binding site for octamer transcription factors in the promoter region. A test for differential transcription from each allele in heterozygous animals showed a 3.9-fold transcription difference (P < 0.0001). OPPV proviral levels were also measured in 351 naturally exposed Rambouillet, Polypay and Columbia sheep. Deletion homozygotes showed reduced OPPV proviral levels among these animals (P < 0.01). The association of this CCR5 promoter deletion with OPPV levels will need to be validated in additional populations before the deletion can be recommended for widespread use in marker-assisted selection. However, because of the large impact on transcription and because CCR5 has roles in inflammation, recruitment of effector cells, and cell-mediated immunity, this deletion may play a role in the control of infections of many diverse pathogens of sheep.
Introduction
Ovine progressive pneumonia virus (OPPV), also known as maedi-visna virus, is a common lentiviral pathogen of U.S. sheep. Almost half of flocks contain at least one infected animal, and overall prevalence is estimated at 26% (Cutlip et al. 1992). OPPV results in persistent, lifelong infection and the virus can be transmitted throughout the lifespan (De Boer et al. 1979). Infected sheep display symptoms which include varying degrees of dyspnoea, mastitis, cachexia, arthritis and/or encephalitis (Narayan et al. 1988). Through these clinical symptoms and histopathological changes in the mammary gland, lung, carpal synovial membranes, and central nervous system, OPPV impacts on the health of the infected animal and the development of lambs nursed by infected ewes.
It has been repeatedly observed that sheep breeds have differing odds of infection with OPPV, which suggests that resistance to infection may have a genetic basis (Gates et al. 1978; Cutlip et al. 1986; Houwers et al. 1989; Snowder et al. 1990). More recently, it has been demonstrated that host control of provirus levels post-infection may also have a genetic basis (Herrmann-Hoesing et al. 2008b). The same study identified Ovar-DRB1 as one potential gene involved in host containment of OPPV (Herrmann-Hoesing et al. 2008b). However, the size of the breed differences suggested that other genes are involved, but these have yet to be identified.
Logical candidates for additional genes involved in responses to OPPV may be resistance genes for related viruses, including human immunodeficiency (HIV). Both HIV and OPPV are macrophage-tropic lentiviruses that cause lifelong persistent infection in the host (Gendelman et al. 1986; Gorrell et al. 1992; Thormar 2005; Alkhatib & Berger 2007). Chemokine (C-C motif) Receptor 5 (CCR5) has been implicated as a source of genetic resistance to HIV. Individuals with the delta-32 deletion have no functional CCR5 protein on the cell surface and are highly resistant to HIV infection (Kaslow et al. 2005; Alkhatib & Berger 2007). Furthermore, other CCR5 sequence variants in regulatory regions have also been shown to influence CCR5 transcription, translation and acquired immune deficiency syndrome (AIDS) progression in humans (McDermott et al. 1998; Bream et al. 1999; Kostrikis et al. 1999; Kaslow et al. 2005; Jin et al. 2008). Sequence variants of ovine CCR5 could likewise impact OPPV infection in sheep.
As CCR5 may play a role in OPPV infection, this study determined the genomic sequence of sheep CCR5. Genetic variants were examined in a discovery group containing several different breeds, and the specific roles of the genetic variants were scrutinized for potential mechanisms by which they might influence OPPV. Further, association with proviral levels was examined in Idaho range sheep that were naturally exposed to OPPV. These initial steps have allowed the investigation of the role of CCR5 variants in OPPV infection in sheep.
Materials and methods
Ovine CCR5 genomic sequence
The genomic sequence of ovine CCR5 was determined from a BAC clone (CCR5-105-J3) representing a Rambouillet ram (Herrmann-Hoesing et al. 2008a). The BAC was subcloned and sequenced using primers designed from bovine CCR5 (Table S1) and BigDye Terminator chemistry (Applied Biosystems).
OPPV proviral level determination
Testing for OPPV was performed on 351 ewes from closed flocks raised under extensive management conditions in Idaho (Herrmann-Hoesing et al. 2008b). Approximately equal numbers of ewes were included from Rambouillet, Polypay and Columbia breeds and for each age (3–6 years) within each breed. Animal care and handling procedures were reviewed and approved by the USSES animal care and use board. DNA was extracted from peripheral blood leucocytes and OPPV proviral levels were determined by a validated qPCR method (Herrmann-Hoesing et al. 2007).
Sequence variants and genotyping
A marker discovery panel was composed of 36 ewes with equal representation of Rambouillet, Polypay and Columbia breeds, and extremely high, extremely low or undetectable proviral levels. PCR was performed using the primers shown in Table S2 and the following standard PCR conditions: an initial denaturation at 92 °C for 5 min, followed by 35 cycles of 92 °C for 30 s, 58 or 60 °C for 30 s and 68 °C for 90 s, and a final extension step at 68 °C for 10 min. Re-sequencing was performed using the primers shown in Table S3 and BigDye Terminator chemistry (Applied Biosystems). Haplotype tagging was performed using haploview (Barrett et al. 2005).
Sixteen markers (Table 1) were genotyped in the full OPPV animal set of 351 ewes which had approximately equal representation from Rambouillet, Polypay and Columbia breeds as previously described (Herrmann-Hoesing et al. 2008b). Full animal set genotyping was performed using TaqMan genotyping assays, restriction fragment length polymorphism (RFLP) assays, or sequencing with BigDye Terminator chemistry (Applied Biosystems) as shown in Table S4. TaqMan assays were performed according to manufacturer specifications (Applied Biosystems) using primer and probe sets as shown in Table S4. RFLP assays were performed using standard PCR conditions (see above) and restriction enzymes listed in Table S4 according to manufacturer specifications (New England Biolabs; Fermentas Inc.). These were visualized on 2% (m/v) agarose gels.
| Marker | NCBI dbSNP | Sequence variant1 | Minor allele | Minor allele frequency (%) | Impact on CCR5 |
|---|---|---|---|---|---|
| 1 | ss105325119 | g.4965C>T | T | 40.7 | |
| 2 | ss105325120 | g.5003G>C | C | 34.0 | |
| 3 | ss105325126 | g.5436_5439delAATG | del | 33.0 | Delete Oct site |
| 4 | ss105325128 | g.5619C>T | T | 33.0 | |
| 5 | ss105325134 | g.6334A>C | C | 41.3 | |
| 6 | ss105325145 | g.7028G>A | A | 24.5 | |
| 7 | ss105325149 | g.7385A>C | C | 7.5 | |
| 8 | ss105325152 | g.7641C>T | T | 7.9 | p.Ser38Leu2 |
| 9 | ss105325155 | g.8173G>A | A | 8.1 | |
| 10 | ss105325156 | g.8315A>T | T | 40.9 | p.Ile263Phe2 |
| 11 | ss105325157 | g.8332C>T | T | 15.2 | |
| 12 | ss105325158 | g.8431G>C | C | 8.0 | |
| 13 | ss105325159 | g.8519T>G | G | 41.0 | p.Ser331Ala2 |
| 14 | ss105325160 | g.8541C>T | T | 41.0 | p.Ala338Val2 |
| 15 | ss105325161 | g.8644T>A | A | 33.0 | |
| 16 | ss105325162 | g.8709C>G | G | 32.9 |
- 1Nucleotide positions in reference to GenBank accession FJ008056.1.
- 2Theoretically deduced amino acid substitutions relative to GenBank accession ACJ46497.1.
Relative transcription of CCR5 mRNA
Relative transcription was measured by allele counting of cDNA clones from each of nine individual sheep heterozygous for marker 3. Nine OPPV- and malignant catarrhal fever virus (MCFV)-free sheep were selected to reduce any pathogen-induced immunomodulatory effects. RNA was isolated from fresh peripheral blood leucocytes, and three first strand cDNA reactions were completed using 100 ng of total RNA, anchored Oligo(dt)20 primers (Invitrogen), and M-MLV reverse transcriptase (Fisher Scientific) incubated at 42 °C for 2 h. The three cDNA preparations were pooled for each animal and used as template for PCR with standard conditions (see above) and primers AGKCTCCAGAGCGAGTGAGC (HG906F+) and GTAGYGAGGGGAGAGCTTGC (HG911R+). Amplification products were cloned using a Topo TA kit (Invitrogen). Overnight cultures of 96 colonies from each animal were prepared, and DNA was extracted using a boiling method.
Marker 3 alleles were identified for each cDNA clone using an allele-specific PCR for marker 16, as marker 16 was expressed in the transcript and was in complete linkage disequilibrium with marker 3 in these animals. One microlitre of DNA and 4 pmol of each primer were used for 10 μl allele-specific PCR. Separate reactions were run with HG906+ and either HG993R (GGCTTTCTAAAATTGATGGC) identifying the marker 3 insertion allele or HG994R (GGCTTTCTAAAATTGATGGG) identifying the marker 3 deletion allele. These reactions used standard PCR conditions (see above) except for the substitution of a 5-s, 68 °C extension time and the use of 40 cycles of amplification. Relative transcription was measured by the proportion of cDNA clones from each chromosome of these heterozygous animals.
Statistical and bioinformatic methods
Hardy–Weinberg proportions were assessed using chi-squared tests prior to additional statistical analyses with marker data. Two types of analyses were performed to assess the association between CCR5 genotypes and OPPV proviral levels. First, logistic regression models from proc logistic of sas v9.1 (SAS Institute) were used to assess genotypic differences in frequency of load-positive animals. The second analytic method used proc glm of sas v9.1 to assess genotypic differences in logarithm (base 10)-transformed proviral loads among positive animals. In both cases, the association models included fixed effects of breed and genotype, a linear covariate of age, and the interaction of breed and age. All reported P-values were nominal and were not corrected for multiple testing.
Relative expression counts of cDNA clones from nine animals were first examined using a chi-squared test for homogeneity of proportions. The pooled counts were tested against a binomial proportion of 0.5 using the frequency procedure of sas v9.1 (SAS Institute).
Transcription factor binding sites were defined based on homology to experimentally verified sites in the human CCR5 promoter, and confirmed using match 12.1, which was set to minimize the sum of the false-positive and false-negative error rates and matrices from the transfac 12.1 Professional database (Biobase GmbH).
Results
A 13.3-kb ovine genomic sequence encompassing CCR5 and surrounding regulatory regions was produced from a Rambouillet ram (GenBank accession FJ008056.1). Ovine CCR5 had two exons with conserved boundaries, and the entire open reading frame was located in the second exon. The open reading frame encoded a CCR5 protein of 352 residues with 95.5% amino acid identity to bovine CCR5, 83.5% to human, 82.4% to canine and 79.0% to mouse. An alignment showing amino acid sequence differences between CCR5 from sheep and several other mammals is shown in Fig. S1.
Fifty-seven sequence variants were observed in 5.2 kb of the CCR5 gene region (dbSNP accessions ss105325111–ss105325167) in the marker discovery animal set. Of these, six were insertion–deletion variants and 51 were single nucleotide polymorphisms (SNPs). Four of the SNPs resulted in amino acid substitutions of leucine for serine at residue 38, phenylalanine for isoleucine at residue 263, alanine for serine at residue 331, and valine for alanine at residue 338 (Table 1). One deletion (g.5436_5439delAATG) eliminated four base pairs from an octamer (Oct) protein binding site in the promoter region at nucleotide positions 5436–5439 in GenBank accession FJ008056.1 (marker 3; Fig. 1). Comparison to cow, pig, horse, human and dog revealed that the insertion was the ancestral allele, with the deletion observed only in sheep.

Ovine CCR5 genomic region. Exons are shown as larger cylinders, with the open reading frame shown as a darker region in exon 2. Surrounding sequences including the intron are depicted as smaller cylinders. Marker positions are shown by vertical lines. Asterisks highlight markers 3, 4, 15 and 16, which were on the haplotypes associated with differential mRNA expression and proviral levels. The Oct site deletion is shown with match core nucleotides for an Oct1 binding site highlighted with bold and underlined text.
To test whether the Oct site deletion interfered with transcription, CCR5 transcripts from peripheral blood leucocytes of nine heterozygous, OPPV- and MCFV-free animals were reverse transcribed, cloned and counted. A preliminary chi-squared test for homogeneity between the animals was not significant (P > 0.05), indicating that none of the animals had significantly differing relative transcription of the two alleles. Therefore, the pooled counts were used to estimate the proportion of transcripts from each allele. A significant 3.9-fold reduction (P < 0.0001; 95% CI: 3.3 to 4.6-fold) in transcription from the deletion allele was observed relative to the ancestral allele (Fig. 2).
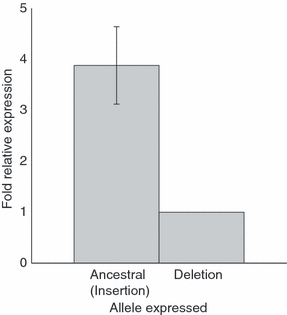
Fold relative expression of CCR5 mRNA transcripts for insertion relative to deletion alleles from heterozygous animals for the Oct site deletion. Expression was significantly different from equal proportions (P < 0.0001), with a 3.9-fold expression difference between alleles. Error bars show 95% confidence interval for relative expression.
To test the whole range of observed CCR5 variation for association with OPPV provirus levels, 16 sequence variants including the Oct site deletion and 15 SNPs were chosen by haplotype tagging to genotype the complete OPPV animal set (Table 1). The genotypes for all sequence variants were in agreement with Hardy–Weinberg proportions (P > 0.05). No sequence variants were associated with differential odds of infection (P > 0.05). However, one haplotype was associated with reduced OPPV levels among positive animals (P = 0.0072; Fig. 3). The haplotype included markers 3, 4, 15 and 16 in tight linkage disequilibrium with each other (r2 > = 0.99; D′ > = 0.99 for all pairwise comparisons). The Oct site deletion allele occurred at a frequency of 33.3% in the overall animal set, with individual breed frequencies of 29.8% in Rambouillets, 25.4% in Polypays and 45.9% in Columbias. Out of the set of 351 animals, there were 36 homozygotes for the Oct site deletion, 162 heterozygotes and 153 homozygotes with the reference allele.
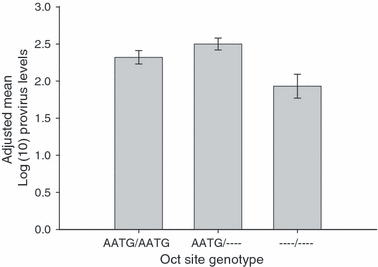
Deletion homozygotes had approximately half the adjusted mean ovine progressive pneumonia virus provirus levels compared to insertion homozygotes or heterozygous animals at the Oct site deletion (P = 0.0072). Error bars show standard error for proviral levels.
Discussion
Chemokine (C-C motif) Receptor 5 is an important gene in the immune response, and this study examined common sequence variants in the CCR5 gene region. Analyses of gene variants, their role in gene expression, and their association with control of OPPV will be discussed below. Because CCR5 impacts the recruitment of leucocytes to combat infection with diverse types of pathogens, the CCR5 variants described here may influence the outcomes of infections with many pathogens of sheep.
To our knowledge, this study provides the first finished genomic sequence for the ovine CCR5 gene region. The gene structure and similarity to other mammalian CCR5 genes were similar to a recently reported bovine CCR5 sequence (Blumerman et al. 2007). More than 50 individual sequence variants were observed in the open reading frame and surrounding regions. One variant was a 4-base pair deletion which removed a transcription factor binding site for Oct proteins (Fig. 1).
The Oct binding site disrupted by this deletion has been shown to be functionally important in the human CCR5 promoter, and it is conserved among mammals. The site is bound by at least two transcription factors, the more ubiquitous Oct-1 and the lymphoid-specific Oct-2 (Moriuchi et al. 1997). Binding by Oct-2 has been shown to upregulate CCR5 transcription and the ability of the protein to fuse with HIV-1 Env, a measure of cell surface CCR5 protein expression (Moriuchi & Moriuchi 2001). Further, there are Oct sites in the same region of the CCR5 promoter in at least seven other mammalian species, including cow, pig, horse, dog, rhesus macaque, chimpanzee and human. The sequence-level conservation in the promoter was lower than the open reading frame, as one would expect for transcription factor binding sites, but the consistent location of Oct sites in CCR5 promoters and the demonstrated biological importance suggested the deletion allele might have direct functional importance for sheep and may reduce expression from the sheep deletion allele.
As the sheep deletion removed a transcription factor binding site in the promoter, we tested relative expression from the ancestral vs. deletion haplotypes. In sheep heterozygous for the Oct site deletion, the deletion haplotype had 3.9-fold reduced expression (P < 0.0001; 95% CI 3.2 to 4.6-fold) compared to the ancestral haplotype (Fig. 2). This reduced expression from the sheep deletion allele was consistent with the observation that binding of this site by Oct-2 enhanced CCR5 expression (Moriuchi & Moriuchi 2001), and suggests that the deletion of the Oct site may be a functional mutation. The possibility exists that other sites on the same haplotype, but further outside CCR5, could also play a role as repressors that could function at a distance. However, the functional importance of the human CCR5 promoter, the conservation of Oct site in the CCR5 promoters, and the greatly reduced expression from the deletion haplotype collectively suggest that this deletion may play a functional role in CCR5 expression.
Several regulatory sequence variants have been shown to influence CCR5 and AIDS progression in humans. The most comparable to the sheep deletion reported here is 59029G, also known as −2459 A/G, which reduces CCR5 transcription by 45% and reduces HIV replication (McDermott et al. 1998; Salkowitz et al. 2003). In the homozygous form, 59029G/G delays the progression time until clinical AIDS by 3.8 years (McDermott et al. 1998). Neither 59029G nor any of the other regulatory sequence variants in human CCR5 change the same Oct site as the sheep deletion (McDermott et al. 1998; Bream et al. 1999; Kostrikis et al. 1999; Jin et al. 2008), but the presence of similar kinds of changes in both human and CCR5 suggest there may be similar effects on lentiviral infection.
Haplotype tagging markers were chosen to efficiently test for genotypic differences in OPPV proviral levels related to any of the variants observed (Table 1). Recent studies have shown that factors such as breed and age are important for OPPV (Herrmann-Hoesing et al. 2008b), so all analyses in this study accounted for those factors explicitly in the association model. After accounting for these factors, the Oct deletion haplotype was associated with reduced proviral levels among OPPV-positive sheep (Fig. 1). Homozygotes for this haplotype had approximately half the OPPV proviral levels compared to insertion homozygotes or heterozygotes, depending on the specific insertion genotype to which it was compared (P < 0.01; Fig. 3).
The lower proviral levels in mature sheep homozygous for the Oct site deletion suggest superior immune containment of the virus post-infection, but the exact mechanisms at work with OPPV remain to be elucidated. Reduced expression of CCR5 could result in reduced chemotaxis of macrophages and other leucocytes to the site of infection (Locati et al. 2002; Ma et al. 2005), which could also slow the rate of cellular infection relative to infected cell death. CCR5 could also function as a coreceptor for OPPV, as it does with HIV (Alkhatib & Berger 2007), and reduced coreceptor availability could slow the rate of cellular infection relative to the rate of infected cell death, which would result in lower proviral levels. Finally, reduced CCR5 expression could lead to changes in cell-mediated immunity that could enhance killing of infected cells (Dolan et al. 2007). The many functional roles of CCR5 suggest a combination of mechanisms as the most likely explanation, and further experiments will be necessary to elucidate the exact role of the Oct site deletion and reduced CCR5 expression in OPPV infection.
Future experiments could validate the Oct site deletion as a selectable genetic marker to reduce the proviral loads of OPPV. If so, the high allele frequency (33%) suggests that rapid genetic progress could be made toward deletion homozygotes that may have lower proviral levels if infected with OPPV. Further, the fact that the allele frequency is high in all breeds examined suggests that rapid genetic progress could be made in many breeds without the need for time-consuming introgression of the allele. However, the Oct site deletion will need to be validated before it can be recommended for wide use in marker-assisted selection.
Beyond its role in OPPV, the fundamental nature of CCR5’s roles in the immune system are demonstrated by the wide range of viral pathogens for which CCR5 variants have been shown to play large roles in resistance/resilience. CCR5 is a critical regulator of viral load and mortality for infections with such diverse infectious diseases as respiratory infection with influenza A and sexually transmitted infection with herpes simplex virus-2 (Dawson et al. 2000; Thapa et al. 2007). CCR5 is also important in inflammation and the development of symptomatic disease for West Nile virus and respiratory syncytial virus (Hull et al. 2003; Lim et al. 2008). Further, CCR5 variants have been associated with differences in odds of clearance vs. chronic infection with hepatitis B (Thio et al. 2007).
The important roles of CCR5 have also been highlighted in responses to bacteria, protozoa and parasitic worms. CCR5 has been associated with Mycoplasma pneumoniae infection in humans (Ungvari et al. 2007). It has also been shown to function as an innate immune receptor for mycobacterial Hsp70 (Floto et al. 2006). CCR5 has been associated with parasite burden of the parasitic protozoa Leishmania major, Trypanosoma cruzi and Toxoplasma gondii (Sato et al. 1999; Hardison et al. 2006; Khan et al. 2006). Finally, CCR5 knockout mice have recently been shown to have enhanced lethality and larger granuloma size when infected with the trematode worm Schistosoma mansoni (Souza et al. 2008), which suggests that CCR5 could play a role in resistance to parasitic worms in sheep. The large variety of pathogens for which CCR5 is important suggests that a sequence variant with large impact on CCR5 function in sheep could play roles in resistance/resilience to a wide range of sheep pathogens.
In conclusion, these results demonstrate a common deletion in the promoter region of ovine CCR5. The location of the deletion suggested differential transcription as a mechanism of action, and 3.9-fold reduced transcription was confirmed from the deletion allele. Homozygotes for the deletion had approximately half the proviral levels of OPPV compared to insertion homozygotes or heterozygotes. The large difference in CCR5 expression and the many immunological functions of CCR5 suggest that this deletion could have roles in resistance or resilience for many diverse pathogens of sheep.
Acknowledgements
The authors gratefully acknowledge Codie Durfee, Heather Garcia, Nic Durfee, Liam Broughton, Tom Kellom, and the farm crews at Washington State University, ADRU, and USSES for technical assistance. We thank Hong Li for providing some of the OPPV-free, MCFV-free sheep used for transcriptional analysis. This research was funded by USDA-ARS CWA No. 5348-32000-029-00D.




