Coordinate action of membrane-type matrix metalloproteinase-1 (MT1-MMP) and MMP-2 enhances pericellular proteolysis and invasion
Abstract
Membrane-type matrix metalloproteinase-1 (MT1-MMP) mediates cleavage of not only MMP-2/gelatinase A for activation, but also a variety of substrates including type I collagen (reviewed in Cancer Sci 2005; 96: 212–7). MMP-2 activation involves tissue inhibitor of MMP (TIMP)-2 as a bridging molecule between MT1-MMP and pro-MMP-2. Thus, net activity of MT1-MMP and MMP-2 is regulated in a complex manner depending on TIMP-2 concentration. During invasive growth of tumor cells in type I collagen matrix, MT1-MMP initiates denaturation of collagen into gelatin, which is subsequently digested further by MMP-2 adjacent to MT1-MMP. Coordinate action of MT1-MMP and MMP-2 may facilitate pericellular proteolysis, and enhance not only tumor invasion/migration but also cell growth. Tetraspanins as binding proteins of MT1-MMP regulate MT1-MMP subcellular localization and compartmentalization, leading to efficient MMP-2 activation and proteolysis coupled with cellular function.
(Cancer Sci 2010; 101: 843–847)
The major physiological role of matrix metalloproteinases (MMPs) is for homeostatic regulation of the extracellular environment, not simply to degrade matrix as their name suggests. MMPs are overexpressed in various human malignancies.(1,2) The expression of MMPs is specifically regulated at the level of transcription. However, the catalytic activity of these proteinases is further controlled at additional levels, including activation of the pro-enzyme and inhibition of the active enzyme by specific inhibitors.(3–5) Because MMPs including MMP-2 (gelatinase A/72-kDa type IV collagenase) are secreted as an inactive zymogen, activation is another key regulatory step for MMP function in vivo. The molecular environments in tumors appear conducive to MMP activation. Activated MMP-2 was specifically observed in a variety of tumor tissues, suggesting the presence of pro-MMP-2 activator(s) in tumor tissues.(6–8) This led to the identification of the first membrane type-MMP (MT1-MMP) as an activator of pro-MMP-2 expressed on the surface of tumor cells.(8) In addition to pro-MMP-2 cleavage for activation, MT1-MMP was shown to cleave variety of substrates, not only extracellular matrix (ECM) macromolecules, but also various biologically important molecules (reviewed in references9,10).
Interaction of MT1-MMP with TIMP
To date, six MT-MMPs have been identified by cDNA cloning, and the proteolytic activation of pro-MMP-2 was shown experimentally for all MT-MMPs.(11–14) MT-MMPs are unique in that they contain a furin recognition motif between the pro- and catalytic domains, and are processed by proprotein convertases such as furin in the secretory pathway.(15) In contrast to secretory form of MMPs, MT-MMPs are presented to the cell surface in active form. MT1-MMP is inhibited by endogenous inhibitors TIMP-2, -3, and -4, but not by TIMP-1.(16,17) This TIMP-1 insensitive nature is common to all MT-MMPs except for MT4-MMP.(18) MT1-MMP causes a rapid auto-degradation in the absence of its inhibitor to produce a 44-kDa form.(19) Due to rapid turnover rate of active MT1-MMP, its concentration on cell surface is very low, and the active form of MT1-MMP predominantly localizes on the cell surface by forming a complex with the inhibitor (Fig. 1). TIMP-2 is the most common inhibitor of MT1-MMP, and MT1-MMP/TIMP-2 complex serves as a receptor for pro-MMP-2. Administration of the synthetic reversible inhibitor of MMP induces accumulation of MT1-MMP on the cell surface, which exerts enzyme activity at a burst by the removal of the inhibitor.(20)
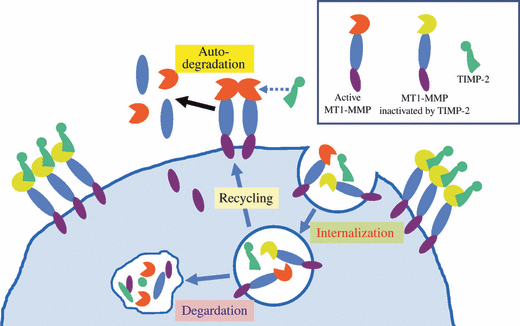
Fate of membrane-type matrix metalloproteinase-1 (MT1-MMP) on the cell surface. MT1-MMP on cell surface rapidly turns over by auto-degradation or clathrin-dependent internalization. The internalized MT1-MMP is recycled or degraded in lysozome. MT1-MMP inactivated by tissue inhibitor of MMP (TIMP)-2 avoids auto-degradation, and accumulates on the cell surface. Accumulated MT1-MMP/TIMP-2 complex serves as a receptor for pro-MMP-2. It should be noted that concentration of active MT1-MMP on cell surface is quite low due to rapid auto-degradation, and only TIMP-2-inactivated MT1-MMP accumulates.
Implications from MMP-deficient mice
MT1-MMP is the most common physiological activator of pro-MMP-2, and the MT1-MMP-deficient mouse produces only a faint level of active MMP-2.(21,22) MT1-MMP-null mice have severe defects in skeletal development and angiogenesis and die within several weeks after birth, suggesting an essential role for MT1-MMP in the process of angiogenesis and bone growth.(21,22) This phenotype is much more severe than that seen in the MMP-2-deficient mice, which have smaller stature, blunted tumor-related angiogenesis, and abnormal lung alveolarization.(23) A hereditary deficiency in MMP-2 has been described in Middle Eastern families who develop a vanishing bone disorder.(24,25) Thus, MT1-MMP functionality significantly exceeds MMP-2 activation in development. However, MT1-MMP and MMP-2 double-knockout mice show more severe phenotype than MT1-MMP-deficient mice, and die immediately after birth with respiratory failure, abnormal blood vessels, and immature muscle fibers reminiscent of central core disease.(26) This indicates that residual MMP-2 activation in MT1-MMP-null mice contributes to prolonged survival by partly complicating MT1-MMP functions in development. MMP-2 degrades types IV and V collagens, gelatins, laminin, fibronectin, and elastin.(27,28) From the analysis of MMP-2-deficient mice, MMP-2 does not seem to be indispensable in development, although it partially complicates MT1-MMP. However, MMP-2 plays critical roles in pathological conditions including tumor growth.(23) For instance, angiogenesis and tumor growth are substantially suppressed in MMP-2 deficient mice.(23) MMP-2-null mice (also MMP-9-null mice) have extremely low incidences of cardiac rupture after acute myocardial infarction.(29,30)
Mechanism of MMP-2 activation by MT1-MMP
Accumulated lines of evidence have demonstrated that MMP-2 is always activated in tumor tissues, in which MT1-MMP is expressed.(8,31,32) Furthermore, MT1-MMP expression and MMP-2 activation levels are well co-related and closely associated with invasiveness and malignancy of tumors, suggesting that MT1-MMP is as critical a factor for tumor invasion and metastasis as MMP-2 activator.(31,32) However, due to the complex MMP-2 activation process, the enzyme activity of MMP-2 and its possible contribution to tumor malignancy remain unclear.
The MT1-MMP mechanism of pro-MMP-2 activation has been well studied and partially defined.(33–35) The suggested model implicates TIMP-2 as a bridging molecule, tethering pro-MMP-2 through binding between the COOH-terminal ends of pro-MMP-2 and TIMP-2 and binding between the NH2-terminal ends of MT1-MMP and TIMP-2 (Fig. 2). The propeptide of pro-MMP-2 is cleaved by an adjacent TIMP-2–free MT1-MMP between Asn37 and Leu38, generating an activated intermediate form that is further processed to the fully activated form by an intermolecular auto-cleavage when an intermediate form is present at a sufficiently high concentration at the cell surface. Pro-MMP-2 activation was expected to occur only at low TIMP-2 concentrations relative to MT1-MMP, which would permit the availability of active MT1-MMP to process the proMMP-2 bound in the ternary complex.(19,34–39) Actually MT1-MMP auto-degradation is suppressed in the presence of TIMP-2, and MT1-MMP/TIMP-2 complex accumulates on cell surface.(40) The accumulated MT1-MMP/TIMP-2 complex serves as a receptor for pro-MMP-2, and the receptor-bound pro-MMP-2 is cleaved for activation by TIMP-2-free active MT1-MMP. In order to induce efficient pro-MMP-2 processing, the molar ratio of MT1-MMP/TIMP-2 complex to TIMP-2-free MT1-MMP is quite high (Fig. 3). Thus, MT1-MMP cannot cleave other direct substrates at the TIMP-2 level that induces efficient pro-MMP-2 processing.(40,41) Furthermore, MMP-2 activated by MT1-MMP at TIMP-2 concentration to induce most efficient pro-MMP-2 processing is inactive. The TIMP-2 concentration to produce TIMP-2-free active MMP-2 is restricted to a narrow range at a low TIMP-2 concentration.(41,42)
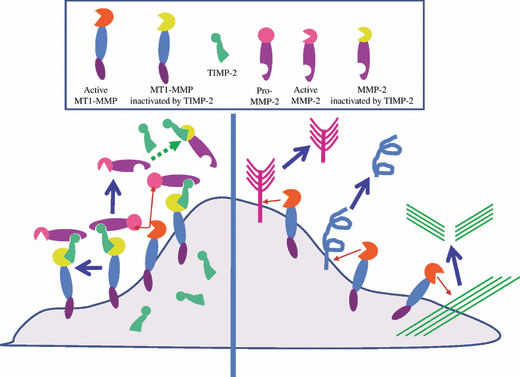
Tissue inhibitor of MMP (TIMP)-2 concentra-tion dictates substrate choice of membrane-type matrix metalloproteinase-1 (MT1-MMP). Left, MT1-MMP forms a complex with TIMP-2, and the complex serves as a receptor for pro-MMP-2. Pro-MMP-2, which binds to MT1-MMP/TIMP-2 complex is then processed by the neighboring TIMP-2-free MT1-MMP. The processed MMP-2 is inactivated by TIMP-2 depending on its concentration. Right, under TIMP-2-free condition, MT1-MMP directly cleaves its own substrates, such as extracellular matrix components, cell-surface molecules and so on.
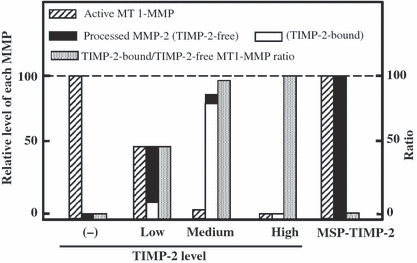
Schematic illustration of tissue inhibitor of MMP (TIMP)-2-regulated membrane-type matrix metalloproteinase-1 (MT1-MMP) and MMP-2 activities. The level of active MT1-MMP and the ratio between MT1-MMP of TIMP-2 complex and TIMP-2-free (active) in the cells expressing MT1-MMP and different levels of TIMP-2 are schematically illustrated. The level of MMP-2 processed by these cells is also shown, which includes TIMP-2-free and TIMP-2-bound forms. In the absence of TIMP-2, MT1-MMP activity is the highest, and decreases proportionally to the TIMP-2 level. MMP-2 activated under a low TIMP-2 level is free from TIMP-2, and shows the highest activity. MMP-2 is processed most effectively at medium level of TIMP-2, in which most of MT1-MMP serves as a receptor for pro-MMP-2 by binding with MT1-MMP and processed MMP-2 is inactivated by TIMP-2. MMP-2 processing is blocked when MT1-MMP is all bound by a high level of TIMP-2. MSP-TIMP-2 does not affect MT1-MMP activity, and generates a maximum level of processed MMP-2 which is free from TIMP-2.
The above observation and interpretation were confirmed by using an artificial receptor for pro-MMP-2 (MSP-TIMP-2), which exposes the TIMP-2 COOH-terminal domain on the cell surface (Fig. 4).(41) Since MSP-TIMP-2 does not have an MMP-inhibition function, the MT1-MMP level required to generate active MMP-2 with MSP-TIMP-2 is much lower than that with TIMP-2. This indicates that in the process of TIMP-2-mediated pro-MMP-2 activation by MT1-MMP, the majority of MT1-MMP functions as a receptor for pro-MMP-2 by making a complex with TIMP-2. Only a minor part of MT1-MMP is free from TIMP-2 and cleaves pro-MMP-2 in a tri-molecular complex. Thus, MT1-MMP is unable to cleave direct substrates effectively at TIMP-2 concentration to induce the most efficient pro-MMP-2 processing. COS-1 cells synthesize relatively higher concentration of TIMP-2 than 293T cells,(14) and MT1-MMP exogenously expressed in COS-1 cells processes pro-MMP-2 more efficiently than those in 293T cells, but cleaves direct substrates less effectively. Furthermore, MMP-2 activated by MT1-MMP in COS-1 cells shows a poor enzyme activity (H.Sato, Y. Okada and M. Seiki, unpublished data, 1994).
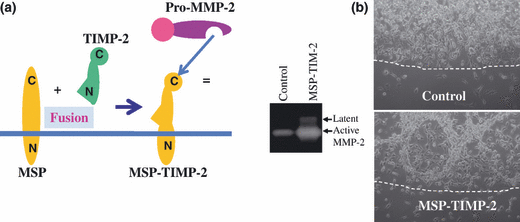
Artificial receptor for pro-matrix metalloproteinaise-2 (MMP-2) (MSP-TIMP-2). (a) MSP-TIMP-2 consists of the transmembrane domain of type II transmembrane protein mosaic serine ptotease (MSP) and tissue inhibitor of MMP (TIMP)-2. Pro-MMP-2 binds to the C-terminus of MSP-TIMP-2. (b) Left, MSP-TIMP-2-expressing HT1080 cells generate more active MMP-2 than mock cells. Right, MSP-TIMP-2-expressing cells were cultured in collagen gel, which caused intensive degradation of collagen gel. Dotted line, border of collagen gel. Mag-nification, ×100.
MT1-MMP turns over very rapidly due to autolysis if not inactivated/stabilized by TIMP-2. The time course of MT1-MMP production and its binding to TIMP-2 on the cell surface is a critical factor; however, this still remains unsolved. The cell-surface concentration of MT1-MMP stabilized by TIMP-2 is regulated by clathrin-dependent internalization (Fig. 1). Furthermore, cell-surface MT1-MMP is regulated temporally and spatially on the cell surface through the interaction with various binding molecules including tetraspanins, which will be discussed below. It should be noticed that such a dynamic cell-surface event may not be interpreted simply in a static stoichiometry, and further analysis is required.
TIMP-2-independent MMP-2 activation
Miyamori et al. reported that tight junction protein claudin mediates the TIMP-2-independent MMP-2 activation pathway by not only MT1-MMP but by other MT-MMPs.(14) Claudin recruits MT-MMP and MMP-2 at a tight junction to achieve elevated focal concentrations, and consequently enhances activation of pro-MMP-2. Among MT-MMP family members, only MT1-MMP can accumulate on the cell surface through the complex formation with TIMP-2, and serves as a receptor for pro-MMP-2 (T. Takino and H. Sato, unpublished data, 2001). An artificial receptor for pro-MMP-2 (MSP-TIMP-2) substitutes for MT-MMP/TIMP-2 complex, and induces activation by all MT-MMPs except for MT4-MMP.(41) MT2-MMP was shown to induce pro-MMP-2 activation in cells derived from TIMP-2-deficient mice.(43,44) Pro-MMP-2 associates with TIMP-2-deficient cells through the hemopexin domain, and is processed by MT2-MMP.(43) Pro-MMP-2 receptor in TIMP-2-deficient cells has not been identified, which cooperates with only MT2-MMP but not MT1-MMP for activation. In any case, all MT-MMPs can functionally cleave pro-MMP-2 for activation, but only MT1-MMP/TIMP-2 complex serves as an efficient receptor for pro-MMP-2 activation (T. Takino and H. Sato, unpublished data, 2001).
Coordinate action of MMP-2 and MT1-MMP
The HT1080 fiborosarcoma cell line expresses MT1-MMP, and produces active MMP-2. MT1-MMP plays a pivotal role in invasive growth in collagen gel, but the role of MMP-2 still remains unclear. Expression of an artificial receptor for pro-MMP-2 MSP-TIMP-2 in HT1080 cells enhances activation of MMP-2 on the cell surface, and results in drastic degradation of collagen gel, which never takes place by overexpression of MT1-MMP or MMP-2 alone (Fig. 4b).(41) A possible explanation for intensive digestion of collagen gel is that MT1-MMP as a collagenase cleaves type I collagen to initiate denaturation into gelatin, which is subsequently digested to pieces by MMP-2 gelatinase. Unlike TIMP-2, MSP-TIMP-2 induces gelatinase (MMP-2) activity without loss of collagenase (MT1-MMP) activity, and its coordinate action may result in the excessive melting of collagen gel. TIMP-2 regulates the balance between MT1-MMP and MMP-2 activity depending on its concentration. HT1080 cells produce a low level TIMP-2, and express both active MT1-MMP and MMP-2 as shown in Figure 4, which may coordinately contribute to the invasive character of HT1080 cells. In fact, down-regulation of either MT1-MMP or MMP-2 expression suppresses invasive growth of HT1080 cells in collagen gel (T. Takino and H. Sato, unpublished data, 2004).
MMP-2 activated through MT1-MMP/TIMP-2 complex as a receptor remains on the receptor. MT1-MMP stimulates integrin-mediated extracellular signal-regulated kinase (ERK) activation by enhancing the turnover rate of focal adhesions on fibronectin or collagen.(45,46) MMP-2 activated by MT1-MMP co-operates with MT1-MMP for ERK activation.(41) Recently, Kenny et al. showed, using small-interfering RNA and neutralizing antibody targeting MMP-2, that MMP-2 plays an essential role in tumor growth and metastasis in nude mice model in ovarian cancer cells.(47) MMP-2 enhances peritoneal adhesion of ovarian cancer cells through cleavage of fibronectin and vitronectin into small fragments, and increases the binding of cells to these fragments and their receptors, α5β1 and αVβ3 integrin.
Tetraspanins as regulators of MT1-MMP.
Tetraspanins are attracting attention as binding proteins of MT1-MMP, which regulate subcellular localization and compartmentalization of MT1-MMP and consequent MT1-MMP activities.(48,49) Tetraspanins of integral membrane proteins with four predicted transmembrane domains comprise a large group of ubiquitously expressed proteins (33 human members). Direct association of MT1-MMP with a tetraspanin family member CD63 was first reported by Takino et al.(50) CD63 was shown to mediate internalization of MT1-MMP from the cell surface to lysosomes, and MT1-MMP undertakes lysosomal porteolysis. Among tetraspanin family members, CD151 in endothelial cells and CD9, CD81, and TSAN12 in cancer cells were reported to be associated with regulation of MT1-MMP functions.(48,49) They support MT1-MMP cell-surface expression and functions including ECM degradation and MMP-2 activation. The CD151-mediated functional association of MT1-MMP with integrin α3β1 regulates MT1-MMP membrane compartmentalization, subcellular localization, and cell-surface concentration as a result in endothelial cells.(48) Since MT1-MMP plays an essential role in angiogenesis, CD151 may contribute to endothelial homeostasis through the regulation of MT1-MMP. CD9, CD81, and TSPAN12 also associate with cell surface molecules including integrins,(51) which may form specialized membrane structures for invasion (e.g. lamelipodia, filopodia, and invadopodia) with MT1-MMP.(52) Tetraspanin-regulated cell surface localization of MT1-MMP increases the local concentration for focal proteolysis within the pericellular environment and leads to efficient ECM degradation and MMP-2 activation. Further study will be necessary to elucidate physiological and pathological roles of tetraspanin-MT1-MMP interaction and functional variations among tetraspanin family members in terms of MT1-MMP regulation.
Conclusion
TIMP-2 dictates substrate choice of MT1-MMP, either direct cleavage of substrate or pro-MMP-2 activation. In the absence of TIMP-2, MT1-MMP mediates only direct cleavage of substrates, but not pro-MMP-2 activation. At an appropriately low concentration of TIMP-2, both MT1-MMP and MMP-2 activated by MT1-MMP can exert enzyme activities. Coordinate action of them induces more efficient tissue degradation and consequent invasive growth compared with that by MT1-MMP or MMP-2 alone. MMP-2 was shown to play a pivotal role in invasive growth of ovarian cancer,(47) in which MT1-MMP should be involved in not only MMP-2 activation but also ECM degradation, and cooperate with MMP-2. Broad-spectrum synthetic inhibitors targeting MMP active sites suffered from harmful side effects in clinical testing. Targeting MMP-2 or MMP-2 activation step may not be excessively toxic, and is promising for the suppression of tumor malignancy.




