Evolutionary history of the devilrays (Chondrichthyes: Myliobatiformes) from fossil and morphological inference
Abstract
The exact affinities of the fossil teeth attributed to the devilrays (mobulids) are critical for resolving the debated origin of these giant pelagic rays amongst Myliobatiformes and the timing of their evolution toward planktivory. We performed the first detailed comparative description of teeth belonging to most of the living and fossil mobulids. Based on a survey of living devilrays, three dental morphologies are newly identified as cobblestone tooth plates, comb-like teeth, and peg-like teeth. In addition, all extinct mobulid species are reviewed with comments on their dentition, fossil record, and geographical distribution. As a result, three fossil mobulid taxa are newly described from the Late Eocene of south-west Morocco (Argoubia barbei gen. et sp. nov., Oromobula dakhlaensis gen. et sp. nov., and Eoplinthicus underwoodi sp. nov.). This has permitted the first assessment of the phylogenetic positions of extinct and extant species of mobulids, using cladistic analyses and a combined data set of nondental anatomical characters from the literature and the dental characters defined here. Our new results support the monophyly of mobulids including all living and most extinct species and indicate that mobulids are closely related to rhinopterids. They also indicate that there was a recent split within Mobulidae into the three tooth morphology groups that we describe in this paper. This work provides clues to the evolutionary history of this clade since the Early Eocene, including the gradual lack in tooth interlocking toward the filter-feeding strategy, whereas the preservation of cusped teeth without feeding function in modern filter-feeder mobulids is interpreted as a tool for precopulatory purposes.
© 2012 The Linnean Society of London, Zoological Journal of the Linnean Society, 2012, 166, 132–159.
INTRODUCTION
Mobulids represent the largest extant rays inhabiting subtropical and tropical waters worldwide. Like other members of the Myliobatiformes, the 11 living mobulid species (belonging to the genera Mobula and Manta) have broad, well-developed pectoral fins and in certain species, a caudal spine and a whip-like tail. Unlike other Myliobatiformes, mobulids are filter feeders (preying on schooling fishes and planktonic crustaceans); these rays direct small food into the mouth through two large cephalic lobes, resembling horns (the origin of the name ‘devilrays’) that, when unfolded, create mobile peribuccal fins. The relatively weak jaws and small teeth are not used for processing prey. Instead, these rays feed by filtering out small-sized prey from the water column that pass through their large mouth and are caught on the branchial filter plates, consisting of soft tissues and located within their internal gill openings (see Notarbartolo di Sciara, 1987 for details). Owing to their atypical feeding behaviour, devilrays do not need the crushing or grinding dentition of the other representatives of the Myliobatiformes (i.e. dasyatids, myliobatids, rhinopterids). This is reflected by the numerous small and often very poorly developed teeth of Recent species. In Manta and some species of Mobula, loss of mastication is extreme and teeth are peg-like and/or only present in the lower jaw (as in Manta). It remains difficult to understand the evolutionary pathway that produced planktophagous rays from supposedly malacophagous ancestors, because the phylogenetic relationships of the living genera (Mobula and Manta) amongst the Myliobatiformes are not fully resolved. Mobulids are sometimes considered a subfamily (Mobulinae) of Myliobatidae Bonaparte, 1838, or as a singular family (Mobulidae) of Myliobatiformes (the taxonomic implication is beyond the scope of this work and the general term mobulid is preferred here rather than Mobulidae or Mobulinae). Two alternative hypotheses are currently presented in several phylogenetic works (Fig. 1). The first hypothesis, most commonly adopted by ichthyologists, considers close relationships between mobulids and rhinopterids (genus Rhinoptera) inside a polyphyletic clade of Myliobatidae (see Fig. 1A). These phylogenetic relationships are supported by DNA sequences (Dunn, McEachran & Honeycutt, 2003, Aschliman et al., 2012) and numerous morphological analyses (Nishida, 1990; Lovejoy, 1996; McEachran, Dunn & Miyake, 1996; Shirai, 1996; Carvalho, Maisey & Grande, 2004; McEachran & Aschliman, 2004; Claeson et al., 2010). The second hypothesis is less familiar and is supported by morphology only (Gonzalez-Isais & Montes Dominguez, 2004). The latter authors, using the first comprehensive sampling of mobulids, considered the mobulids as the sister clade of a myliobatid–rhinopterid clade (Fig. 1B; see Gonzalez-Isais & Montes Dominguez, 2004:11 for details on synapomorphies).

Body shapes of pelagic Myliobatiformes are expanded to show familial differences in head shape. The two main phylogenetic hypotheses (see text for references) concerning the myliobatoids are reported on both sides: according the first one (A), more derived myliobatoids possess more enlarged cephalic lobes (shaded grey), according the second one (B), more derived myliobatoids possess a complete fusion of cephalic lobes. Line drawing inspired by Sasko et al. (2006).
This inconsistency in phylogenetic assumptions is notable and leads us to consider the two alternative scenarios for the evolution of cephalic lobes in myliobatids, rhinopterids, and mobulids (Fig. 1). These alternatives also concern the dental types (grinding-type in myliobatids/rhinopterids and reduced clutching-type in mobulids) corresponding to two extreme trophic adaptations. Consequently, it remains uncertain whether the grinding tooth plate of shell-predators (with cephalic lobes distally fused together) pre-dated a reversal to the smaller teeth that are typical of filter-feeders (with enlarged cephalic lobes) or not.
Unlike the undefined taxonomic positions of taxa amongst the myliobatid–mobulid clade (corresponding to the Myliobatoidea of Nishida, 1990, or aquilopelagic rays of Compagno, 1990), the monophyly of living mobulids (species belonging to Mobula and Manta) is strongly supported by morphology (Gonzalez-Isais & Montes Dominguez, 2004), suggested by DNA sequences (Dunn, McEachran & Honeycutt, 2003), and also attested to by parasitism evolution (Benz & Deets, 1988; Olson et al., 2010). The peculiar feeding behaviour and associated morphological features (e.g. filter plates, cephalic lobes, dysfunctional spiracle) of all living mobulids strongly support monophyly. Conversely, the precise relationships amongst living mobulid species are still subject to debate. Some authors consider that the genus Mobula could be in fact paraphyletic (Herman et al., 2000) on the basis of closer dental morphologies between Mobula japanica/ Mobula mobular and Manta birostris (Herman et al., 2000: Group 3) than with other Mobula species (Herman et al., 2000: Group 4).
To test both interspecific and intraspecific relationships of mobulids compared to the myliobatids and rhinopterids (e.g. Myliobatis, Rhinoptera), we propose a global cladistic revision based on tooth morphology. This is inclusive of most living species and of all the extinct taxa referred to mobulids (reviewed here in the Systematic palaeontology section). Secondly, we compare the strength of these two phylogenetic hypotheses when fossil data are added to our matrix including new dental characters as performed here on living species. Finally, we discuss the implications of the more strongly supported hypothesis in the evolutionary history of the plankton-feeding strategy within the devilrays.
TOOTH MORPHOLOGY OF LIVING MOBULIDS
Tooth bands and isolated teeth of some living species have been previously described (Notarbartolo di Sciara, 1985, 1987; Herman et al., 2000). However, these studies were mostly based on small samples and tooth morphology was only partially documented. The main difficulties in identifying teeth of both living and fossil species derive from the family's strong heterodonty, which is a general rule in Elasmobranchii. Intraspecific tooth differences fall into three main categories (Cappetta, 1987). One of them is related to sexual dimorphism (gynandric heterodonty of Compagno, 1970), particularly notable in many batoids, mobulids included. Males generally have teeth with cuspidate crowns, whereas females have teeth with rounded crowns. However, this may be more related to seasonal dynamics of mating than to sexual differences only, as observed by Kajiura & Tricas (1996) in some Myliobatiformes. We illustrate a sample of isolated teeth of all species available in our collection with one male and one female specimen for each (Appendix 1; 2-4). The complete list of the specimens examined together with information on capture, sex, and size is given in Notarbartolo di Sciara (1985, 1987) and partially reported here (e.g. sex and disc width in mm). The material consists of 50 fresh specimens recovered by two of the authors (G. Notarbartolo di Sciara and H. Cappetta). We followed the taxonomic framework proposed in Notarbartolo di Sciara (1987), who synonymized numerous invalid species. Eight out of the 11 valid species are illustrated here: Mobula mobular, Mobula japanica, Mobula munkiana, Mobula rochebrunei, Mobula hypostoma, Mobula thurstoni, Mobula tarapacana, and Manta birostris. Two additional species, Mobula eregoodootenke and Mobula kuhlii, were summarily illustrated by Notarbartolo di Sciara (1987: figs 12, 16C) and are not reported here in the absence of sufficient material, currently limited to juvenile specimens (see Appendix 1). The species Manta alfredi (Krefft, 1868), which has been recently resurrected from Indo-West Pacific specimens and previously attributed to Ma. birostris (Marshall, Compagno & Bennett, 2009; Kashiwagi et al., 2012), could not be considered in this paper because of the lack of appropriate material. A first overview of jaws and teeth allowed us to aggregate a priori the living species into three ‘morphotype dental groups’, according to their similarity in tooth morphology and independently of taxonomy. Contrary to most other elasmobranchs, there is no real difference in tooth morphology according to the tooth position on the lower and upper jaws, except for a slight decrease of the tooth height/length ratio toward the commisure of the jaws.

Peg-like tooth morphology of sexed specimens of A-N, Mo. mobular (UM-REC 26M: ♂ 2400 mm DW; UM-REC 27M: ♀ 1400 mm DW); O-W, Mo. japanica (NS 83-024: ♂ 2077 mm DW; NS 83-070: ♀ 2108 mm DW); X-G′, Ma. birostris (NS 83-003: ♂ and NS 83-160: ♀ size unknown). For each species and sex, teeth are sorted from anterior (right) to lateral (left) positions. A-J, O-W, upper teeth; K-N, X-G′, lower teeth. Abbreviations of the view are indicated on the right hand side of each tooth: o, occlusal view; li, lingual view; la' labial view; p, profile; b, basal view. Detail of specimens in Appendix 1.

Cobblestone and comb-like tooth morphologies of sexed specimens of A-S, Mo. tarapacana (UM-REC 30M: ♂ 2500 mm DW, NS 83-141: ♀ 3015 mm DW) and T-M′, Mo. thurstoni (NS 83-077: ♂ 1770 mm DW, NS 83-020: ♀ 1626 mm DW). For each species and sex, teeth are sorted from anterior (right) to lateral (left) positions. A-E, J-N, T-X, D′-H′, upper teeth; F-I, O-S, Y-C′, I′-M′, lower teeth. Abbreviations of the view are indicated on the right hand side of each tooth, o, occlusal view; li, lingual view; la, labial view; p, profile; b, basal view. Detail of specimens in Appendix 1.

Comb-like tooth morphology of sexed specimen of A-T, Mo. munkiana (NS 82-015: ♂ 895 mm DW, NS 84-004:♀ 931 mm DW); U-L′, Mo. rochebrunei (UM-REC 25M: ♂ 1170 mm DW, UM-REC 14M : ♀ 1310 mm DW); M′-Z′, Mo. hypostoma (UM-REC 28M: ♂ size unknown, UM-REC 29M: ♀ size unknown). For each species and sex, teeth are sorted from anterior (right) to lateral (left) positions. A-E, K-O, U-X, A′-F′, M′-P′, U′-W′, upper teeth; F-J, P-T, Y-Z, G′-L′, Q′-T′, X′-Z′, lower teeth. Abbreviations of the view are indicated on the right hand side of each tooth, o, occlusal view; li, lingual view; la, labial view; p, profile; b, basal view. Detail of specimens in Appendix 1.
Mobulids with peg-like teeth (Fig 2)
This group encompasses three species, Mo. japanica, Mo. mobular, and Ma. birostris, characterized by teeth with a high tubular crown carried by a quite globular root. Mobula mobular (Fig. 2A–N) and Mo. japanica (Fig. 2O–W) have teeth with very similar morphologies. Sexual dimorphism is minimal in these species, with male teeth slightly higher than female teeth. Dignathic heterodonty (morphological differences between lower and upper teeth) was not conspicuous in the material examined here. In both jaws, teeth are higher than wide. The crowns are medially compressed with a pipe-like aspect in median files and labiolingually flattened in lateral files. The labial edge of the occlusal face is slightly marked (in Mo. mobular) or salient, divided into an inclined oral part and a vertical labial part separated by a rounded crest (in Mo. japanica). Teeth are often unicuspidate (except on some median files of male specimens of Mo. japanica) but the cusp is never well developed lingually. The labial and lingual faces are laterally continuous. The crown surface is completely smooth except in the upper part of the labial face. The holaulacorhize root is thick and broader than the crown, with a deep median groove pierced by one or a pair of basal nutritive foramina. Dental differences between Mo. japanica and Mo. mobular are tenuous but it seems that Mo. japanica possesses teeth with a distinct occlusal surface (demarcated from the lower part of the labial face by a transversal crest), a strongly ornamented enamel (in males and females), and a higher root than in Mo. mobular. These two species are very similar in external appearance, and until now could be differentiated only on the basis of maximum size (Mo. mobular becoming larger), and morphometrics (Mo. mobular reaching greater disc width relative to the rest of the body). The validity of separate species status for Mo. mobular and Mo. japanica has been questioned, with the overlap in their morphological features potentially indicative of single species status (Notarbartolo di Sciara, Serena & Mancusi, 2006). Based on tooth morphology we confirm that Mo. japanica and Mo. mobular are indeed different species.
Manta birostris (Fig. 2X–G') and Ma. alfredi (see Marshall et al., 2009) are characterized, in part, by the lack of upper teeth compared to Mobula. No evidence of sexual dimorphism was detected on the observed material and/or figured teeth (see Herman et al., 2000) and monognathic heterodonty is quite limited in Ma. birostris. The crown has a pipe-like aspect, except on lateral files where the crown may be wider than high. Teeth are not cuspidate and an occlusal surface, labially inclined or slightly concave, is clearly separated from the vertical labial face by a transversal crest. The enamel surface is strongly ornamented with numerous irregular granules and peaks that are sometimes labiolingually aligned. The lingual crest is irregular. Labial and lingual faces are laterally continuous with totally smooth enamel. The anaulacorhize root is thick, wider, and higher than the crown. A few small nutritive foramina open on the basal face.
Mobulids with cobblestone tooth plates (Fig. 3A–S)
Only one species of mobulids observed here, Mo. tarapacana, presents jaws with an imperfect interlocking of tooth files, as particularly observed in myliobatids. Teeth are numerous (more than 80 tooth files) and are clearly broader than long. The crown is somewhat hexagonal in oral view, enabling a contact, even partially, with the surrounding teeth (file and row).
The teeth of Mo. tarapacana (Fig. 3A–S) are quite peculiar. Sexual dimorphism is well marked but seems to be reduced to a stronger ornamentation of the enamel on the labial face in males compared to females. Dignathic heterodonty is not obvious from the material examined. The crown is two or three times higher in size than the root. The apical part of the crown is often wider than the collar in labial or lingual view. Particularly flat, the labial face is however drilled by numerous labiolingually directed furrows, particularly on male teeth. These furrows strongly indent the sharp transversal crest and sometimes the labial extremity of the labial visor. A slight reticular ornamentation occupies the whole labial face above its visor. The lower part of the labial face (beneath the labial visor) is high, almost vertical, and its enamel is free of ornamentation. The labial visor is rounded and strongly overhangs the root in lateral view. The reduced root is at the polyaulacorhize stage, the grooves are large and irregularly distributed along the root width.
Mobulids with comb-like teeth (3, 4)
This morphology is represented by all Mobula species with small, relatively low, and very slightly or not interlocking teeth. The teeth are relatively sharp with an indented or cuspidate crown probably used to hold small prey. Two subgroups may be provisionally separated between species with ornamented enamel and short cusps (Mo. thurstoni, Mo. eregoodootenke, and Mo. kuhlii) and those with smoother enameloid and long cusps (Mo. munkiana, Mo. rochebrunei, and Mo. hypostoma). Tooth morphological differences between these two subgroups are tenuous and some species (e.g. Mo. munkiana) can be easily considered in either of the two.
In the first sub-group (Fig. 3T–M'), only Mo. thurstoni was analysed in detail. The tooth morphology of Mo. eregoodootenke seems to be quite similar to Mo. thurstoni, according to the illustrations of the neotype provided by Notarbartolo di Sciara (1987: fig. 12). In Mo. thurstoni (Fig. 3T–M'), the sexual dimorphism is reduced and limited to a labial face that is more deeply scored by furrows in males than in females. Ontogenetic variation is relatively pronounced with young males resembling females. The crown is low and wider than high. The labial face is slightly concave and labially inclined; its upper part is drilled by numerous labiolingually orientated furrows in males, affecting the transversal crest with irregular and developed cusps. The enamel of the labial face is strongly ornamented above its visor with numerous enamel ‘blisters’ that tend to fuse on the rounded visor. The labial visor strongly overhangs the root in lateral view. The root is commonly holaulacorhize or occasionally polyaulacorhize with a low number of irregular grooves (principally two). Mobula kuhlii was not observed in this study. Notarbartolo di Sciara (1987: fig. 16C), however, figured juvenile teeth of this species that resemble those of Mo. thurstoni, considered by the author as sister species of the former. Indeed, we provisionally attributed Mo. kuhlii to this group pending further investigation.
The second subgroup (Fig. 4) includes the living species Mo. munkiana (Fig. 4A–T), Mo. rochebrunei (Fig. 4U–L'), and Mo. hypostoma (Fig. 4M'–Z'). Teeth are quite similar in overall shape to those belonging to the previous subgroup; however, sexual dimorphism is strongly marked in this subgroup, with males having some well-developed cuspidate teeth. The enameloid of the crown is relatively smooth in all species compared to the previous subgroup, except the visor of female teeth, in which short vertical grooves may be deeply marked (e.g. in Mo. rochebrunei). Amongst these species, Mo. munkiana possesses the less derived and cuspidate teeth. These teeth are reminiscent of those of Mo. thurstoni, except for the lack of enameloid ornamentation on the labial face of crown. Teeth of Mo. hypostoma and Mo. rochebrunei are more specialized with very elongate cusps in males, sometimes with a fully individualized pair of cusps on the lateral extremities of the crown (e.g. in Mo. hypostoma). Although single-cuspidate teeth are usually bilobate, they have a polyaulacorhize root, particularly marked in mesiodistally elongate teeth.
SYSTEMATIC PALAEONTOLOGY
Review of fossil mobulids and resembling taxa
Fossil remains of mobulids are limited to isolated teeth. They are relatively scarce in marine deposits and are extremely fragile as a consequence of the reduced enameloid layer on the tooth crown. Both extant genera (Manta and Mobula) have fossil representatives. Between five and nine fossil genera have usually been attributed to mobulids depending on the points of view of palaeoichthyologists, and consist of the following genera: †ParamobulaPfeil, 1981, †EomobulaHerman, Hovestadt-Euler & Hovestadt, 1989, †EomantaPfeil, 1981, †EoplinthicusCappetta & Stringer, 2002, †PlinthicusCope, 1869, †BurhmaniaCappetta, 1976, and possibly †ArchaeomantaHerman, 1979, †CretomantaCase, Tokaryk & Baird, 1990, and †BrachyrhizodusRomer, 1942.
Concerning the extinct species of living genera, the oldest occurrences of true Mobula are known from the Oligocene (Müller, 1999; Picot et al., 2008; Cicimurri & Knight, 2009) and eight extinct species are currently counted. They are summarized here:
GenusMobula Rafinesque, 1810
†Mobula loupianensisCappetta, 1970
Occurrences: Langhian of southern France (Cappetta, 1970) and North Carolina (Purdy et al., 2001). Serravallian of Portugal (Jonet, 1976).
Remarks: Particularly well illustrated by Jonet (1976: figs 1–13) with male (monocupsidate teeth) and female (not well cuspidate) specimens, the morphology of †Mo. loupianensis is clearly close to that observed in the living species Mo. hypostoma. The tooth crown of males has a massive central cusp with sometimes a pair of small denticles located at each extremity in occlusal view. The tooth crown of females is devoid of a cusp but the visor can occasionally be very slightly irregular in occlusal view. Dental differences with the living species are tenuous but the teeth of †Mo. loupianensis (particularly the females) present a smoother enameloid than in Mo. hypostoma, in which the labial face is slightly marked by some folds. Cicimurri & Knight (2009: fig. 9A–F) reported a large variety of morphotypes of mobulids from the upper Chattian of South Carolina. The authors considered that they all belong to a unique species attributed to †Mo. cf. loupianensis and suggested that all the Neogene species such as †Mo. pectinata, †Mobula irenae, and †Mo. loupianensis are possibly conspecific. However, their material differs from the distinctive Miocene Mo. loupianensis in the presence of an irregular surface, sometimes dotted by deep folds on the labial face of the crown and by the root being higher than wide. If the Chattian species belongs to the mobulid group with comb-like teeth (close to the Mo. hypostoma/Mo. rochebrunei subgroup), they probably belong to a species other than †Mo. loupianensis.
†Mobula pectinata (Cappetta, 1970)
Occurrences: Langian of southern France (Cappetta, 1970) and Serravalian of Portugal (Jonet, 1976). ? Late Oligocene–Early Miocene of the Old Church Formation in Virginia (USA).
Remarks: Cappetta (1970) first suggested a close relationship with the living Mo. tarapacana (formerly Mobula coilloti in the text) but it appears that the resemblance to the extant Mo. thurstoni is stronger. Compared to the material attributed to †Mo. loupianensis, the labial surface is slightly folded. The lingual visor is generally multicuspidate with two cusp-like extensions located at each crown extremity, as observed in teeth of male specimens of †Mo. loupianensis. Müller (1999) figured three teeth (as Mobula sp.) from the Late Oligocene–Early Miocene of the Old Church Formation in Virginia (USA), that could be cautiously referred to †Mo. pectinata.
†Mobula fragilisCappetta, 1970
Synonymy: Manta fragilis Cappetta, 1970; †Paramobula fragilis (Pfeil, 1981), †Mobula cappettaiJonet, 1976.
Occurrences: Late Oligocene of South Carolina, USA (Cicimurri & Knight, 2009), Middle Miocene of USA (Müller, 1999; Purdy et al., 2001), southern France (Cappetta, 1970), and Serravallian of Portugal (Jonet, 1976).
Remarks: Pfeil (1981: 366) erected a new genus †Paramobula from the holotype of †Mo. fragilis, considering that it represents a peculiar mobulid characterized by mesiodistally elongate teeth with a hollow labial face of crown marked by numerous folds and grooves, precluding a strong interlock. Cappetta & Stringer (2002) and Cappetta (2006) subsequently considered †Paramobula as congeneric with Mobula contrary to Cicimurri & Knight (2009) who accepted the generic distinction, although the latter authors did not exclude that †Paramobula fragilis could be included in the unclear heterodonty of †Plinthicus. Cicimurri & Knight (2009) included in this genus part of the material published by Case (1980: pl. 10, fig. 2a–e) from the Middle–Late Oligocene. †Mobula cappettaiJonet, 1976 was described from isolated teeth from Costa de Caparica (Portugal) and recovered with †Mo. fragilis, †Mo. loupianensis, and †Mo. pectinata (Jonet, 1976). However, after examination of material coming from the type locality we consider it a synonym of †Mo. fragilis based on the shared convex oral face and straight root, considered as diagnostic by Jonet (1976: 60–61).
†Mobula melanyae (Case, 1980)
Synonymy: Manta melanyae Case, 1980
Occurrences: Only known from the River Bend Formation in North Carolina (USA) possibly dated as Middle–Late Oligocene (Ward, 1985 in Müller, 1999).
Remarks: Teeth are broken (no root thoroughly preserved) but their morphologies are unusual, having a labiolingually compressed root, a high crown with an inclined upper labial face that displays a strongly ornamented enamel (except in its apical part) with numerous grooves, at least in adult specimens (Case, 1980: pl. 10, fig. 1), and an irregular transversal crest. Cicimurri & Knight (2009) considered that only the holotype (large specimen) is referable to Mobula whereas the paratype, nearly two times smaller, belongs to the dubious genus †Paramobula. Without additional evidence beyond size, we disagree with such splitting and consider that both the holotype and paratype belong to the same species (adult and juvenile, respectively).
Other Mobulid Fossil Remains
Other short reports of fossils of Mobula or Manta are available in the literature. Laurito Mora (1999) illustrated teeth of Mobula cf. hypostoma recovered from the Late Miocene–Early Pliocene of Alto Guayacan, Costa Rica. Despite the unexpected absence of female teeth in this sample, these fossils are currently the oldest representatives of the living form. In the same fossil deposits, the author reported different mobulid teeth that he attributed to †Mobula lorenzolizanoiLaurito Mora, 1999. These teeth present several features (ornamented labial face, transversal crest sinuous to ragged) that are reminiscent of those of Mo. thurstoni. Their validity as extinct species remains also questionable. Numerous other fossil mobulid species were falsely attributed to the genus Manta before the use of iconography devoted to the tooth morphology of living species. Most of them must be subsequently reattributed to Mobula. It is the case for †Manta hynei (Bourdon, 1999) from the Pliocene of Yorktown (Virginia, USA) because the teeth display dental features that are reminiscent of those of Mo. japanica or Mo. mobular (e.g. holaulacorhize root, occlusal surface of crown without ornamentation). Although the current distributions of Mo. japanica and Mo. mobular do not include the western Atlantic realm as argued by Bourdon (1999), the morphological characters better fit those of these two peculiar Mobula than Manta. Laurito Mora (1999) revealed many fossil teeth of Manta sp. in the Late Miocene–Early Pliocene deposits of Costa Rica. If one excludes one specimen (Laurito Mora, 1999: fig. 2 pl. 38), which seems to possess a root with two separated lobes (holaulacorhize stage as in Mo. japanica or Mo. mobular), there is no doubt that other fossils are really affiliated to Manta. The oldest representative of Manta in the fossil record is possibly dated as Early Oligocene (Picot et al., 2008) even though the unique tooth recovered (cf. Manta in Picot et al., 2008: fig. 8) displays an unusually lower crown and a narrower root compared to the younger forms.
In addition to the extinct Mobula and Manta species discussed above, several fossils have been compared or affiliated to mobulids on the basis of their tooth morphologies, although quite similar to those of some rhinopterids or myliobatids. Their systematic positions are still debated, often speculatively, and are supported by few features. These potential mobulids are reviewed below.
Genus†CretomantaCase, Tokaryk & Baird, 1990
Included species: †Cretomanta canadensisCase et al., 1990.
Occurrences: The genus occurs from the early Late Cretaceous (Cenomanian) of Canada and North America (Cappetta & Case, 1999; Case et al., 1990; Cicimurri, 2001; Shimada et al., 2006; Underwood & Cumbaa, 2010) to the Maastrichtian of Morocco (Noubhani & Cappetta, 1997).
Remarks: The monospecific genus †CretomantaCase et al., 1990 is only known by isolated teeth with a ‘peg-like’ morphology. There is no individualized labial face on the crown; the cusp is medially compressed, the enameloid is smooth except on the rounded labial face that presents faint longitudinal scratches (Underwood & Cumbaa, 2010); the root is anaulacorhize with numerous nutritive foramina as observable in recent Manta. Some teeth show a bifid cuspidate crown, as observed in the dentition of some living mobulids. Case et al. (1990) originally attributed this taxon to a microphageous batoid close to the Recent Manta, considering the global similarity between tooth shapes of both taxa. However, this tooth morphology is regarded as possibly convergent with Manta as observed in other enigmatic Cretaceous batoids such as Duwibatis (Cappetta, 1991), and has also led some authors to consider Cretomanta as a planktivorous lamniform (Noubhani & Cappetta, 1997; Cappetta, 2006) or with uncertain familial affinities amongst batoids (Underwood & Cumbaa, 2010).
Genus†ArchaeomantaHerman, 1979
Included species: †Archaeomanta hermaniKozlov, 2001; †Archaeomanta melenhorstiHerman, 1979; †Archaeomanta priemiHerman, 1979.
Occurrences: Widely recorded in Palaeogene deposits from north Europe to East and West Africa (e.g. Herman, 1979; Cappetta, 1987; Noubhani & Cappetta, 1997; Kozlov, 2001; Strougo, Cappetta & Elnahas, 2007; Van Den Eeckhaut & De Schutter, 2009; Adnet, Cappetta & Tabuce, 2010; Underwood et al., 2011),
Remarks: The genus †ArchaeomantaHerman, 1979, resembles †Cretomanta and is more diversified with at least three different species (see above). Their remains, also restricted to isolated teeth, are scarce in Palaeogene deposits (except in some Moroccan localities) but widely distributed in the tropical zone from North Europe to East and West Africa, as expected with a supposed filter-feeding behaviour. Morphologically very close to those of †Cretomanta, the teeth are distinctive in having a broad and deep axial groove on the root (sometimes with secondary labiolingually orientated grooves) that separates two rather mesiodistally inclined lobes and a labiolingual crest on the occlusal part of the crown. However, this crest is irregular and sometimes disappears in lateral files, thus resembling the morphology observed in some putative contemporaneous fossil Gymnuridae such as in males of the genus Ouledia. Moreover, Underwood et al. (2011) observed a well-developed pulp cavity, which extends to the tip of the cusp in Archaeomanta, making their supposed position within the mobulids doubtful. Another uncertainty concerns the monospecific genus †Eomobula, which was originally described from a small tooth sample from the Lower Eocene of Egem Formation (Belgium) but was secondarily recorded by rare specimens in some Early–Middle Eocene localities (e.g. Kemp, 1994; Gheerbrant et al., 2003; Tabuce et al., 2005). Having very small teeth, this genus was attributed to a mobulid despite its teeth that show a very large convex occlusal face compared to the extremely reduced lingual and labial faces and a wide polyaulacorhize root as in Myliobatid. The authors themselves (Herman et al., 1989) remarked that there is no real affinity in tooth morphology with other mobulids such as †Burhnamia (see below) or Mobula and suggested that this primitive mobulid probably represents an intermediate group between living representatives of the Myliobatidae and mobulids.
Genus†BrachyrhizodusRomer, 1942
Included species: †Brachyrhizodus wichitaensisRomer, 1942.
Occurrences: Erroneously reported from the Carbo-Permian as a petalodontiform (Romer, 1942), this genus occurs only in the Campanian–Maastrichtian of North America (Cappetta & Case, 1975; Welton & Farish, 1993).
Remarks: Claeson et al. (2010) recently suggested that the Cretaceous †B. wichitaensisRomer, 1942, is a stem-mobuline and not a stem-myliobatid as previously reported by Cappetta (1987). According to these authors, a unique character state supports this reattribution: teeth of †Brachyrhizodus lack any curvature in basal or occlusal view, as observed in living devilrays (Mobula and Manta) and contrary to the genus Rhinoptera. However, observations made by us from the available figurations (Cappetta & Case, 1975; Welton & Farish, 1993) and on dozens of specimens of †Brachyrhizodus allow us to refute the hypothesis of Claeson et al. (2010).
Genus†PlinthicusCope, 1869
Included species: †Plinthicus stenodon (Cope, 1869); †Plinthicus kruibekensisBor, 1990.
Occurrences: Genus known from the Rupelian of the Boom Clay Formation, Belgium (Bor, 1990) and Late Chattian of South Carolina, USA (Cicimurri & Knight, 2009), until the middle Miocene of southern France (Cappetta, 1970) and of Maryland and North Carolina, USA (Cope, 1869; Müller, 1999; Purdy et al., 2001).
Remarks: The extinct genus †PlinthicusCope, 1869 was tentatively placed in the mobulids (Cappetta, 1987; Cappetta & Stringer, 2002) although these teeth recall those of some rhinopterids (Purdy et al., 2001; Cicimurri & Knight, 2009). The largest species †P. stenodon (Cope, 1869) appears to have had a rhinopterid-type dentition with multiple, weakly interlocked columns of teeth. Most striking in these teeth is the 45° angle of recline seen in lateral profile, the concave occlusal surface, and the labiolingually compressed root with regular and fine root lamellae. The species †P. kruibekensisBor, 1990 is known from a unique tooth that strongly differs from the younger species by a flat occlusal surface (as in some Mobula), a concave lingual face (as in †Mo. fragilis), and anastomosing enameloid laminae on the labial and lingual faces of crown.
Genus†BurnhamiaCappetta, 1976
Included species: †Burnhamia daviesi (Woodward, 1889); †Burnhamia fetahiCappetta, 1985; †Burnhamia glikmani (Pfeil, 1981).
Occurrences: This genus is known from the Late Palaeocene to the Late Eocene and was largely distributed in the Northern Hemisphere (see Cappetta, 1987, and references therein).
Remarks: This genus was erected on material originally placed in the genus Rhinoptera by Woodward (1889, formally as †Rhinoptera daviesi). Cappetta (1976) attributed this material to a new genus amongst the mobulids on the basis of: an evident reduction of tooth size, an increase in file number (more than eight), and a fine ornamentation rarely planed on the concave occlusal surface, leading to a supposed lack of biomechanical stress as observable in teeth of filter-feeders and contrary to the benthic batoids with grinding-type dentition as the rhinopterids. †Burnhamia fetahi illustrates the extreme reduction in tooth size and one can clearly observe anterior cuspidate teeth, lacking in rhinopterid or myliobatid taxa. Numerous Palaeogene fossils attributed to the genus Rhinoptera belong in fact to the different species of Burnhamia. The extinct species †Mobula glikmaniPfeil, 1981, was only named in the text (Pfeil, 1981) from the material recovered in the Eocene of Kazakhstan (Glikman, 1964). Cappetta (2006) refuted this attribution and reported this material as belonging to the genus †Burnhamia.
Genus†EomantaPfeil, 1981
Included species: †Eomanta kowaldiPfeil, 1981.
Synonymy: †Mobula irenaePfeil, 1981.
Occurrences: Early Oligocene of Galon-Grabens, south Germany (Pfeil, 1981).
Remarks: The extinct genus †EomantaPfeil, 1981 is probably the most overlooked fossil belonging to the mobulids. This taxon occurs in the Early Oligocene of Galon-Grabens, south Germany and was described from a unique tooth attributed to the species †E. kowaldiPfeil, 1981.Pfeil (1981) reported a short diagnosis for the genus †Eomanta. It is characterized by: a high crown with extended labial and lingual faces; a flat occlusal surface and a dejected labial face, many small deep anastomosing furrows on the upper part of the labial face; a relatively straight transversal crest; an unnoticeable labial visor and a well-centred root in the middle of the basal face of the crown, relatively thick without exceeding the labiolingual extremities of basal edge of the crown. With the exception of the lack of labial visor, all these characters are noticeable in many Recent and fossil mobulids as in the living Mo. tarapacana or †Mo. fragilis, respectively. Pfeil (1981) excluded other mobulid-like teeth recovered in the same fossil layer that he attributed to †Mobula irenaePfeil, 1981. Since this time, †Eomanta has been considered alternatively as an intermediate form between Mobula and Manta (Pfeil, 1981), a synonym of Mobula (Cappetta, 1987, 2006; Cappetta & Stringer, 2002), or as a nomen dubium (Cicimurri & Knight, 2009). The two teeth attributed to †Mo. irenae by Pfeil (1981: figs 1–2, pl. 1) show a relatively high crown; a flat occlusal surface (especially in the holotype) with a dejected labial face, some small deep furrows on the apical part of the labial face and a labial visor; a transversal crest relatively straight; an unnoticeable labial visor and a well-centred root in the middle of the basal face of the crown, relatively thick but never exceeding the labiolingual extremities of the basal edge of the crown. Pfeil (1981) suggested that the teeth attributed to †Mo. irenae are distinct from those attributed to †E. kowaldi in having a more salient lingual and labial visor and a lower crown. Based on improved sampling and broader comparisons of mobulid heterodonty and on the overview of the fossil record, we consider that the original material attributed to †Mo. irenae cannot be excluded from †E. kowaldi and should be considered as conspecific (contrary to Cicimurri & Knight, 2009, who considered †Mo. irenae as a possible junior synonym of †Mo. loupianensis). Other isolated teeth are sometimes attributed to the mobulids but should be considered with serious caution. They often represent damaged teeth that resemble mobulid dental material but actually belong to other batoid taxa (e.g. in Van Den Eeckhaut & De Schutter, 2009).
New mobulids from the Late Eocene of south-west Morocco
Three new taxa belonging to mobulids have been recognized in the dental remains recovered south of Ad Dahkla in the so-called ‘Western Sahara’, currently in south-western Morocco. The preliminary report of these faunas (Adnet et al., 2010) suggested a late Middle Eocene–Late Eocene age, although Underwood et al. (2011) referred these faunas to the Late Eocene only, based on common occurrences with the well-dated Fayum area. The attribution of the taxa described here to three different genera is based on morphological comparisons with previously detailed taxa.
Argoubiagen. nov.
Type horizon: Samlat Formation, Gerran member (Ratschiller, 1967) – late Middle Eocene/Late Eocene.
Etymology: From the close souk of El Argoub.
Type species: Argoubia barbei gen. et sp. nov.
Occurrences: Only known from the type locality, Late Eocene.
Diagnosis: Mobulid only known by isolated teeth of small size. The crown is much higher than the root. Occlusal face rather flat and less developed transversally than the base of the crown in occlusal view. Some smooth and little salient folds labiolingually disposed. The lingual margin of this face is irregularly cut out. The labial face of the crown is oblique in profile and is strongly ornamented, with vertical folds bearing many small spines or tubercles irregularly disposed. These folds are separated by deep grooves. The ornamentation of the lingual face is very similar to that of the labial face, but with fewer spines and tubercles. The basal bulge is thick but not very salient. The lower part of the labial visor is oblique and well developed. The root is not high, with three to four lobes separated by broad and deep furrows. Their basal face is flat with marginal lobes of triangular outline.
Argoubia barbeisp. nov. (Fig. 5)

Argoubia barbei gen. nov., sp. nov. A-E, Antero-lateral tooth, holotype, DAK3-1. A, lingual view; B, profile; C, labial view; D, occlusal view; E, basal view. F-J, Antero-lateral tooth, paratype, DAK3-2. F, lingual view; G, profile; H, labial view; I, occlusal view; J, basal view.
Material: Two complete teeth and several fragments.
Type locality: Locality DAK3. Unit 2 level B1, close to El Argoub, south of Ad Dakhla, south-western Morocco (Adnet et al., 2010).
Type horizon: Samlat Formation, Gerran member (Ratschiller, 1967) – late Middle Eocene/Late Eocene.
Etymology: Species dedicated to Mr Gérard Barbe for his help in the field and for donation of material.
Holotype: DAK3-1 (Fig. 5A–E) housed at the University of Montpellier.
Diagnosis: Same as the genus.
Description: The teeth of this species are rather large, reaching between 3 and 4 mm width for anterolateral teeth. The holotype (Fig. 5A–E) is broader than high in labial view and rather thick in profile (3.2 mm wide, 2.4 mm high). The maximum width is located above the middle of the tooth and the base of the crown is practically as broad as the occlusal face. In labial view (Fig. 5C), the edges of the crown are generally convex, and distinctly and irregularly cut off above the limit of the visor. In occlusal view (Fig. 5D), the occlusal face, very slightly depressed, is less developed transversely than the rest of the crown. The enameloid of this face is completely smooth and bright. The labial margin of the face is irregular. Its lingual edge is more salient and cut off by well-developed irregular cusps with rounded extremities and separated by weak to deep notches. The labial face (Fig. 5C) of the crown bears a very strong ornamentation of more or less vertical ridges separated by deep vertical hollows. These ridges are very salient, irregular, and bear villi directed upwards. These villi have oblique directions when the tooth is observed in labial view. The basal part of this labial face is limited by a distinct crest that corresponds to a labial visor. The lower part of this visor is well developed and oblique in profile (Fig. 5B). It is smooth and, in labial view (Fig. 5C), it is higher and slightly depressed marginally on both sides.
The lingual face is oblique in profile view (Fig. 5B), parallel to the labial face. It is restricted at its base by a distinct bulge, rounded in profile. This bulge overhangs the root in occlusal view. The lingual face is covered by vertical ridges that are shorter, less developed, and less irregular than those of the labial face. They stop above the bulge. Marginally, some ridges are oblique.
The root is not very high, about a quarter of the tooth in profile view (Fig. 5B), and distinctly narrower than the crown. In lingual view, the width of the root itself is greater just below the crown than basally. The root is higher medially than on the margins. There are three lobes (Fig. 5E): two marginal, with a flat basal face of triangular outline, and one median that is narrow and also has a flat basal face. The lobes are separated by broad and rather deep and Ω-shaped furrows. Irregularly placed foramina open on the labial, lingual, and basal faces of the root.
The paratype (Fig. 5F–J) is smaller and exhibits some differences. The occlusal face (Fig. 5I) is less distinctly separated from the labial face, with a lingual edge more strongly cut off. In profile view (Fig. 5G), the tooth is less high than the holotype, and, in occlusal (or basal) view (Fig. 5I–J), an angular labial outline can be observed medially. The lingual face of the crown is less ornamented, with only three smooth vertical ridges. The labial face bears strong vertical crests but less irregular than in the holotype. The marginal outlines of the crown are not cut off in labial view. The root shows four lobes and three furrows (Fig. 5J). It is broader just below the crown than at the level of the basal face.
Discussion: Despite the morphological differences observed between the holotype and paratype, one can consider that they fall within the range of intraspecific variation. It is possible that the more salient and more cut off edge of the lingual face observed on the paratype corresponds to a dental sexual dimorphism. In this case, the paratype may have belonged to a male. Several other fragmentary teeth of this species have been collected. However, they were not illustrated because of their poor state of preservation. Some teeth are more transversally developed with a distinctly polyaulacorhize root, indicating that the teeth described above would be anterior or anterolateral teeth. These more lateral teeth are also characterized by a less high crown.
Argoubia barbei gen. et sp. nov. can be easily separated from Oromobula dakhlaensis gen. et sp. nov. by its relatively less high and much thicker teeth. The ornamentation of the labial and lingual faces of the crown is also stronger, and the root is more massive with less high lobes in the former. It is also important to note that these taxa do not co-exist in the same level.
Oromobulagen. nov.
Type horizon: Samlat Formation, Gerran member (Ratschiller, 1967) – late Middle Eocene/Late Eocene.
Etymology: Genus named after the old Spanish spelling of locality ‘Rio del Oro’, ‘Western Sahara’ in south-western Morocco.
Type species: Oromobula dakhlaensis gen. et sp. nov.
Occurrence: Only known on type locality, Late Eocene.
Diagnosis: Mobulid only known by isolated teeth of small (near 3 mm) to moderate size (up to 6 mm). Teeth (crown and root) are quite to strongly flattened labiolingually (according to age and file position) without any possibility of interlocking. Occlusal face rather flat with enameloid layer extremely thin and strongly rippled by numerous deep furrows, partially interlaced on the convex labial face. The labial visor is proportionally high and does not overlap the root–crown boundary. The apical extremity of the labial face is irregularly flat and horizontal because of scouring by functional wear. The transversal crest is always ragged and the notches are toughened by short and scattered lingual folds. The root is medium to high and principally polyaulacorhize in large forms with three to five well-spaced lamellas that can be strongly compressed labiolingually. Their basal face is relatively salient.
Oromobula dakhlaensissp. nov. (Fig. 6)
Oromobula dakhlaensis gen. nov., sp. nov. A-D, DAK2B-4, tooth position unknown. A, lingual view; B, profile; C, labial view; D, occlusal view. E-H, holotype DAK2B-5, tooth position unknown. E, lingual view; F, profile; G, labial view; H, occlusal view; I-L, DAK2B-6, tooth position unknown; I, lingual view; J, profile; K, labial view; L, occlusal view. M-N, young element DAK2B-7, tooth position unknown. M, lingual view; N, profile. O-P, young element DAK2B-8, tooth position unknown. O, lingual view; P, occlusal view. Vertical scale bar for A-H, horizontal scale bar for I-P.
Type locality: DAK3. Unit 2, level B2; from two sites south of Ad Dakhla (35.5 km and 41.5 km), south-western Morocco (Adnet et al., 2010).
Type horizon: Samlat Formation, Gerran member (Ratschiller, 1967) – late Middle Eocene/Late Eocene.
Synonymy: Mobula sp. (Adnet et al., 2010) in text only.
Etymology: Species named after the peninsula of Dakhla, south-western Morocco.
Holotype: DAK 2B-5 (Fig. 6E–H).
Diagnosis: Same as the genus.
Description: Crown is three or more times higher than root. Its lateral extremities are flared as in holotype (Fig. 6E) to convex (Fig. 6A) in labial or lingual view. Strongly labiolingually flattened, its lingual face is concave between a basal transversal roll of enameloid, overlapping the root/crown boundary, toward the transversal crest. Labial face is however, principally convex with a curve that follows those of lingual face in profile (Fig. 6B). Labial visor is only visible on the smallest tooth (Fig. 6N) but disappears in larger ones (Fig. 6B, F) and root is totally overhung in labial view (Fig. 6C, G). Nearly all the labial surface (visor included) is ragged by numerous deep vertical folds that form partially anastomosing lamellae around the mid part of labial face. The basal part is devoid of grooves, especially on the small teeth (Fig. 6M–P) considered as the youngest specimen. The apical part of the labial face that edges the transversal crest (making an occlusal surface) is flat, nearly horizontal, and smooth (except on the smallest elements, Fig. 6M–P) because of scouring by functional wear. Occlusal contour is chaotic (Fig. 6D, H, L), with no geometric structure indicating possible interlocking columns of teeth, even weakly. The transversal crest is irregular and largely overlaps the lingual face. The lingual face is relatively smooth except under the transversal crest where a few pronounced vertical folds extend below the transversal crest (Fig. 6E, M). Root is extremely reduced in size, both in height and depth. Except on some of the youngest specimens of the type series (Fig. 6M, N), root is principally at the polyaulacorhize state. Root lobes are lamella-like and are well-spaced, except on the youngest specimens (Fig. 6M, N), in which the root shows a more compact shape. Small scattered foramina are sometimes visible on the labial and lingual faces of root, as in holotype (Fig. 6E, G).
Discussion: Teeth of †O. dakhlaensis gen. et sp. nov. come only from the level B2 (see Adnet et al., 2010 for geological details) and have never been found together with those of A. barbei gen. et sp. nov. from the underlying level B1. Distinct in time, these two taxa are also distinct in morphology with the youngest (†O. dakhlaensis gen. et sp. nov.) having more strongly labiolingually compressed teeth with a higher crown without well-marked bulge on the labial face, a non-ornamented lingual face and a higher root strongly compressed labiolingually compared to the crown with well-spaced short lamellae.
Neither sexual nor monognathic heterodonty are seen in our limited sample. However, ontogenetic heterodonty is substantial within the smallest specimens (Fig. 6I–P), which display teeth showing more morphological affinities with those of Mobula (e.g. holaulacorhize to polyaulacorhize root, labial face partially ornamented, presence of an individualized labial visor). The labiolingual flattening as well as the enameloid ornamentation is moreover only slightly marked on the smallest teeth. Affinities with the Oligocene †E. kowaldi and †P. kruibekensis are marked as they share a number of similar characters such as a reduced labial visor combined with a concave lingual face in large teeth and a flat occlusal surface with a transversal crest often ragged (contrary to †Plinthicus). However, the enameloid layer on the lingual face is globally smooth (except some scattered folds) as in †Eomanta and contrary to †Plinthicus. The teeth are strongly constricted labiolingually with a high crown/root ratio and the root possesses thin regular lamellae as in †Plinthicus and contrary to †Eomanta. Indeed, this genus represents an intermediate form with a mixed suite of characters existing in both enigmatic genera. Comparisons may be extended with care to †P. stenodon, as some small specimens of this species with large teeth recovered from the mid Miocene of North Carolina, USA (H. Cappetta, unpubl. data), resemble those of †O. dakhlaensis gen. et sp. nov. However, we remain unsure whether these unstudied and unfigured teeth really represent juvenile specimens of †P. stenodon or a different lineage.
GenusEoplinthicusCappetta & Stringer, 2002
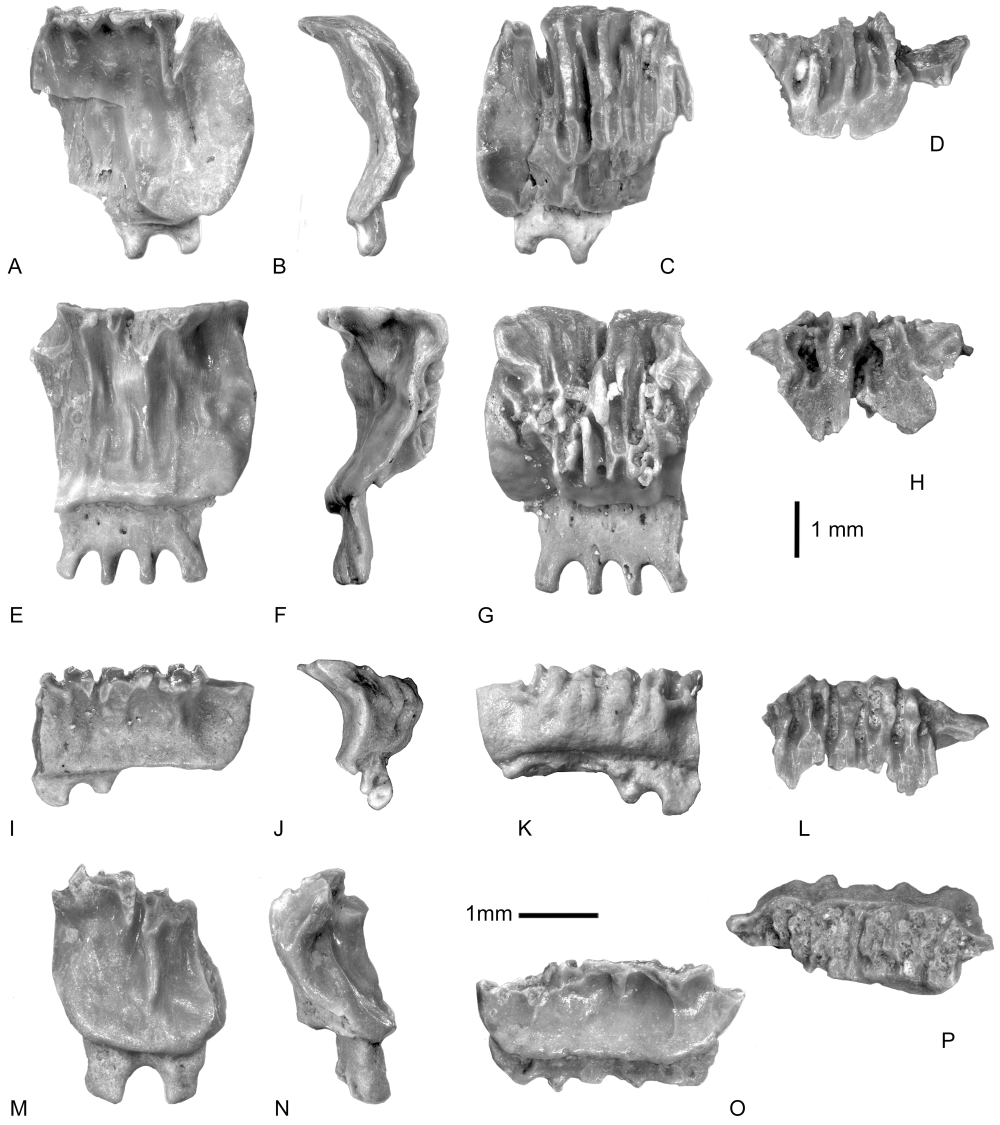
Eoplinthicusunderwoodisp. nov. (Fig. 7)

Eoplinthicus underwoodi sp. nov. A-E, DAK2A-2, holotype: tooth position unknown. A, lingual view; B, labial view; C, occlusal view; D, basal view; E, profile. F-G, DAK2A-3, paratype, tooth position unknown. F, lingual view; G, occlusal view. H-J, DAK2B-2, tooth position unknown. H, lingual view; I, labial view; J, occlusal view. K-O, DAK2B-3: young element, tooth position unknown. K, lingual view; L, labial view; M, occlusal view; N, basal view; O, profile.
Material: Four complete teeth and many fragments.
Type locality: Locality DAK2. Unit 2, levels B1 and B2, south of Ad Dakhla, south-western Morocco (Adnet et al., 2010).
Type horizon: Samlat Formation, Gerran member (Ratschiller, 1967) – late Middle Eocene/Late Eocene.
Etymology: After C. J. Underwood, for his work on the Fayum faunas in Egypt.
Holotype: DAK2A-2 (Fig. 7A–E).
Diagnosis: The teeth have a small size, less than 5 mm broad, with a roughly hexagonal outline in occlusal view. Marginolabial and marginolingual angles very obtuse and rounded. Occlusal face as developed or less developed than the complete width of the crown, and very hollowed transversally. Oblique and well-developed labial face covered by corrugated enameloid. Sharp and salient labial visor and well-marked lingual bulge at the base of the crown. Root as high as the crown, with about six lobes separated by rather deep and broad Ω-shaped furrows. Sharp basal face of the lobes. The two marginal lobes may be shorter and diverging more than the others.
Description: The holotype (Fig. 7A–E) is about 2.4 times broader (4.8 mm) than long (2 mm) and 2.3 mm high. In occlusal view (Fig. 7C), the outline is roughly hexagonal, with marginal angles less than 90°. The occlusal face is narrower than the crown at the level of marginal angles and its shape is elliptical on the whole. This face has a deep transverse hollow with an angular bottom. This hollow is asymmetrical, with a labial wall more abrupt than the lingual one. Its surface is sometimes irregular, with a shiny enameloid. Its lingual margin is more irregular than its labial margin, which is rather convex. The labial face (Fig. 7B) is oblique in profile (Fig. 7E) and slightly convex. The labial visor is sharp and distinctly overhangs the root. The lower part of the visor is rather flat and well developed labiolingually. In occlusal view, the visor outline consists of a labial transversal segment slightly convex and of two shorter and straighter marginal segments, with strongly marked and rounded angles. The labial face is strongly corrugated. The lingual face is less developed than the labial one and more vertical in profile (Fig. 7E). It is also very ornamented, with smooth vertical folds mainly in the median part of the face. In lingual view (Fig. 7A), oblique segments unite the marginal angles with the extremities of the occlusal face of the crown. The transversal bulge of the base of the lingual face is well developed, rounded, and salient. The root is practically as high as the crown. It consists of six lobes separated by broad and deep Ω-shaped furrows. The marginal lobes are shorter than the median ones and even diverging. Their basal face is sharp and not flattened (Fig. 7D).
The other specimens (Fig. 7F–O) have a similar morphology. Their occlusal face is sometimes more transversally developed, with vertical segments joining their extremities and the marginal angles in lingual view (Fig. 7F). The largest specimen (Fig. 7H–J) has a polyaulacorhize root that consists of nine lobes, irregularly spaced, whereas the smallest (Fig. 7K–O) has a less concave occlusal face.
Discussion: Compared with the type species †Eoplinthicus yazooensisCappetta & Stringer, 2002, from the Priabonian of Louisiana, USA, several differences can be noted. The teeth of †E. underwoodi sp. nov. are smaller and much less transversally elongated. Their occlusal face is more lingually developed, with a deeper transverse hollow that does not show the ‘lip-like’ design of the type species. The ornamentation of the labial face is less corrugated. The differences are more marked at the level of the root. In the type species, the lobes are more numerous (six versus 11/12), with a flat basal face, and the base of the root is parallel to the base of the crown in labial or lingual view. Such differences seem to indicate a less derived morphology in †E. underwoodi sp. nov. compared to †E. yazooensis and species of †Plinthicus, resembling the morphology of some species of †Burnhamia. Cappetta & Stringer (2002) suggested that the crown was little marked by functional wear as in mobulids and contrary to the rhinopterids and myliobatids. The same functional assumption could be seriously made with the concave occlusal surface observed in material from Dakhla. At present, the new species †E. underwoodi sp. nov. is known only from the Bartonian–Priabonian deposits of south-western Morocco although its presence may be spatially extended to the contemporaneous deposits from Fayum, Egypt (Underwood et al., 2011 as †Eoplinthicus sp.). The occurrence of this species elsewhere is suspected but currently remains debatable. Case & West (1991) reported a single broken tooth as Rhinoptera sp. from the Late Eocene of Pakistan that shows a deflected extremity of the crown in labial view (Case & West, 1991: pl. 4, fig. 1), indicating that the occlusal face is straighter than the base of the crown, as in †Eoplinthicus and contrary to Rhinoptera.
PHYLOGENETIC RELATIONSHIPS OF LIVING AND FOSSIL MOBULIDS
Cladistic analysis
Material and methods
Most of the living and extinct taxa previously described were included in the analyses. They consist of five modern genera (Mobula, Manta, Rhinoptera, and outgroups: Aetobatus and Myliobatis), eight living species, and 15 extinct taxa (genus or species) usually attributed to mobulids described above (†Cretomanta, †Archaeomanta, †Mo. loupianensis, †Mo. pectinata, †Mo. melanyae, †Mo. fragilis, †E. kowaldi, †A. barbei gen. et sp. nov., †O. dakhlaensis gen. et sp. nov., †Plinthicus krubekiensis, †P. stenodon, †Eoplinthicus, †Burhnamia, †Eomobula, †Brachyrhizodus). Most of the characters were selected from the literature (see Appendix 2 for details) directly available in matrix format from the two main discordant analyses of living taxa, i.e. that of Gonzalez-Isais & Montes Dominguez (2004) and that of McEachran & Aschliman (2004). Differences between the previous studies concern the number and the affiliation of living taxa analysed and the coding of soft body characters.
In addition, seven characters (labelled A–G) were newly coded from morphological data available in the literature devoted to living species (e.g. Notarbartolo di Sciara, 1987; Summers, 2000). These characters are visible on fresh and/or complete specimens only. They are: character A: absence (0) or presence of trabecular cartilage in jaw (1) according to Summers (2000); character B: complete interlocked teeth forming a pavement-like (0), labiolingual contact of teeth forming independent rows of interlocked teeth (1), teeth separated from each other (2); character C: low number of tooth files (< 10:0), high number of tooth files (> 10:1); character D: presence (0), absence of upper teeth (1); character E: no obvious sexual dental heterodonty (0), significant difference between male and female (1), not applicable on restricted sample; character F: absence (0), presence of soft branchial filter plates (1); character G: presence of a barbed caudal spine, possibly encased in calcified mass (0), absence (1).
We added 17 newly elaborated characters (labelled H–X) focusing on tooth morphology only, keeping in mind that they must be noticeable on all recent and fossil isolated teeth (e.g. in 2-7). Some of these characters were deduced from Claeson et al. (2010). Character H: crown size two times wider than long (0), longer than wide or subquadratic (1) in anterior files; character I: root tilted and elongated lingually beyond the crown (0), moderately elongated lingually (1), root vertical (2); character J: presence of a transversal crest between the labial and lingual faces: straight (0), sinuous or slightly ragged (1) or pointed (2); character K: enameloid of the lingual face: smooth or slightly ornamented (0), with strong vertical folds (1); character L: lack of labial visor (0), presence of a small labial visor that overlaps the root with collar not visible in labial view (1), presence of a labial visor well detached (2); character M: labial face of crown vertical or subvertical in profile (0), labially inclined (1), convex with two equal parts divided by a median bulge (2), lingually inclined (3); character N: absence of occlusal face of crown (0), presence of occlusal face (1); character O: occlusal face of crown rounded and convex in profile (0), flat (1), or concave (2); character P: labial face of crown with smooth enameloid (0), presence of slight ornamentation (spikes) (1), presence of folds and grooves (2); character Q: the lingual transversal shelf is absent (0), little developed (1), well developed (2), or rounded forming a transversal bulge (3); character R: crown two times higher than root (0), equal to the root (1), or lower (2); character S: root is principally at the polyaulacorhize stage with grooves and lamellae regularly distributed (0), polyaulacorhize stage with grooves anarchically distributed (1), holaulacorhize stage (2) or at the anaulacorhize stage (3); character T: root extremities are subvertical in labial or lingual view (0), flared in labial or lingual view (1); character U: root is longer than crown (0) or narrower than crown (1) in labial or lingual view; character V: root lobes are wider than high with flat basal faces (0), are higher than wide with basal faces (1) are extremely thin without basal face (2); character W: tooth is higher than long (0), is longer than high (1); character X: root curvature significant in basilar view (0), not significant (1).
These fine anatomical characters were deduced from previous observations, using the nomenclature chart available for batoid teeth in Cappetta (1987). Owing to the great intraspecific variation in mobulid teeth, we considered that the occurrence of a character state is significant when it is displayed in both adult males and females (if available for extinct taxa). Amongst the 101 characters from Gonzalez-Isais & Montes Dominguez (2004) and 47 from McEachran & Aschliman (2004), 21 are dental characters (characters B–E, H–X). Missing characters (mainly from soft anatomy) represent nearly 50% in the two compiled data matrices including fossils only known by teeth. Thirty characters (amongst the 124 compiled here) have more than two character states. Most of the characters have not been ordered a priori, except for some dental characters (B, J, M, Q, and R), which represent morphological series. All characters are formerly unweighted. The data matrices are available in nexus format in Appendix 2.
The data were treated under the assumption of the minimal model of unweighted parsimony, using PAUP 4.0 (Swofford, 1993), with a heuristic search (1000 replications with random taxa addition, 1000 treeholds by replication). The polymorphism option was considered for 18 characters to support the morphological variability inside each taxon (genus or species). Trees were only rooted by using the outgroup method (outgroup including Myliobatis–Aetobatus) to test the two primary alternative hypotheses concerning the place of Rhinoptera (see Fig. 1) and no a priori character polarization was retained. Analyses of the whole data set considering the two morphological analyses, as discussed previously, were successively performed removing or not some ambiguous fossil mobulids. The phylogenetic signal provided by the tooth characters only was also compared with the whole data set through partial analysis restricted to the dental characters available on isolated teeth (17 characters, H–X). The reweighting option was used (automatically performed by the software, Farris, 1969) after each first search to reinforce the weight of informative characters and to resolve the polytomies.
Results
Three of the strict consensus trees from the three different analyses that we ran are illustrated in Figure 8. Few differences were detected in the most parsimonious trees generated when including or excluding extinct taxa. Differences mainly concern the phylogenetic relationships between one outgroup (Myliobatis) to the rhinopterids or the relative position of †Mobula melanyae, which appears resolved (Fig. 8A, B) or unresolved (Fig. 8C) according to the data and taxa considered. Bremer indices are low (values of 1 for nodes A and F) despite relatively high values of the retention (RI) and consistency (CI) indices. We did not retrace the synapormorphic characters deduced from soft body anatomy; instead we direct readers to refer to Gonzalez-Isais & Montes Dominguez (2004) and McEachran & Aschliman (2004). We only focused on new dental characters performed for the present study (A–X) and supporting affiliation of fossils with living mobulids.

A, Strict consensus tree of the most parsimonious trees with all taxa analysed and recovered whatever the dataset used [CI = 0.61, RI = 0.82 with modified data sets from McEachran & Aschliman (2004); CI = 0.73, RI = 0.8 with modified data sets from Gonzales-Isais & Montes Dominguez (2004); CI = 0.54, RI = 0.8 with dental characters A-X only]. B-C, Strict consensus tree performed after exclusion of ambiguous fossil taxa †Cretomanta, †Archaeomanta and successive weighting, considering modified data sets from: B, Gonzalez-Isais & Montes Dominguez (2004) (Length = 155, CI = 0.87, RI = 0.89) and C, McEachran & Aschliman (2004) (Length = 74, CI = 0.74, RI = 0.86). Whole data sets are available in Appendix 2.
- •
The case of †Cretomanta and†Archaeomanta. The two ambiguous mobulid taxa (†Cretomanta and †Archaeomanta) were included in the first analysis. Both genera are grouped into the clade represented by node F (Fig. 8A). However, we removed them from the secondary analyses (Fig. 8B, C) because they probably do not represent true mobulids (see Systematic palaeontology section) and because their phylogenetic relationships within the mobulids sharing peg-like teeth require very large gaps in the fossil record. No major incongruence was observed after their removal regardless of the data sets used, indicating that their tooth morphologies did not induce equivocal changes in character states.
- •
Other ambiguous fossil mobulids ( Fig. 8 , node A). Recent assumptions considering the genus †Brachyrhizodus as a stem-mobulid (Claeson et al., 2010) or †Eomobula as representative of a transitional step toward mobulids (e.g. Herman et al., 1989) are rejected by the trees resulting from the present analyses. Concerning †Brachyrhizodus, this incongruence is however supportable if one considers that Claeson et al. (2010) reduced their comparative sample of rhinopterids/mobulids to the living genera, excluding many fossil relatives (e.g. †Eoplinthicus, †Plinthicus, †Burhnamia) showing all the dental features that define the rhinopterids in their analysis (Claeson et al., 2010: characters 623: roots in basal view are fine edges; 630: distance between roots is broad, groove wider than root; and 650: root grooves are regularly spaced between laminae) and those that define mobulids (Claeson et al., 2010: character 521: direction of tooth curvature horizontal). Concerning †Eomobula, this genus is currently regarded as a peculiar myliobatid sensu stricto (Cappetta, 2006) or possibly as a juvenile of an unknown myliobatid.
- •
Sister group to mobulids ( Fig. 8 , node B). The common assumptions considering ‘myliobatids’ as the sister group to a rhinopterid/mobulid clade (McEachran & Aschliman, 2004) or as a derived sister group (with rhinopterids) to mobulids (Gonzalez-Isais & Montes Dominguez, 2004) were tested. Results support the former, considering the rhinopterids as sister clade to mobulids comparatively to the myliobatids, whatever the data sets used. However, no synapomorphies in the dental characters clearly support this node.
- •
Stem mobulid ( Fig. 8 , node C). The monophyly of mobulids sensu lato (extant mobulids + extinct taxa) is retained in all the most parsimonious trees, whatever the assumptions concerning the data sets and/or ambiguous extinct taxa removed. In addition to the numerous synapomorphies already revealed in McEachran & Aschliman (2004) and/or Gonzalez-Isais & Montes Dominguez (2004) for the living mobulids, three unambiguous dental synapomorphies support this clade, including living and extinct taxa. These synapomorphies include the lack of interlocked teeth forming a pavement-like structure (B0→1); the increase of tooth file number (C0→1), and the flattening of the occlusal face of the crown (O0→1/2). The first two characters are assumed but not observed in most of the extinct taxa essentially known by isolated teeth. The Palaeogene taxa †Burnhamia and †Eoplinthicus differentiate early amongst the mobulids s.l., sharing tooth features of nondurophageous mobulids, which are counterbalanced by numerous dental similarities with rhinopterids. Despite an increase in crown height (W0→1), other Palaeogene taxa do not group together, which indicates that these genera form a paraphyletic assemblage. For instance, although teeth of †P. stenodon are quite similar to †Eoplinthicus (as noted by Cappetta & Stringer, 2002), differing from the latter by an unusual long extension of the tip of crown toward the lingual side (W0→1), they show more differences from those of the congeneric †P. krubekiensis that present a flat occlusal surface (O0→1) and other trends recovered in more derived mobulids. The attribution of the latter species to the genus †Plinthicus is thus problematic and the two species should be considered as possibly distinct. The intermediate genera †Eomanta, †Oromobula gen. nov., and †Argoubia gen. nov. mainly differ from †Plinthicus in having tooth roots at the polyaulacorhize stage with grooves irregularly distributed (S0→1) and longer than high (V1→0) as observable in some extant mobulids.
- •
Mobula clade sensu lato ( Fig. 8 , node D). Contrary to Gonzalez-Isais & Montes Dominguez (2004), all the most parsimonious trees clearly show the paraphyly of the genus Mobula. As previously observed with tooth morphology, Mo. japanica and Mo. mobular are close to the genus Manta (node F), including or not the fossil †Archaeomanta and †Cretomanta. This clade, grouping all the extinct and extant species of Mobula, is supported by a tooth root with flared extremities (T0→1), contrary to the subvertical lamellae observed in teeth of non-Mobula species. The elevation of the species †Mo. fragilis to the genus †Paramobula proposed by Pfeil (1981) is still disputable and no real autapomorphies clearly support this.
- •
Modern mobulids ( Fig. 8 , node E). As observed in fresh jaws of Mo. tarapacana, the largest and highest teeth observable on this species represent the last evidence of multiple and weakly interlocked columns of teeth. This node is marked by a polytomy and the incongruence between the different topologies affects the relative position of †Mo. melanyae, equally considered with taxa sharing peg-like teeth (node F) or comb-like teeth (node G). No dental synapomorphies support this clade, which contains all other living species of Mobula and Manta (nodes F and G).
- •
‘Peg-like teeth group’ ( Fig. 8 , node F). All the most parsimonious trees support this clade with at least four unambiguous dental synapomorphies; three of them being nonhomoplastic. They concern the total disappearance of interlocked ‘peg-like’ teeth (B1→2) that display an occlusal face of the crown newly concave (O1→2), a root at the holaulacorhize stage (S1→2) and that is wider than the crown (U1→0). Living Manta are confined in a polytomy with two living Mobula species (Mo. japonica and Mo. mobular), despite their teeth with anaulacorhize roots (S2→3) and the lack of toothed band on the upper jaw (D0→1). Evolution of tooth morphology does not better support one of the three possibilities suggested by this polytomy (Manta sister group of Mo. mobular and Mo. japonica and the two other alternatives). Although beyond the scope of this work, the validity of the genus Manta and the monophyly of the genus Mobula appear debatable (see Herman et al., 2000) considering the phylogenetic relationships obtained here, including or not the extinct taxa in the analysis.
- •
‘Comb-like teeth group’ ( Fig. 8 , node G). Being the sister group to mobulids with ‘peg-like’ teeth, the clade including node G is supported by the secondary reduction of crown height (W1→0) and well-marked sexually dimorphic dentition (E0→1). Intraclade relationships are still unresolved although Mo. thurstoni is in a basal position in the majority of the most parsimonious trees. Reasons for such a particularly well-marked sexually dimorphic dentition in this clade are still unclear.
DISCUSSION
Systematic implications for the extinct mobulids
The fossil record was mapped (Fig. 9B) on the tree illustrating the phylogenetic relationships discussed above with the exclusion of the ambiguous †Cretomanta and †Archaeomanta (see Fig. 8B). The choice to remove them was mainly motivated by the fact that their tooth morphologies are often considered convergent by numerous authors who advanced serious evidence based on unstudied characters, such as the root vascularization (Underwood & Cumbaa, 2010) or the lack of occlusal face, when this character is shared by all the extant and fossil myliobatids, rhinopterids, and mobulids. As previously argued from many unstudied morphological incongruences (see also the Systematic palaeontology section above), the exclusion of †Cretomanta and †Archaeomanta from mobulids is also justified by the large gaps induced by their position in the most parsimonious trees in comparison to the fossil record. Despite a tooth morphology quite comparable to that of mobulids with peg-like teeth (e.g. Ma. birostris or Mo. japanica), we conclude that both genera belong to another selachian family and their real affinities remain unclear. However, such a convergence in tooth morphology probably reveals a similar diet and behaviour between the Cretaceous †Cretomanta/Palaeogene †Archaeomanta and living mobulids with peg-like teeth. This seems to indicate that other selachians were probably planktivores before the rise of modern mobulids sensu stricto dated at least from Late Palaeogene. These enigmatic selachian lineages filled in the large ecological niche after the extinction of the Mesozoic filter-feeding Pachycormiformes (Friedman et al., 2010) and before the rise of large modern planktivorous elasmobranchs and whales in the Late Palaeogene (Cavin, 2010). In addition, our results confirm the affiliation of most of the fossils attributed to mobulids but exclude the genera †Eomobula and †Brachyrhizodus, which we consider representatives of extinct myliobatid lineages and not as ‘primitive’ mobulids.

A: Probable evolution of toothed jaw in mobulids, compared to sister groups of Myliobatidae (Myliobatis) and Rhinopteridae (Rhinoptera). Except for the living taxa, layout of teeth and number of tooth files were artificially reconstructed based on tooth morphologies and phylogenetic relationships performed on isolated teeth (number of tooth files and scales were not respected between taxa). B, Phylogenetic relationships from Fig. 8B constrained by the fossil record of Mobulidae (see text). Vertical bar, stratigraphical occurrences; dashed bar, uncertain records.
As previously hypothesized, the results of the cladistic analysis strengthen the monophyly of living mobulids (species of Mobula and Manta) relative to the myliobatids and rhinopterids. The hypothesis considering close relationships between mobulids and the rhinopterid/myliobatid clade (Gonzalez-Isais & Montes Dominguez, 2004) is not supported, whatever the data set analysed. Divergence time between mobulids and rhinopterids was recently estimated to 22.6–29.9 Mya based on molecular analyses (Aschliman et al., 2012). Although this age fits the oldest record of the genus Mobula, we cannot support this estimated divergence time for Rhinoptera and we consider the latter nearer to 50 Mya, as evidenced by the first occurrence of fossil rhinopterids and the oldest supposed mobulid †Burhnamia.
Implications for mobulid origins and evolution of planktivory
The peculiar incongruence in food habits (and thus in tooth morphology) between living myliobatids/rhinopterids and mobulids is more easily justifiable when fossils are taken into account. Focusing on tooth morphology, the results indicate that the grinding tooth plate of shell-predators probably pre-dated a reversal to the smaller teeth that are typical of filter-feeders. Moreover, phylogenetic relationships and fossils both suggest a gradual lack of tooth interlocking as represented in fossil and extant rhinopterids or myliobatids with grinding tooth plate, possibly correlated with the increase in the number of tooth files (Fig. 9A), as currently observable in the development of term-embryos of living Mo. thurstoni (Notarbartolo di Sciara, 1987) and Ma. birostris (Cadenat, 1960). We consider that †Burnhamia and †Eoplinthicus are the first representatives of mobulids s.l. even if we suspect they were not strictly planktivores. Indeed, although these taxa still have a rhinopterid-type dentition, dental features suggest a lack of biomechanical stress as observable in batoids that feed on soft prey, including plankton-feeders. The specialized lineage of †Burhnamia (e.g. †B. fetahi) illustrates the extreme reduction in tooth size with the presence of cuspidate teeth, lacking in all hard-shell consumers. Following the lack of dentition with multiple weakly interlocked columns of teeth, fossil teeth of †Plinthicus, †Argoubia gen. nov., †Oromobula gen. nov., and †Eomanta illustrate a second step toward a plankton-feeding strategy with a labiolingual compaction and thinning of the crown and flattening of the occlusal surface coupled with the emergence of sinuous to slightly ragged transversal crests between the labial and lingual faces, providing a new gripping ability in some living mobulids. The earliest modern mobulids (e.g. genus Mobula) probably displayed weakly interlocked columns of teeth as evidenced by †Mo. fragilis or living Mo. tarapacana, despite a reduction in tooth length in correlation with a possible increase in tooth files (up to 130 in Mo. tarapacana). This increase in tooth files may be correlated with the developmental features seen in embryos of some modern mobulids (see above). The morphological link toward mobulids displaying either peg-like teeth (living Mo. japanica, Mo. mobular, and Manta) or comb-like teeth (other living and fossil species of Mobula) remains obscure, even though one can assume that two changes possibly occurred respectively during the late Palaeogene: (1) an extreme reduction of tooth width from species with high crown (e.g. †Mo. melanyae) leading to peg-like teeth, enhanced extremely in Manta, and (2) a reduction of the crown height with development of cuspidate teeth in males distinctive of species with comb-like teeth, such as Mo. thurstoni or Mo. rochebrunei.
Considering that soft tissues of branchial filter plates do not preserve in the fossil record, precise timing of the appearance of strict planktophagy amongst mobulids remains difficult to identify, however, dental correlates can be dated to at least the Late Palaeogene (first Mobula s.s.). Mobulid teeth have evolved through time from grinding plates to long bands of numerous small homodont teeth, suggesting a gradual shift in feeding behaviour. Neogene species and most living species affiliated to modern mobulids (e.g. Mobula and Manta) exhibit teeth with functional inability for predation, probably substituted by the soft branchial filter plates. As recovered in Cretaceous species of unaffiliated selachians (e.g. †Cretomanta), living Ma. birostris represents an extreme case of loss of dental function with peg-like teeth on the lower jaw only, a feature present since the foetal state (Marshall, Pierce & Bennett, 2008). The smaller species Ma. alfredi possesses this unusual feature with a slightly more reduced tooth band compared to Ma. birostris (Marshall, Compagno & Bennett, 2009). Without ascribed functional roles, teeth of mobulids are however preserved and have evolved during the last 40 million years. The reasons for this remain unclear but inheritance of mating behaviours must be considered in evolutionary scenarios. In fact, the presence of cuspidate teeth in males confers a better grip during the courtship and mating, maintaining intromission. Such behaviour is usual in sharks and also occurs in batoids, Myliobatiformes included (McCourt & Kerstitch, 1980; Reed & Gilmore, 1981; Taniuchi & Shimizu, 1993; Nordell, 1994; Kajiura & Tricas, 1996). Living myliobatids, rhinopterids, and mobulids are known to have such complex precopulatory activities and courtships (Carrier, Pratt & Castro, 2004) with males grasping pectoral fin tips (Tricas, 1980; Uchida, Toda & Kamei, 1990), Manta included (Yano, Sato & Takahashi, 1999; Deakos, 2010). Therefore, the usefulness of teeth in mobulids would be restricted to mating activities, which allows the development of ornamentations and other atypical shapes (e.g. in Oromobula, Eoplinthicus, and Manta) as the grasping capacity is the only character retained by selectivity. In the mouth of Ma. birostris, Marshall et al. (2009) reported the presence of two rows of enlarged denticles of the same width as the lacking upper jaw tooth band. This can be interpreted as an extreme case of tooth functional loss, where modified mouth denticles probably replaced the upper teeth, offering a comparably efficient grasping capacity. The other outcome is that modern mobulids have relatively homodont teeth showing no major changes in size from the mesial to the distal regions of the jaw, a feature only observable in batch feeders, like sharks of the genera Cetorhinus or Rhincodon (Motta, 2004). This may be explained by the fact that these taxa do not need their teeth to process prey but require a full grasping potential for mating, for which heterodonty is ineffective.
Finally, as for the development of the free cephalic lobes described in the Introduction, another peculiarity of these pelagic myliobatids–mobulids is their locomotory mode, referred to as oscillatory (Webb, 1984) (i.e. pectoral fins flapping up and down). It has been suggested that batoids with oscillatory (flapping) locomotion that feed on benthic items (i.e. myliobatids, rhinopterids) extend the pectoral fins above but not below the body axis during swimming, whereas pelagic filter feeders batoids (i.e. mobulids) do. Therefore, it is conceivable that the transition from benthic feeding locomotion to pelagic feeding locomotion did not demand important modifications of the locomotory apparatus but only higher oscillation amplitudes. These modifications have probably been gradual through evolution and followed the tooth evolution towards soft bodied and then planktonic prey and it is likely that taxa such as †Burnhamia and †Eoplinthicus still had to use their lateral line canals for feeding and orientating relative to the substrate and therefore had limited oscillation amplitudes.
ACKNOWLEDGEMENTS
The authors wish to thank L. Cavin (Geneva) and an anonymous reviewer for their critical reading of the manuscript and very helpful suggestions and corrections. The authors are indebted to Gérard Barbe for donation of the fossil material. The authors are grateful to C. Cazevieille (CRIC/IURC-Montpellier) for scanning electron microscope (Hitachi S 4000) technical assistance. This research was supported by the French ANR-PALASIAFRICA Program (ANR-08-JCJC-0017, ANR-ERC). N° ISEM 2012-075.
Appendices
APPENDIX 1
List of fresh material and fossils examined
Abbreviations:* indicates if teeth are presently figured; DW, disc width; NS, indicates that material is referenced in Notarbartolo di Sciara (1985, 1987); UM-REC indicates that fresh material is housed at the University of Montpellier; UM-, indicates that this fossil material was figured and is housed at the University of Montpellier.
Mobula japanica: ♂ 1316 mm DW (NS 84-005), ♂ 1507 mm DW (NS 83–213), *♂ 2077 mm DW (NS 83-024), ♂ 2125 mm DW (NS 83-018), *♀ 2108 mm DW (NS 83-070), ♀ 2200 mm DW (NS 83-068), ♀ 2259 mm DW (NS 83-023); Mobula mobular: *♂ 2400 mm DW (UM-REC 26 M), Algeria, *♀ 1400 mm DW (UM-REC 27 M), Algeria; Mobula tarapacana:♂ 2476 mm DW (NS 83–143), ♂ 2494 mm DW (NS 84-009), *♂ 2500 mm DW (UM-REC 30 M) Ivory Coast, ♀ 2704 mm DW (NS 83–216), ♀ 2831 mm DW (NS 83–161), *♀ 3015 mm DW (NS 83–141); Mobula eregoodootenke: 250 mm DW (UM-REC 31 M) Queensland, Australia; Mobula kuhlii:♂240 mm DW (UM-REC 32 M), Zanzibar; Mobula thurstoni:♂ 630 mm DW (NS 83–212), ♂ 940 mm DW (83-015), ♂ 1147 mm DW (NS 83-022), ♂ 1437 mm DW (NS 83-008), ♂ 1675 mm DW (NS 83-059), *♂ 1770 mm DW (NS 83-077), *♀ 1626 mm DW (NS 83-020), ♀ 1719 mm DW (NS 83-055). Mobula rochebrunei:*♂ 1170 mm DW (UM-REC 25 M) Senegal, *♀ 1310 mm DW (UM-REC 14 M) Senegal; Mobula hypostoma:*♂ size unknown (UM-REC 28 M), *♀ size unknown (UM-REC 29 M); Mobula munkiana:♂ 826 mm DW (NS 82-028), ♂ 851 mm DW (NS 82-020), ♂ 885 mm DW (NS 82-027), *♂ 895 mm DW (NS 82-015), ♂ 864 mm DW (NS 83-001), ♂ 871 mm DW (NS 83-002), ♀ 843 mm DW (NS 83-022), ♀ 881 mm DW (NS 83-010), ♀ 877 mm DW (NS 83-023), ♀ 883 mm DW (NS 83–201), ♀ 906 mm DW (NS 83-025), ♀ 931 mm DW (NS 83-024), ♀ 971 mm DW (NS 83-026), ♀ 983 mm DW (NS 83-017), *♀ 931 mm DW (NS 84-004); Manta birostris:*♂ size unknown (NS 83-003), *♀ size unknown (NS 83–160); †Mobula loupianensis: holotype (UM-LPN 481) and paratypes (UM-LPN 478 to 480), S France, uncatalogued material (UM) coming from Costa de Caparica (Portugal); †Mobula pectinata: holotype (UM-LPN 484), paratypes (UM-LPN 482 to 483) and supplementary material (UM-MAZ 34) south of France, uncatalogued material (UM) coming from Costa de Caparica (Portugal); †Mobula fragilis: Holotype (UM-LPN 485) and paratype (UM-LPN 486) S France, uncatalogued material (UM) coming from Costa de Caparica (Portugal); †Cretomanta canadensis: (UM-MID 3) Midlothian Tx Quarry (Texas, USA) and uncatalogued material (UM); †Archaeomanta melenhorsti: (UM-DYD 2 and 3) phosphate basins (Morocco) and uncatalogued material (UM); †Archaeomanta priemi: uncatalogued material (UM) phosphate basins (Morocco); †Brachyrhizodus wichitaensis: (UM-HBR 137 to 147) Hop Brook (New Jersey, USA) and uncatalogued material (UM); †Plinthicus stenodon: (UM-LEE 11) Lee Creek (North Carolina, USA) and uncatalogued material (UM); †Burnhamia daviesi: (UM-ZOY 2 and 3) phosphate basins (Morocco) and uncatalogued material (UM), uncatalogued material (UM) from Burnham on Crouch (England), H. Cappetta sampling, uncatalogued material (UM) from diverse European localities; †Burnhamia fetahi: holotype (UM-MYB 22) and paratypes (UM-MYB22 to 29) phosphate basins (Morocco), uncatalogued material (UM) from other localities in Morocco; †Eoplinthicus yazooensis: holotype (UF 206580) and paratype (UF 206581) Yazoo Clay (Louisiana, USA), loan of G. Stringer.
APPENDIX 2
Data matrix for the cladistic analyses
| Taxa | Data 1 | Data 2 | ABCDEFGHIJKLMNOPQRSTUVWX |
|---|---|---|---|
| Aetobatus | 203220011111111311201?4 | 11131101212011110020113410001101011201111313102010111111100122300012212211002 | 10000000000001001(0+1)001100 |
| Myliobatis | 1132200101(0+1)1111(2+3)1120104 | 1112100021201(2+3)1100111133000011010101021001121120(0+1)0011111100022200011112211002 | 10000000000001002(0+1)001101 |
| Rhinoptera | 30322111111111131020144 | 1113111221101(2+3)110130113310101101111401(1+3)12112112120111112000002210011312211002 | 100000000000210031001101 |
| Manta | ??????????????????????? | 1113110221401522034001331000111001?????????2002?2002100201?211101103402211122 | 121101(0+1)1210001210(0+1)31001- |
| Mjap | ??????????????????????? | 1113111221111422034001331000111101130420121200222101100201?2111?0103402211012 | ?2100101220001210(0+1)21001- |
| Mmob | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ?2100101220001210(0+1)21001- |
| Mtar | ??????????????????????? | 11131112210114220140013310001111011304211313002220011002010211100003402211032 | ?01001101100211200111011 |
| Mroc | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ?11011111201111(0+1)01111001 |
| Mhyp | 40??21111110101310211?4 | ????????????????????????????????????????????????????????????????????????????? | ?11011111201111(0+1)01111001 |
| †Mlou | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ????1??112011110(0+1)1111001 |
| †Mpect | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ????0??01201111011111001 |
| †Mfra | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ????1??010+101111210111011 |
| Mmunk | ??????????????????????? | 11131112213113221140013310001111011304201313112220011002011211000004402211012 | ?110111(0+1)1201111(0+1)01111001 |
| Mthu | ??????????????????????? | 11131112213113221140013310001111011303101212002220011002011211100003402211012 | ?11011101201111(1+2)01111001 |
| †Mmelanyae | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ?????????20021120?????11 |
| †Eomobula | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ????0??0200021(0+1)011(1+3)01000 |
| †Eomantakow | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ???????011(0+1)(0+1)21121(0+1)1010?? |
| †Orodak | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ???????0(1+2)100211210001211 |
| †Argbarb | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ???????011(0+1)1211210101111 |
| †Plikru | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ???????0101?211210001011 |
| †Pliste | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ????0??01011112210001211 |
| †Burnhamia | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ????(0+1)??01(0+2)02112(0+1)3(1+2)001201 |
| †Eoplinthicus | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ???????01001112221001101 |
| †Archaeomanta | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ????0??12-0000-00121001- |
| †Cretomanta | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ????0??12-0000-00131001- |
| †Brachyrhizodus | ??????????????????????? | ????????????????????????????????????????????????????????????????????????????? | ????0??02000010001101000 |
- Two databases (based on soft tissues analyses) are detailed in Gonzalez-Isais & Montes Dominguez (2004) and McEachran & Aschliman (2004). They were compiled here after minor modifications detailed below.
- Data 1: Amongst the 82 characters of McEachran & Aschliman (2004), only 23 characters were conserved (numbered 9, 15–17, 20, 26, 29, 31, 33–34, 36–38, 41, 43–44, 51, 60, 62, 67, 77–78, 82 in McEachran & Aschliman, 2004) because the others are uninformative for the limited sample of taxa that we considered (genera Myliobatis, Rhinoptera, and Mobula hypostoma). Only two characters in McEachran & Aschliman (2004) are devoted to tooth morphology (their characters 16–17) and concern the root histology only. Contrary to these authors who did not formally consider the secondary loss of serrated caudal spines as informative in myliobatiforms, we conserved this character in the seven additional anatomical characters described in the text.
- Data 2: The 77 characters used by Gonzalez-Isais & Montes Dominguez (2004) were conserved.
- Abbreviations: Mjap, Mobula japonica; Mmob, Mobula mobular; Mtar, Mobula tarapacana; Mroc, Mobula rochebrunei; Mhyp, Mobula hypostoma; †Mlou, Mobula loupianensis; †Mpect, Mobula pectinata; †Mfra, Mobula fragilis; Mmunk, Mobula munkiana; Mthu, Mobula thurstoni; †Mmelanyae, Mobula melanyae; †Eomantakow, Eomanta kowaldi; †Orodak, Oromobula dakhlaensis gen. et sp. nov.; †Argbarb, Argoubia barbei gen. et sp. nov.; †Plikru, Plinthicus krubekensis; †Pliste, Plinthicus stenodon. ?, character state unknown or unavailable; -, character inapplicable; ( ), polymorphism.




