Phylogenetic implications of the morphology of the braincase of caecilian amphibians (Gymnophiona)
Abstract
Caecilian morphology is strongly modified in association with their fossorial mode of life. Currently phylogenetic analyses of characters drawn from the morphology of caecilians lack resolution, as well as complementarity, with results of phylogenetic analyses that employ molecular data. Stemming from the hypothesis derived from the mammal literature that the braincase has the greatest potential (in comparison to other cranial units) to yield phylogenetic information, the braincase and intimately associated stapes of 27 species (23 genera) of extant caecilians were examined using images assembled via microcomputed tomography. Thirty-four new morphological characters pertaining to the braincase and stapes were identified and tested for congruence with previously recognized morphological characters. The results reveal that when added to previous character matrices, characters of the braincase and stapes resolve generic-level relationships in a way that is largely congruent with the results of molecular analyses. Analysis of a combined data set of molecular and morphological data provides a framework for conducting ancestral character state reconstructions, which resulted in the identification of 95 new synapomorphies for various clades and taxa, 27 of which appear to be unique for the taxa that possess them. Together these data demonstrate the utility of the application of characters of the braincase and stapes for resolving phylogenetic relationships for a group whose morphology is largely confounded by functional modifications. In addition this study provides evidence of the utility of the braincase in resolving problematic morphology-based phylogeny outside of Amniota, in an amphibian group.
© 2012 The Linnean Society of London, Zoological Journal of the Linnean Society, 2012, 166, 160–201.
INTRODUCTION
Caecilians (Gymnophiona), one of three living orders of lissamphibians, remain one of the most poorly understood tetrapod groups in terms of many basic aspects of their biology and evolution. In a recent review, Wilkinson & Nussbaum (2006) presented a phylogenetic tree that reflected the current state of the contribution of morphological data to the understanding of caecilian phylogeny (Fig. 1A). Although this tree was not the result of a computer-based analysis, rather a consensus of separate analyses and descriptions, the rooting of most species in a large polytomy underscored the central challenge that faces progress in the understanding of caecilian morphology-based phylogeny and taxonomy; that is, the general paucity of morphological synapomorphies useful for identifying low taxonomic levels.
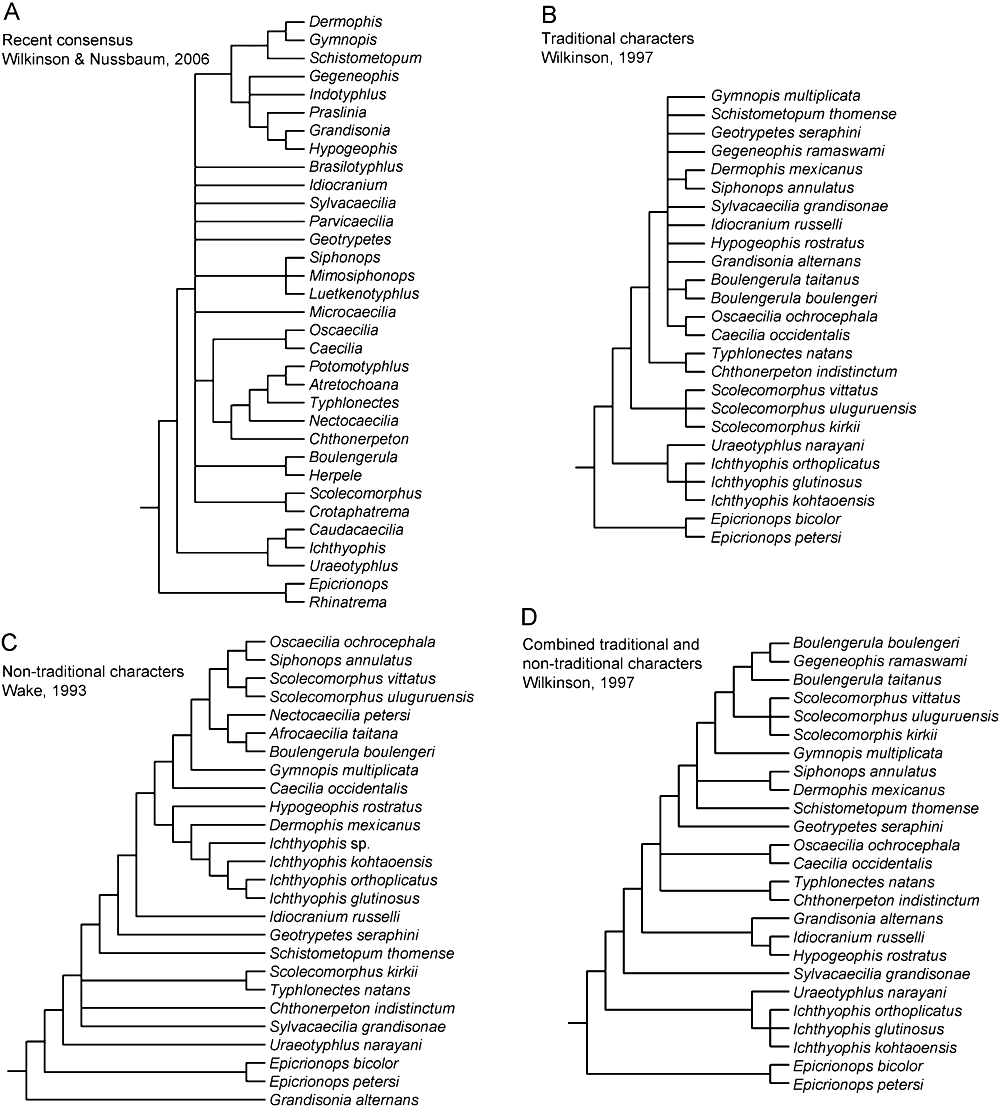
Previous morphology-based phylogenies of caecilians. A, a consensus tree derived from individual component trees computed in separate smaller analyses and taxonomic descriptions representing the current state of morphology-based caecilian systematics (Wilkinson & Nussbaum, 2006); B, tree derived from ‘traditional characters’ (Wilkinson, 1997); C, tree derived from ‘nontraditional characters’ (Wake, 1993); D, tree derived from combined ‘traditional’ and ‘nontraditional’ characters, revealing an unconventional topology wherein eye-reduced taxa (Boulengerula, Gegeneophis, Scolecomorphus, and Gymnopis) form a clade (Wilkinson, 1997).
Several reasons for the inability of morphological data to yield a resolved pattern of phylogeny of caecilians have been proposed. Firstly, externally caecilians are morphologically conserved across the clade (Taylor, 1968). Features from which to draw meaningful character data from the external anatomy (often the only region available because of the rarity of specimens) are limited to traits such as colour, numbers of annuli (body rings), position of the tentacular aperture relative to the eye and nostril, and tooth counts (e.g. Nussbaum & Gans, 1980; Gower et al., 2002; Gower & Wilkinson, 2007). The character states derived from these traits tend to be non-unique and some, such as numbers of annuli, are variable within genera and species. Secondly, the general scarcity of morphological work focusing on low-level taxonomic ranks has been cited as an obstacle that limits further phylogenetic resolution (Wilkinson, 1997). Detailed morphological analyses are available for only a few species (e.g. Wiedersheim, 1879; Sarasin & Sarasin, 1890; Ramaswami, 1941; Wake & Hanken, 1982). Conversely, only a few anatomical systems are very well known for a wide variety of species (e.g. Wake, 1968, 1985; Fritzsch & Wake, 1988; Schmidt & Wake, 1990), and many of these systems necessitate destructive techniques in order to evaluate the condition for additional species. Finally, caecilian morphology is considered highly adapted to their predominantly fossorial mode of life (Wake, 2003). The skulls of caecilians are robust in comparison to those of frogs and salamanders, and there appear to be several instances of morphological convergence associated with the evolution of the caecilian skull including multiple instances of orbit loss and temporal closure/opening (i.e. stegokrotaphy/zygokrotaphy).
Previous computer-based morphological analyses of caecilian phylogeny have yielded either very poor low-level taxon resolution (e.g. Fig. 1B; Wilkinson, 1997) or results that are largely at odds with hypotheses of relationships based on taxonomic descriptions and analyses using molecular data (Fig. 1C; Wake, 1993). An earlier combined analysis of all available morphological characters resulted in a well-resolved phylogeny (Fig. 1D; Wilkinson, 1997); however, several of the more unconventional hypotheses of relationship were retained (such as the clustering of reduced-eyed species). Therefore much of what we know of caecilian phylogeny is derived from molecular-based approaches (Roelants et al., 2007; Zhang & Wake, 2009); and these approaches preclude the ability to include fossil taxa, and, therefore, address broader evolutionary questions.
Recent studies have demonstrated the superior performance of the morphology of the braincase in resolving phylogenetic relationships, in comparison to other units of the skull such as the face, cranial vault, and lower jaw, in certain groups of mammals, using quantitative measures of form (Cardini & Elton, 2008; Goswami & Polly, 2010). It remains unknown, however, if the same applies to other groups of amniotes, or even to more distantly related non-amniote tetrapods. Early ossification and ossification from a cartilaginous precursor have been proposed as explanations as to why the braincase may be a more reliable source of phylogenetic information than the other regions of the skull, these latter regions being more susceptible to nongenetic selection pressures such as function and environment (Lieberman, Ross & Ravosa, 2000). Because these features of the braincase are shared across tetrapods, there is reason to hypothesize that the braincase would be informative in resolving phylogenetic relationships of other tetrapods too.
The goal of this study was to determine whether the morphological characters extracted from the braincase and associated stapes in many species of caecilians could contribute to the resolution of relationships amongst caecilian genera, and possibly even species. A total evidence approach combining morphological and molecular data provides a test of congruence amongst all known characters, and permits the identification of new synapomorphies that aid in defining clades of taxa, some of which previously lacked morphological synapomorphies. The data generated here provide a framework within which further hypotheses of various aspects of caecilian morphological evolution can be explored and confirm the utility of the braincase for resolving morphology-based phylogeny in a non-amniote group with skulls greatly confounded by function and homoplasy. This work reveals that there remains substantial amounts of phylogenetically informative morphological variation concealed within the skulls of caecilians, and further work will elucidate these patterns and refine our understanding of morphological evolution within the group.
MATERIAL AND METHODS
Microcomputed tomography
The current study focused on the acquisition of new morphological data that can contribute to resolving relationships amongst caecilian taxa. To accomplish this morphological data were extracted by digital dissection of the braincase and stapes using nondestructive microcomputed tomography (µCT).
Ninety-eight specimens belonging to 27 extant species (23 genera), from each of the nine currently recognized families within Gymnophiona (as per Wilkinson et al., 2011), covering a broad geographical distribution, including Central and South America, Africa, and Asia, were available for study (Table S1). These specimens were either obtained as preserved specimens from museum collections, and subjected to µCT scanning at the University of Calgary (SkyScan 1174 or Scanco µCT35; 60 to 80 kVp and 60 to 72 µA), or were obtained as already compiled µCT data sets courtesy of E. Sherratt, M. Wilkinson, and D. Gower (Natural History Museum, London, UK; Table S1). These latter specimens were scanned on a Metris X-Tek HMX ST 225 system (Natural History Museum, London), with settings ranging from 85 to 120 kVp and 140 to 190 µA. Three additional specimens that were synchrotron-scanned at the DORIS III accelerator ring at the German Electron Synchrotron (DESY) in Hamburg, Germany, were obtained as already compiled data sets courtesy of T. Kleinteich (University of Hamburg, Hamburg, Germany; Table S1). Parameters of the scan can be found in Kleinteich, Haas & Summers (2008). A specimen of Ascaphus truei (RM 2184) and Hynobius naevius (UAMZ 3635) were µCT scanned on the Scanco µCT35 system, at the settings described above. The scans of these specimens were examined and these data formed the basis for the morphological scores of the outgroup taxa.
Data visualization
All scan data were down-sampled to a maximum of 512 pixels in any orientation, rendered as eight-bit greyscale TIFFs using the batch processing function in PHOTOSHOP CS2, and imported into AMIRA v.4 and v.5 (Visage Imaging, San Diego, CA) as a series of stacked images. The elements of the braincase were isolated by labelling structures using the LabelFields module, and visualized by applying the SurfaceGen and SurfaceView modules to the labelled data. The morphology of the braincase and stapes of all of the species is described here based on three-dimensional SurfaceView models generated from the µCT data sets.
Measurements and morphometric analysis
Some aspects of braincase morphology were quantified in order to generate measures of the observed morphological variation. The digitally isolated braincases were measured using the 3D measurement tool in the AMIRA v.5 software package. Twelve linear measurements were taken from the braincases and one measure of skull length was taken to evaluate the influence of size on the measured features (Fig. S1). Multiple measurement replicates were taken, and the coefficient of variation (cov) for each set of repeated measurements was used to assess the repeatability of the measurements (in all cases cov values were < 5%, suggesting accuracy).
For the morphometric analyses, a single value representing the adult condition for each measurement for each species in the sample was desired. To provide this, average values for specimens that fell within a 10% range of variation in skull length from the largest specimen of a species were used. Average values for each species were logarithmically transformed to linearize the data for further analysis.
All analyses of the morphometric data were conducted in the R software package (R Development Core Team, 2010). Ordinary least squares regression analysis (car and plotrix packages: R Development Core Team, 2010) was performed to explore the relationship between each measure and size, using skull length as proxy for size. In instances of poor relationship (high scatter and r-squared value less than 0.80) the data were further explored by plotting residual values of the measured feature against fitted values predicted by the regression line (sfsmisc package: R Development Core Team, 2010), and inspecting the outliers.
Phylogenetic analyses
The new qualitative and quantitative morphological data were developed into characters and incorporated into phylogenetic analyses of caecilians. Morphological and combined (morphological plus molecular data) phylogenetic analyses were performed.
Morphological analyses
The matrix used in the phylogenetic analyses of morphological characters is derived from that of Wilkinson (1997). This matrix incorporates 25 species of caecilians scored for 52 ‘traditional characters’[derived from the analyses of Nussbaum (1979) and Wilkinson & Nussbaum (1996)] and 26 ‘nontraditional characters’ (derived from the analysis of Wake, 1993), for a total of 78 characters. Several modifications to the traditional portion of the matrix were made (Table S2). Additionally, Caecilia occidentalis was excluded because it was scored as a composite taxon by Wilkinson (1997) and there was a risk of redundancy with two new species of Caecilia included here.
An additional 34 characters pertaining to the braincase and stapes were identified here (Appendix 1) and were included in the previous list of morphological characters. Additionally, seven species were included (Caecilia tentaculata, Caecilia volcani, Caudacaecilia asplenia, Crotaphatrema lamottei, Herpele squalostoma, Ichthyophis beddomei, Microcaecilia albiceps) that have not been previously included in morphological phylogenetic analyses. Rhinatrema bivittatum was included by Nussbaum (1979), but it was not included in the more recent analysis by Wilkinson (1997). These new taxa were scored for the characters employed by Wilkinson (1997) from primary observation of specimens, and from descriptive accounts in the literature. Unfortunately these new taxa could only be scored for the osteological, cranial characters, as dissection of specimens to get to internal soft tissue structures was not permitted or only skull data were available. Additionally, specimens from the analysis of Wilkinson (1997) that were not available for the current analysis were retained in the matrix, but were scored as missing data for the new characters. Ascaphus truei (RM 2184) and Hyn. naevius (UAMZ 3635) were scored for the morphological characters, and designated as outgroups for rooting.
Phylogenetic analysis of the morphological data was performed by applying maximum parsimony and Bayesian inference. For the parsimony analysis, PAUP* v.4.0b10 (Swofford, 2002) was used to analyse the matrix of 112 morphological characters (traditional, nontraditional, plus new braincase and stapes characters). The heuristic search option was used, the tree bisection and reconnection (TBR) option was selected and the multiple trees (MulTrees) option was in effect. Multistate taxa were treated as polymorphic and all characters were unordered and equally weighted. Bootstrap support was determined using the full heuristic search option for 500 replicates in PAUP. Indices of goodness of fit of the character data to the topology [e.g. consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI)] were calculated in PAUP. An index of tree resolution [consensus fork index (CFI); Colless 1980; Wortley & Scotland 2006] was calculated manually by dividing the number of nodes observed by the total number of nodes possible.
Bayesian inference of phylogeny was performed on the morphological data using MrBayes v.3.1.2 for OSX (Huelsenbeck & Ronquist, 2001). The Markov k (Mk) model and the gamma shape distribution prior was applied to the morphological data. The analysis was run three separate times, each for three million generations, sampled every 1000 generations. Stationarity of the runs and convergence of the posterior probabilities were assessed using TRACER v.1.5 for OSX (Rambaut & Drummond, 2007). TRACER was also used to assess the appropriate duration of the run via the effective sample size value, and to determine the burn-in value (25%). Support for family-level clades that were not retrieved in the summary trees was calculated as a per cent of the post-burn-in trees in which the clade was retrieved, using a constraint tree for each family and filtering post-burn-in trees in PAUP.
Combined analysis of morphological and molecular data
The molecular data for the caecilian portion of the combined matrix are those of Zhang & Wake (2009; the matrix was obtained courtesy of the authors). The matrix is composed of a concatenated sequence containing four partial nuclear gene sequences (RAG1, NCX1, SLC8A3, and CXCR4) and three partial mitochondrial gene sequences (12S, 16S, cytb) available from GenBank (see Zhang & Wake, 2009). Two frogs (Ascaphus truei and Leiopelma archeyi) and two salamanders (Hynobius formosanus and Andrias davidianus) were added to the matrix by downloading corresponding sequences from GenBank (Table S3) and performing alignments of the sequences with the caecilian species using the ClustalW (Thompson, Higgins & Gibson, 1994) extension in the program BioEdit (Hall, 1997). The aligned gene sequences were then reconcatenated, resulting in a molecular matrix composed of 45 species (41 caecilians, two frogs, and two salamanders) and 6332 nucleotide characters, 1883 of which are phylogenetically informative.
The 112 morphological characters were added to the matrix of molecular data. Three species (Ichthyophis kohtaoensis, Idiocranium russeli, and Sylvacaecilia grandisonae) that are present in the morphology matrix but are absent from the molecular matrix of Zhang & Wake (2009) were added to the combined matrix and assigned missing data values for the molecular portion of the matrix. Additionally, the molecular portion of Epicrionops niger was combined with the morphological portion of Epicrionops bicolor and Epicrionops petersi, forming a composite Epicrionops terminal taxon because no regions of overlap existed in the character scores between species. The same was carried out with Microcaecilia sp. and M. albiceps, and Hyn. formosanus and Hyn. naevis (outgroup salamander). One species, Caecilia sp., was deleted from the molecular matrix as there was risk of redundancy with the two species of Caecilia added here. The resulting combined matrix therefore consists of 47 species (43 caecilians, two frogs, and two salamanders) with a total of 6444 characters, 1995 of which are phylogenetically informative.
The combined data were analysed using MrBayes v.3.1.2 for OSX as a matrix of mixed datatype (Huelsenbeck & Ronquist, 2001). Each of the seven genes were defined as a character set, and the morphological characters were specified as an eighth character set. The matrix was partitioned according to the eight defined character sets, allowing each partition to vary independently of the others. The general time reversible with a proportion of invariable sites and gamma distributed rate heterogeneity (GTR+I + Γ) model (Tavaré, 1986) was applied to the RAG1, SLC8A3, CXCR4, 12S, 16S, and cytb genes and the Hasegawa-Kishino-Yano with a proportion of invariable site and gamma distributed rate heterogeneity (HKY+I + Γ) model (Hasegawa, Kishino & Yano, 1985) was applied to the NCX1 gene as per the recommendation of MrModeltest v.2.3 (Nylander, 2004). This differs from the analysis of Zhang & Wake (2009) in which the GTR+I + Γ model was applied to all seven partial genes. The Mk model was applied to the morphology partition, and it received the gamma-shaped distribution option for the rate setting.
The rate prior was set to variable and all parameters were unlinked. The outgroup species was specified as Ascaphus truei for rooting purposes. The analysis was run for ten million generations, sampled every 1000 generations, and the entire analysis was repeated three times. Stationarity of the runs and convergence of the posterior probabilities were assessed as described above for the Bayesian analysis of morphology.
Ancestral character state reconstruction and phylogenetic signal
Ancestral character state reconstructions (ACSRs) were performed under the maximum parsimony (MP) and maximum likelihood (ML) approaches in MESQUITE v.2.72 (Maddison & Maddison, 2010). The Trace Character History function was applied to the morphological characters under both MP and ML approaches using the 50% majority-rule consensus tree generated in the combined analysis as the topology. The hypothesized character states for the 34 braincase and stapes characters were recorded for each node at which they appeared. These are discussed below as they relate to identifying new potential synapomorphies.
The ACSRs were also used to assess the characters for phylogenetic signal. The approach of Maddison & Slatkin (1991) was adopted. An analysis of molecular data alone was conducted (same models and parameters as for the molecular portion of the matrix in the combined analysis described above), providing a consensus tree that did not incorporate the morphological characters (topology identical to that of Zhang & Wake, 2009, except that Boulengerula was retrieved as monophyletic here). The minimum number of steps observed for each character reconstruction was recorded in MESQUITE and compared to a null distribution of the number of steps that was generated for each character by randomly reshuffling (1000 times) the distribution of states at the terminal tips (simulating no influence of phylogeny). The hypothesis of no phylogenetic signal was rejected if the number of observed steps fell within the lower 5% of the null distribution (Stone & Cook, 1998; Crespi & Sandoval, 2000).
RESULTS
Description of the morphology of the caecilian braincase
The braincase of adult caecilians is composed of two bony elements: the anteriorly located sphenethmoid and the posteriorly located os basale (Fig. 2). The sphenethmoid consists of a main body that forms the floor and walls around the anterior portion of the brain. From the main body extend two pairs of variably present/ossified processes, the anterolateral processes and sola nasi, as well as the dorsomedial process and nasal septum that are present in all species (Fig. 3). In most species two pairs of foramina pierce the anterior wall of the sphenethmoid and these transmit the paired dorsal and ventral trunks of the olfactory nerve (Maddin, 2011). The os basale forms the floor, walls, and the posterior-most portion of the roof over the brain. The antotic region of the os basale is comprised of the antotic walls, which contain foramina that transmit cranial nerves V to VII and various blood vessels (Maddin, 2011). Descriptions of these and other braincase foramina come from Maddin (2011). It was shown that patterns ranged from five to one antotic foramina (with variants of three and two foramina), and these different patterns are numbered here as Patterns 1 to 8 based on this previous treatment. The otic-occipital complex is comprised of the otic capsules and occipital surface (Fig. 2). The bulbous otic capsules house the membranous labyrinth of the inner ear. The dorsal surface of the otic capsules is smooth and slightly tilted posteroventrally, providing an attachment site for the neck musculature. The paired occipital condyles extend to varying degrees from the posterior surface of the otic-occipital complex.

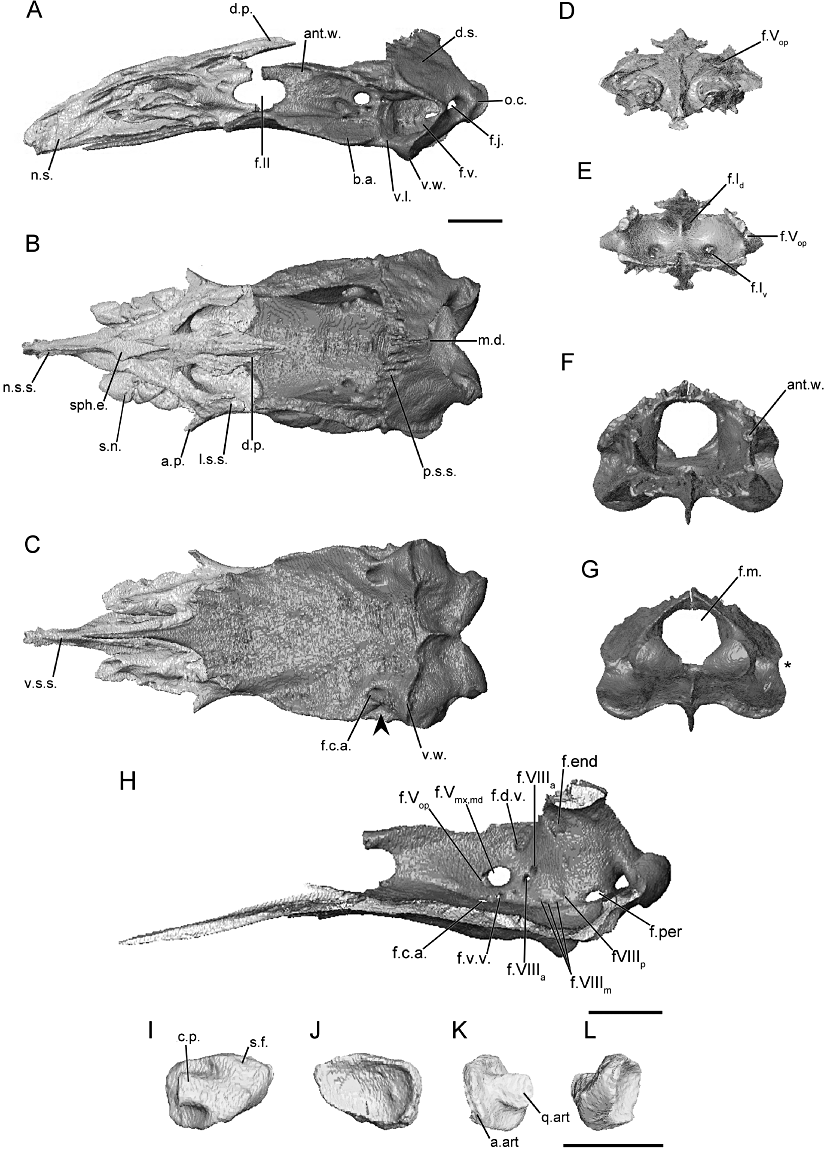
The morphology of the braincase (sphenethmoid and os basale) and stapes, as revealed through microcomputed tomography, of Caecilia tentaculata (FMNH 63833). A, the braincase in lateral view; B, dorsal view; and C, ventral view (arrowhead indicates location of the constriction of floor of the os basale, if present). D, the sphenethmoid in anterior view; and E, posterior view. F, the os basale in anterior view; and G, posterior view (asterisk indicates the location of the incision of the margin of the otic capsule by the fenestra vestibuli). H, the right medial surface of the os basale revealing the foramina of the antotic wall and of the medial wall of the otic capsule. I–L, the left stapes in natural position. I, lateral view; J, medial view; K, anterior view; and L, posterior view. Scale bars = 1 cm. Abbreviations for all figures: a.art., anterior articular surface of the footplate of the stapes; a.e., anterolateral expansion of the sphenethmoid; ant, antotic region; ant.w., antotic wall; a.p., anterolateral process; b.a., basicranial articulation; c.d.r., columellar distal ridge; c.l.r., columellar lateral ridge; c.p., columellar process of the stapes; c.VIIpal, canal for the palatal ramus of the facial nerve; d.p., dorsomedial process; d.s., dorsal surface of otic occipital complex; f.c.a., foramen for the carotid artery; f.d.v., foramen for the dorsal vein; f.end, endolymphatic foramen; f.j., jugular foramen; f.m., foramen magnum; f.per, perilymphatic foramen; f.s., stapedial foramen; f.v., fenestra vestibuli; f.v.v., foramen for the ventral vein; f.I, foramen for both trunks of the olfactory nerve; f.Id, foramen for the dorsal trunk of the olfactory nerve; f.Iv, foramen for the ventral trunk of the olfactory nerve; f.II, optic nerve foramen; f.Vmx,md, foramen for the maxillary and mandibular branches of the trigeminal nerve; f.Vop, foramen for the ophthalmic branch of the trigeminal nerve; f.VII, foramen for the facial nerve; f.VIIIa, foramen for the anterior branch of the vestibulocochlear nerve; f.VIIIm, foramen for the medial branch of the vestibulocochlear nerve; f.VIIIp, foramen for the posterior branch of the vestibulocochlear nerve; l.s.s., lateral wall of the sphenethmoid dorsal sutural surface; m.b., main body of the sphenethmoid; m.d., median depression for the hypophysis of the brain; n.s., nasal septum; n.s.s., nasal septum dorsal sutural surface; o.c., occipital condyle; oto, otic-occipital complex; p.s.s., parietal sutural surface on os basale; q.art., articular surface for the quadrate; r.o., ridge on the lateral surface of the otic capsule; s.d.p., stapes, dorsal process on the footplate; s.f., stapes footplate; s.n., sola nasi; s.o., shelf on the lateral surface of otic capsule; sph.e., sphenethmoid dorsal exposure; v.f., ventral flange on the nasal septum; v.l., ventral lip on the fenestra vestibuli; v.s., ventral sheet of bone on sphenethmoid; v.s.s., ventral sutural surface of the nasal septum; v.w., ventral wing-like process on the otic capsule.
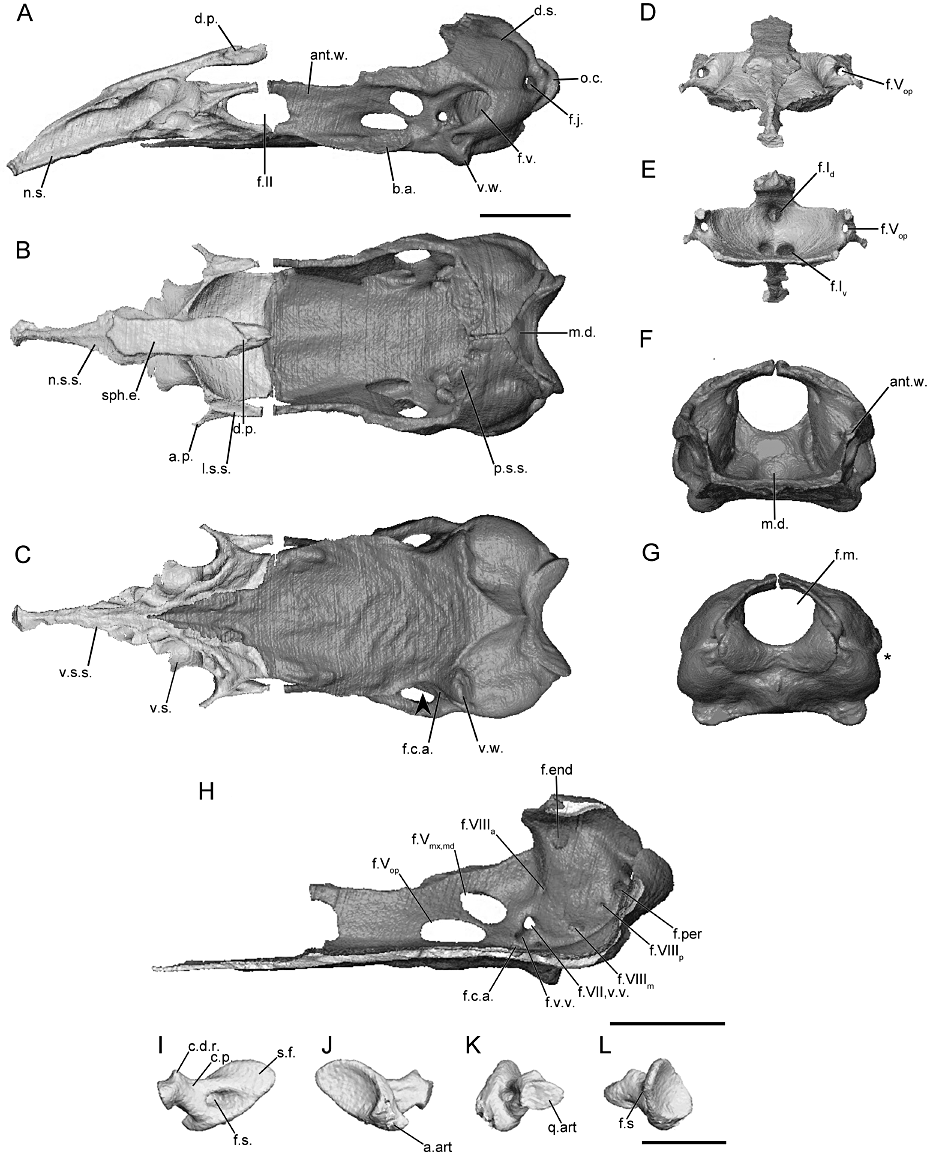
The morphology of the braincase (sphenethmoid and os basale) and stapes, as revealed through microcomputed tomography, of Gymnopis multiplicata (BM 1907.10.9.10). A, the braincase in lateral view; B, dorsal view; and C, ventral view (arrowhead indicates location of the constriction of floor of the os basale, if present). D, the sphenethmoid in anterior view; and E, posterior view. F, the os basale in anterior view; and G, posterior view (asterisk indicates the location of the incision of the margin of the otic capsule by the fenestra vestibuli). H, the right medial surface of the os basale revealing the foramina of the antotic wall and of the medial wall of the otic capsule. I–L, the left stapes in natural position. I, lateral view; J, medial view; K, anterior view; and L, posterior view. Scale bars = 1 cm. See Figure 2 for abbreviations.
Comparison amongst species reveals high levels of subtle variation in features of the braincase and stapes across Gymnophiona. Detailed descriptions of the morphology of the braincase and stapes are given in a family by family account below. Figures of representative species for each family are provided below (2-10), and figures of the remaining species examined are provided in the Supplementary Material (Figs S2–S19).
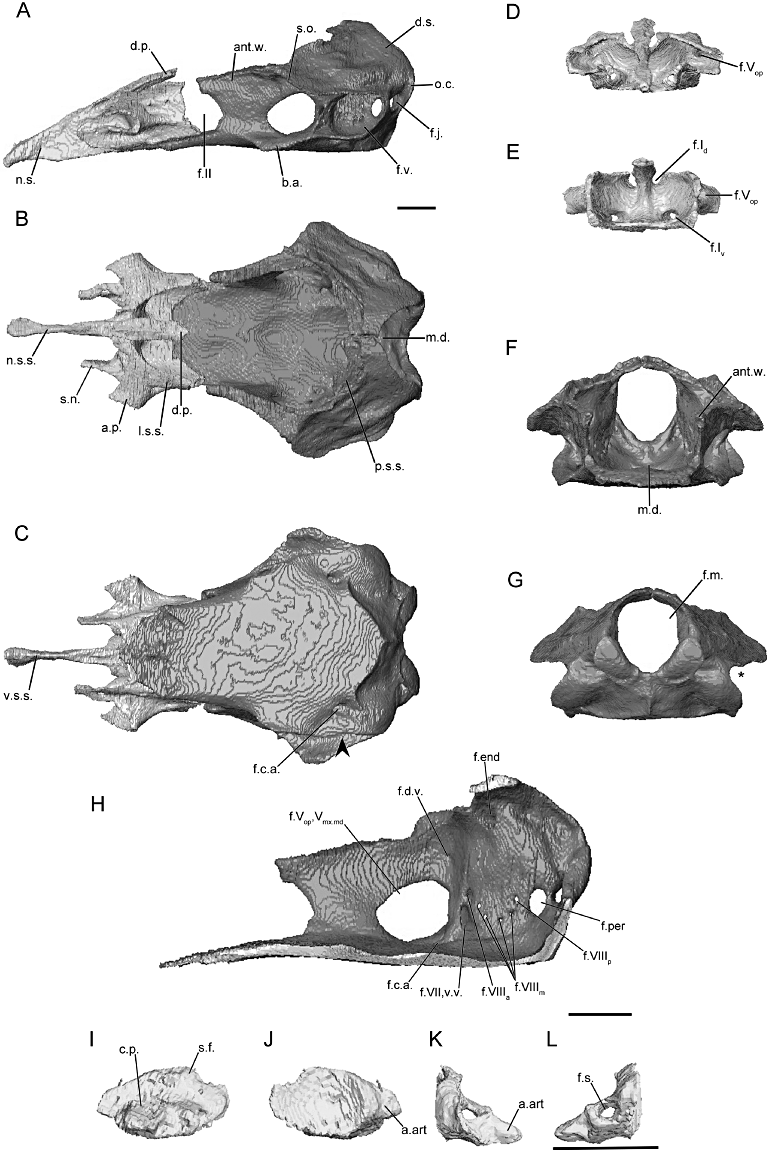
The morphology of the braincase (sphenethmoid and os basale) and stapes, as revealed through microcomputed tomography, of Boulengerula boulengeri (BM 2002.776). A, the braincase in lateral view; B, dorsal view; and C, ventral view (arrowhead indicates location of the constriction of floor of the os basale, if present). D, the sphenethmoid in anterior view; and E, posterior view. F, the os basale in anterior view; and G, posterior view (asterisk indicates the location of the incision of the margin of the otic capsule by the fenestra vestibuli). H, the right medial surface of the os basale revealing the foramina of the antotic wall and of the medial wall of the otic capsule. I–L, the left stapes in natural position. I, lateral view; J, medial view; K, anterior view; and L, posterior view. Scale bars = 1 cm. See Figure 2 for abbreviations.
The morphology of the braincase (sphenethmoid and os basale) and stapes, as revealed through microcomputed tomography, of Ichthyophis kohtaoensis (ZMH A08981). A, the braincase in lateral view; B, dorsal view; and C, ventral view (arrowhead indicates location of the constriction of floor of the os basale, if present). D, the sphenethmoid in anterior view; and E, posterior view. F, the os basale in anterior view; and G, posterior view (asterisk indicates the location of the incision of the margin of the otic capsule by the fenestra vestibuli). H, the right medial surface of the os basale revealing the foramina of the antotic wall and of the medial wall of the otic capsule. I–L, the left stapes in natural position. I, lateral view; J, medial view; K, anterior view; and L, posterior view. Scale bars = 1 cm. See Figure 2 for abbreviations.
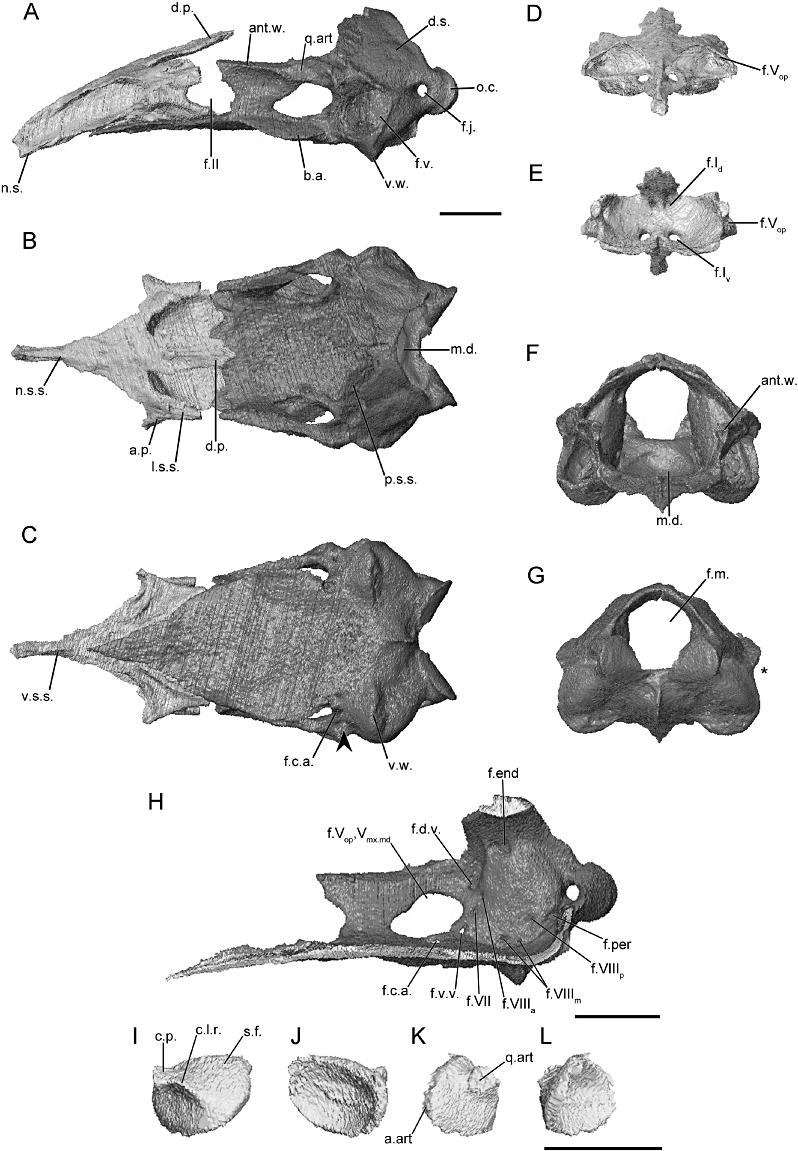
The morphology of the braincase (sphenethmoid and os basale) and stapes, as revealed through microcomputed tomography, of Gegeneophis ramaswami (KU 203038). A, the braincase in lateral view; B, dorsal view; and C, ventral view (arrowhead indicates location of the constriction of floor of the os basale, if present). D, the sphenethmoid in anterior view; and E, posterior view. F, the os basale in anterior view; and G, posterior view (asterisk indicates the location of the incision of the margin of the otic capsule by the fenestra vestibuli). H, the right medial surface of the os basale revealing the foramina of the antotic wall and of the medial wall of the otic capsule. I–L, the left stapes in natural position. I, lateral view; J, medial view; K, anterior view; and L, posterior view. Scale bars = 1 cm. See Figure 2 for abbreviations.

The morphology of the braincase (sphenethmoid and os basale) and stapes, as revealed through microcomputed tomography, of Epicrionops bicolor (BM 1946.9.5.66). A, the braincase in lateral view; B, dorsal view; and C, ventral view (arrowhead indicates location of the constriction of floor of the os basale, if present). D, the sphenethmoid in anterior view; and E, posterior view. F, the os basale in anterior view; and G, posterior view (asterisk indicates the location of the incision of the margin of the otic capsule by the fenestra vestibuli). H, the right medial surface of the os basale revealing the foramina of the antotic wall and of the medial wall of the otic capsule. I–L, the left stapes in natural position. I, lateral view; J, medial view; K, anterior view; and L, posterior view. Scale bars = 1 cm. See Figure 2 for abbreviations.

The morphology of the braincase (sphenethmoid and os basale), as revealed through microcomputed tomography, of Crotaphatrema lamottei (UMMZ 174496). A, the braincase in lateral view; B, dorsal view; and C, ventral view (arrowhead indicates location of the constriction of floor of the os basale, if present). D, the sphenethmoid in anterior view; and E, posterior view. F, the os basale in anterior view; and G, posterior view (asterisk indicates the location of the incision of the margin of the otic capsule by the fenestra vestibuli). H, the right medial surface of the os basale revealing the foramina of the antotic wall and of the medial wall of the otic capsule. Scale bars = 1 cm. See Figure 2 for abbreviations.

The morphology of the braincase (sphenethmoid and os basale) and stapes, as revealed through microcomputed tomography, of Siphonops annulatus (UMMZ 150624). A, the braincase in lateral view; B, dorsal view; and C, ventral view (arrowhead indicates location of the constriction of floor of the os basale, if present). D, the sphenethmoid in anterior view; and E, posterior view. F, the os basale in anterior view; and G, posterior view (asterisk indicates the location of the incision of the margin of the otic capsule by the fenestra vestibuli). H, the right medial surface of the os basale revealing the foramina of the antotic wall and of the medial wall of the otic capsule. I–L, the left stapes in natural position. I, lateral view; J, medial view; K, anterior view; and L, posterior view. Scale bars = 1 cm. See Figure 2 for abbreviations.

The morphology of the braincase (sphenethmoid and os basale) and stapes, as revealed through microcomputed tomography, of Chthonerpeton indistinctum (FMNH 206622). A, the braincase in lateral view; B, dorsal view; and C, ventral view (arrowhead indicates location of the constriction of floor of the os basale, if present). D, the sphenethmoid in anterior view; and E, posterior view. F, the os basale in anterior view; and G, posterior view (asterisk indicates the location of the incision of the margin of the otic capsule by the fenestra vestibuli). H, the right medial surface of the os basale revealing the foramina of the antotic wall and of the medial wall of the otic capsule. I–L, the left stapes in natural position. I, lateral view; J, medial view; K, anterior view; and L, posterior view. Scale bars = 1 cm. See Figure 2 for abbreviations.
Caeciliidae (Figs 2, S2–S3)
The main body of the sphenethmoid of caeciliids accounts for less than half of the total length of the element (Fig. 2A). The lateral wall of the main body is short and is capped by a broad sutural surface for the reception of a large anterolateral lappet of the parietal. The anterolateral corner of the dorsal surface is slightly expanded; however, an ossified anterolateral process is absent (Fig. 2B). A broad dorsomedial process is present and has a large dorsally exposed region (Fig. 2B). The exposure is bounded by the nasals, frontals, and parietals. The process does not reach the level of the antotic wall of the os basale in Cae. tentaculata (Fig. 2A), but does so in Cae. volcani and Oscaecilia ochrocephala (Figs. S2A and S3A). The posterior margin of the lateral wall is quite deeply incised by the anterior margin of the oval optic foramen (Fig. 2A). The posterior margin of the floor of the sphenethmoid is almost completely divided by a deep medial incision. Otherwise the floor is extensive and broad, and its posterior margin is only slightly concave. The ventral surface of the main body bears a pair of grooves that form the dorsal portion of a canal for the palatal ramus of the facial nerve (Fig. 2C).
The nasal region is divided by a moderately long, ossified nasal septum (Fig. 2A) terminating just posterior to the external naris. It is relatively uniform in height throughout its length in the species of Caecilia (Fig. 2A), but it tapers dramatically in O. ochrocephala (Fig. S3A). The broad dorsal sutural surface along the main body of the sphenethmoid extends onto the nasal septum (Fig. 2B). The surface tapers in width distally, but terminates relatively more broadly than those of several other species. Sola nasi are absent.
The foramina in the anterior wall, which transmit the dorsal and ventral branches of the olfactory nerve (Maddin, 2011) are paired, as they are in the majority of species examined here (Fig. 2D, E). The foramina of each pair are located close to either side of the midline of the sphenethmoid. The dorsal foramina lead to canals just beneath the sutural surface of the nasal septum, exiting almost half way along the length of the nasal septum. The ventral foramina also extend into short canals before exiting into the nasal capsule. An anterolateral foramen transmitting the ophthalmic branch of the trigeminal nerve (Maddin, 2011) pierces the base of the anterolateral expansion of the sphenethmoid.
In dorsal view the anterior portion of the antotic wall of the os basale runs parallel to the contralateral antotic wall (Fig. 2B). Posteriorly it is bowed laterally at the level of the antotic foramina. In anterior view the antotic wall is vertical (Fig. 2F). The dorsal sutural surface is extremely thin, but expands posteriorly over the antotic foramina, and is continuous with that along the anterior margin of the otic capsule. The anterior margin of the antotic wall is incised by the posterior margin of the oval optic foramen (Fig. 2A). There are two foramina present in each antotic wall (Fig. 2H). Both trunks of the trigeminal nerve, as well as the facial nerve and ventral vein, pass through the large antotic foramen (Maddin, 2011). A dorsal vein exits through a much smaller, posterodorsal foramen. This is referred to here as Pattern 6.
The dorsal surface of the otic-occipital complex is strongly inclined posteroventrally. The surface tapers slightly in width towards the midline. A broad region is exposed dorsally posterior to the parietal, and it also bears an extensive sutural surface along its anterior margin that receives the parietal (Fig. 2B). The dorsal surface extends laterally, creating a thin shelf dorsal to the fenestra vestibuli for the species of Caecilia (Fig. 2A), but not for O. ochrocephala (Fig. S3A). The fenestra vestibuli is small and ovoid longitudinally and orientated roughly horizontally. In posterior view the fenestra vestibuli deeply incises the lateral margin of the otic-occipital complex for the species of Caecilia (Figs 2G, S2G), but does not for O. ochrocephala (Fig. S3G). In lateral view the occipital condyle is extremely well developed, protruding far posterior to the otic capsule. A large jugular foramen is present in the base of the occipital condyle, and is visible in lateral view. The foramen magnum is diamond-shaped.
The medial wall of the otic capsule contains seven or eight foramina (Fig. 2H). The endo- and perilymphatic foramina, and the smaller foramina for the anterior and posterior branch of the vestibulocochlear nerve, are present in their typical locations, as described in Maddin (2011). Variably within the family three or four small foramina form an arc along the ventral margin of the medial wall of the otic capsule and these conduct the medial branch of the vestibulocochlear nerve.
The floor of the os basale of caeciliids is concave dorsally, rather than flat. The anterior margin tapers continuously to a point, and terminates beneath the nasal septum of the sphenethmoid. A central ridge corresponds to the medial incision of the floor of the sphenethmoid, and the main body of the sphenethmoid overlies much of the remainder of the anterior portion of the floor. A pair of weak depressions is located between the antotic walls. This corresponds to the location of the paired cerebral hemispheres. A deeper depression is located posteriorly, between the otic capsules. This corresponds to the location of the hypophysis of the brain (Maddin, 2011), and is particularly well defined in caeciliids (Fig. 2B, F). Lateral to the antotic wall the floor expands dramatically into well-developed basicranial articular surfaces (Fig. 2A, B). In ventral view the lateral margin of the floor is constricted slightly, just posterior to the basicranial articulation (arrowhead; Fig. 2C). A well-developed, wing-like projection is present under each otic capsule (Fig. 2A, C), demarcating the boundaries of the attachment site for the m. longus capitis (Bemis et al., 1983). Posteriorly the floor of the os basale terminates at a rounded point, which almost reaches the ventral margin of the foramen magnum.
A foramen that leads to a canal for the carotid artery perforates the floor of the os basale anterior to the otic capsule (Fig. 2C). The canal coursing anteriorly from this foramen bifurcates into lateral and medial canals, which terminate at foramina located on the lateral and medial sides of the antotic wall, respectively. Both foramina occur at roughly the equivalent positions on either side of the antotic wall, ventral to the large antotic foramen (Fig. 2H).
The footplate of the stapes of caeciliids is markedly elongated anteroposteriorly, with its long axis orientated horizontally (Fig. 2I, J). The footplate is closely applied to the anterior margin of the fenestra vestibuli. The articulation between the footplate and margin of the fenestra vestibuli has previously been described as a synchondrosis (de Jager, 1939; Wever, 1975). The remainder of the margin of the footplate does not contact the cartilaginous joint with the fenestra vestibuli, but it closely approaches its margins. The medial surface of the footplate is concave. The columellar process extends anterodorsally from the lateral surface of the footplate. It is long and slightly depressed dorsoventrally, and greatly expanded distally into a broad articular surface that contacts the quadrate (Fig. 2K). The lateral margin of the columellar process is reduced to a thin ridge (Fig. 2L). There is no stapedial foramen present.
Dermophiidae (Figs 3, S4–S6)
In dermophiids the main body of the sphenethmoid accounts for 30% or less of the total length of the sphenethmoid (Fig. 3A). The short lateral wall of its main body is capped by a broad sutural surface (less broad in Geotrypetes seraphini) that receives the anterolateral lappet of the parietal (Fig. S5B). The anterolateral corner bears an ossified anterolateral process (Fig. 3B), except for Geo. seraphini in which only a small anterolateral expansion is present (Fig. S5B). The dorsomedial process is thick and long, extending well beyond the anterior limit of the lateral walls of the os basale (Fig. 3A). The process is variably exposed dorsally in specimens of Dermophis mexicanus (Fig. S4B), a portion is exposed in Geo. seraphini (Fig. S5B) and Schistometopum thomense (Fig. S6B), and the dorsal exposure of the sphenethmoid in Gymnopis multiplicata corresponds to a region over the nasal septum (Fig. 3B). The posterior margin of the lateral wall of the sphenethmoid is deeply incised by the optic foramen in all dermophiids. The posterior margin of the floor of the main body of the sphenethmoid is deeply incised at the midline, but otherwise the floor is quite broad and only slightly concave (Fig. 3B). Geotrypetes seraphini is distinct amongst dermophiids in the presence of a deeply concave posterior margin of the floor (Fig. S5B).
The ossified portion of the nasal septum is long, and reaches the posterior limit of the external naris. The dorsal surface of the nasal septum is broad near its base (less broad in Geo. seraphini), and tapers to a thin ridge towards the tip (Fig. 3B). The height of the nasal septum remains roughly uniform throughout its length (Fig. 3A). Long, tubular sola nasi extend anteriorly from the main body of the sphenethmoid (Fig. 3B, C); those in Geo. seraphini are less robust (Fig. S5C) and those in Sc. thomense are connected to the nasal septum via a thin ventral sheet of bone (Fig. S6C).
The anterior wall contains paired dorsal and ventral foramina (Fig. 3D, E). The foramina of the dorsal pair are located close to the midline, and those of the ventral pair are offset laterally from the midline. The dorsal foramina lead to short canals before exiting onto the anterior surface of the sphenethmoid. The ventral pair leads to the tubular sola nasi. A single anterolateral foramen is present in the base of each anterolateral process (Fig. 3D, E) in dermophiids. An additional small foramen is also present in the anterolateral expansion of Geo. seraphini (Fig. S5D).
The antotic wall of the os basale is orientated parallel (angled slightly anteromedially in Geo. seraphini) to its contralateral wall in dermophiids (Fig. 3B). In anterior view the wall is vertical (Fig. 3F), except for Geo. seraphini in which it is angled slightly dorsolaterally. The dorsal sutural surface is narrow anteriorly and expands dramatically at the level of the antotic foramina into a broad surface in all species, except for Geo. seraphini (Fig. S5B) in which it remains narrow. The surface is continuous with the sutural surface along the anterior margin of the otic capsule. The anterior margin of the antotic wall is deeply incised by the posterior margin of the oval optic foramen; in Geo. seraphini it is posteroventrally obliquely so (Fig. S5B). Five foramina are present in the antotic wall of all dermophiids (Fig. 3H), except for Geo. seraphini in which there are three (Fig. S5H). The difference occurs because the two trunks of the trigeminal and the ventral vein all exit a large common foramen in Geo. seraphini (referred to here as Pattern 3), but discrete foramina are present in the other dermophiid species (referred to here as Pattern 1).
The dorsal surface of the otic-occipital complex of the os basale is posteroventrally tilted, tapered towards the midline (Fig. 3A, B). The anterior portion bears the sutural surface that receives the parietal, which is broad, except for Geo. seraphini. A laterally extending shelf, continuous with the dorsal surface, is present dorsal to the fenestra vestibuli in all dermophiids except Geo. seraphini (Fig. S5B). The fenestra vestibuli is elongate anteroposteriorly. Its ventral margin is thick in D. mexicanus and Gy. multiplicata (Fig. 3A). It opens in the lateral direction and only gently incises the lateral margin of the otic-occipital complex when viewed posteriorly (Fig. 3G), except for D. mexicanus in which it does so very deeply (Fig. S4G). In lateral view the occipital condyle projects far beyond the posterior limit of the otic capsule (Fig. 3A), and the jugular foramen is visible in its base. The foramen magnum is large and subcircular (Fig. 3G).
The medial wall of the otic capsule is perforated by six to eight foramina. Four of these are common to those of all species (endo- and perilymphatic foramina, and anterior and posterior vestibulocochlear nerve foramina; Maddin, 2011). Variably within the family two to four additional foramina transmit portions of the medial branch of the vestibulocochlear nerve (Fig. 3H).
The anterior margin of the floor of the os basale tapers dramatically to a thin, rod-like tip in D. mexicanus (Fig. S4C) and Gy. multiplicata (Fig. 3C), but not the other species (Figs S5C, S6C). The floor extends far anteriorly, terminating in the anterior half of the nasal septum (Fig. 3C), except for Geo. seraphini in which it just barely underlaps the nasal septum (Fig. S5C). The floor of the os basale is smooth and slightly concave dorsally, and a well-defined median depression is present in the posterior region of the floor (Fig. 3B). A well-developed basicranial articulation, complete with an ovoid sutural surface, is present in all dermophiids (Fig. 3B); however, those in Geo. seraphini are distinctive in their tab-like appearance (Fig. S5C). In ventral view the lateral margin of the floor, just posterior to the basicranial articulation, is slightly constricted (arrowhead; Fig. 3C), except in Sc. thomense in which it is strongly constricted (arrowhead; Fig. S6C). A well-developed ventral wing-like projection is present on the ventral surface of the otic capsule, marking the lateral limit of the ventral muscle attachment site (Fig. 3A, C). In D. mexicanus and Gy. multiplicata the posterior margin of the floor terminates at transverse ridge that does not closely approach the foramen magnum (Fig. 3C). In Geo. seraphini and Sc. thomense the floor terminates at a rounded point, the apex of which closely approaches the foramen magnum (Fig. S5C).
The foramen for the carotid artery that leads to a canal in the floor of the os basale is located just anterior to the otic capsule (Fig. 3C). Its lateral and medial exit points are located ventral to the midpoint of the antotic foramina, on either side of the antotic wall.
The footplate of the stapes is elongate anteroposteriorly (Fig. 3I, J), and is fairly tall in dermophiid species, except for Geo. seraphini (Fig. S5I, J). Its long axis is orientated horizontally to slightly anteroventrally inclined. The anterior margin of the footplate is straight and closely applied to the margin of the fenestra vestibuli, and the rest closely approach its margins, except for Geo. seraphini in which the footplate is somewhat inset from the margin of the fenestra. The medial surface of the footplate is strongly concave. The columellar process is short and robust. It extends anteriorly from the footplate in roughly the horizontal plane. There is no foramen in the base of the columellar process (Fig. 3L).
Herpelidae (Figs 4, S7–S8)
The main body of the sphenethmoid accounts for roughly 30% of the total length of the sphenethmoid in herpelids (Fig. 4A). The lateral wall of the main body is short, and it is capped by a moderately broad sutural surface that receives an anterolateral lappet of the parietal (Fig. 4B). An ossified thin, rod-like anterolateral process is present in herpelids, although it is variably developed in the specimens of He. squalostoma (Fig. S8B). The dorsomedial process is broad. In Boulengerula boulengeri and He. squalostoma the process is exposed dorsally (as well as a region over the nasal septum) and reaches the level of the antotic wall of the os basale. It extends well beyond the antotic wall in He. squalostoma (Fig. S8A). In lateral view the posterior margin of the lateral wall of the main body of the sphenethmoid is deeply incised by the optic foramen (Fig. 4A). In He. squalostoma the outline of the foramen is anterodorsally oblique (Fig. S8A). The posterior margin of the floor is moderately to gently concave in herpelids (Fig. 4B).
The nasal region is divided by a long nasal septum (Fig. 4A), which is dorsoventrally short, and tapers in height towards the tip of the snout (Fig. 4A). In the species of Boulengerula it terminates in a small rounded tip (Figs 4A, S7A). The tip of the nasal septum closely approaches the anterior extent of the snout, with only a small gap separating it from the nasopremaxilla. The sutural surface over the nasal septum tapers to a thin, blade-like surface (Fig. 4B). A thin shelf of bone extends laterally that forms a shelf that covers the ventral olfactory foramen dorsally and medially (weakly developed in He. squalostoma, along with a small shelf ventral to the olfactory foramen). This appears to differ from the solum nasi of other species.
The anterior wall contains paired dorsal and ventral foramina in He. squalostoma (Fig. S8E), but only a single dorsal foramen and paired ventral foramina in the species of Boulengerula (Figs 4E, S7E). The dorsal foramen divides distally into the left and right foramina. The ventral pair of foramina is located close to either side of the midline, except for Boulengerula taitana in which they are more laterally placed (Fig. S7E). The anterolateral foramen is present in the base of each anterolateral process (Fig. 4D, E).
In dorsal view the antotic wall of the os basale of herpelids is bowed laterally at the level of the antotic foramina and is roughly parallel to the contralateral antotic wall anteriorly (Fig. 4B). In anterior view the antotic wall of the os basale is orientated slightly dorsolaterally (Fig. 4F). The dorsal sutural surface is slender for its entire length (Fig. 4B). The anterior margin of the antotic wall is incised by the posterior margin of the oval optic foramen (Fig. 4A), more deeply so in He. squalostoma (Fig. S8A). The antotic wall contains three or four foramina (Fig. 4H). This is referred to here as Pattern 5. The two trunks of the trigeminal nerve exit through their own large foramina. The facial nerve, along with a ventral vein, exit through an elongate foramen located posterior and ventral to the larger two (Maddin, 2011). Variably an additional branch of the ventral vein is present and exits through a foramen slightly anterior to that for the facial and the other branch of the ventral vein in B. boulengeri (Fig. 4H) and a dorsal vein and foramen is present in B. taitana (Fig. S7H) and He. squalostoma (Fig. S8H).
The dorsal surface of the otic-occipital complex is posteroventrally sloped. The dorsally exposed portion of this surface is broad in the species of Boulengerula (Fig. 4B), but less so in He. squalostoma (Fig. S8B). The fenestra vestibuli is small and has an ovoid outline. It is orientated anteroventrally and is located in the anterior-most portion of the otic capsule. In lateral view the occipital condyle protrudes well beyond the posterior limit of the otic capsules. A large jugular foramen is present, and is visible in lateral view (Fig. 4A).
The medial wall of the otic capsule contains five to seven foramina. These include the four foramina common to all species examined here (endo- and perilymphatic foramina, anterior and posterior vestibulocochlear nerve foramina). Variably within the family one to three additional foramina are present that transmit the medial branch of the vestibulocochlear nerve (Fig. 4H).
The anterior portion of the floor of the os basale is very narrow, and is triangular in outline (Fig. 4C). The floor extends to reach the ventral surface of the nasal septum of the sphenethmoid (Fig. 4C). A well-developed, medial depression is present in the posterior-most region of the floor of the os basale, just ventral to the foramen magnum (Fig. 4B). Lateral to the antotic wall is a small, moderately well-developed basicranial articulation (Fig. 4A). In ventral view the lateral margin of the floor of the os basale is moderately to weakly constricted medially, posterior to the basicranial articulation (arrowhead; Fig. 4C). A ventral projection is present on the ventral surface of the otic capsule. The posterior extent of the floor of the os basale terminates at a median point in B. boulengeri (Fig. 4C) and a more rounded point in the other species. Only in B. taitana (Fig. S7C) does the apex of the point approach the margin of the foramen magnum.
The foramen that leads to a canal for the carotid artery enters the ventral surface of the os basale (Fig. 4C). This foramen is in a much more posterior and lateral location in B. boulengerula than in the other herpelids. Anteriorly the canal divides into lateral and medial canals, which in B. taitana and He. squalostoma terminate at foramina located ventrally to the trigeminal antotic foramina (and more posteriorly so in B. boulengerula).
The footplate of the stapes is elongate anteroposteriorly (Fig. 4I, J) and its long axis is inclined anteroventrally. The anterior margin of the footplate bears a large articular facet for contact with the margin of the fenestra vestibuli in the species of Boulengerula (Fig. 4J). The medial surface of the footplate is strongly concave. The columellar process is long with a distal ridge (Fig. 4I). A foramen pierces the base of the columellar process (Fig. 4I).
Ichthyophiidae (Figs 5, S9–S11)
The main body of the sphenethmoid is short in ichthyophiids, accounting for less than half to roughly 30% of the total sphenethmoid length (Fig. 5A). The lateral wall of the main body is capped by a variably broad sutural surface that receives the frontal only in Cau. asplenia, or both the frontal and parietal in the other species. The anterolateral corner bears a very broad ossified process in all ichthyophiids (Fig. 5B) except for Cau. asplenia in which there is only a slight expansion (Fig. S9B). A thin dorsomedial process is present that is not exposed dorsally. The process does not extend beyond the level of the lateral wall of the sphenethmoid (Fig. 5B). The posterior margin of the lateral wall is slightly incised by the optic foramen in all species (Fig. 5A), except for Uraeotyphlus narayani in which it is more deeply so (Fig. S11A). The posterior margin of the floor of the sphenethmoid is deeply incised, exposing a great area of the os basale ventral to it (Fig. 5B). In Cau. asplenia and the species of Ichthyophis there is a median extension of the margin of the floor (not visible here).
The nasal septum is tall and blade-like (Fig. 5A). The dorsal sutural surface is uniformly narrow, except for U. narayani in which it is slightly broader at the base (Fig. S11B). The nasal septum tapers in height distally, and terminates posterior to the external naris. Sola nasi are present, except for U. narayani. Additionally in U. narayani a pair of anterolaterally projecting flanges extends from the ventral surface of the nasal septum (Fig. S11C).
The dorsal pair of anterior foramina is not confined within the wall of the sphenethmoid in Cau. asplenia and the species of Ichthyophis (Fig. 5E) and form troughs instead. The ventral foramina are located somewhat laterally from the midline (Fig. 5E), except for U. narayani in which they are much closer to the midline (Fig. S11E). A single anterolateral foramen is present in the anterolateral corner of the main body (Fig. 5E).
In dorsal view the antotic wall is angled towards the midline anteriorly and is vertical in anterior view. The dorsal surface is capped by a narrow sutural surface. In the species of Ichthyophis and U. narayani the surface expands slightly at the level of the antotic foramina before becoming continuous with the surface along the anterior margin of the otic-occipital (Fig. 5B). The anterior margin of the antotic wall is weakly to deeply incised by the posterior margin of the optic foramen (Fig. 5A). The antotic region is perforated by three foramina, constituting Pattern 4. Both trunks of the trigeminal nerve plus the abducens nerve exit through a large subcircular foramen (Maddin, 2011). A dorsal vein exits through a foramen posterior and dorsal to the large foramen (except for U. narayani), and the facial nerve and a ventral vein exit through a common foramen just posterior and slightly ventral to the large foramen (Fig. 5H).
The dorsal surface of the otic-occipital complex of the os basale is tilted slightly posteroventrally. It tapers towards the midline and bears an anterior sutural surface for the parietal (except for Cau. asplenia). A laterally extending shelf, located dorsal to the fenestra vestibuli, is present (Fig. 5A). The fenestra vestibuli is elongate anteroposteriorly, and in posterior view it incises the lateral margin of the otic-occipital complex to varying degrees amongst ichthyophiid species (Fig. 5G). A large articular facet receives the footplate of the stapes. The occipital condyle projects only slightly posterior to the otic capsule, and its margin is continuous with that of the dorsal surface of the complex when viewed laterally. The jugular foramen is present in the base of the condyle, but it is only partially visible in lateral view (Fig. 5A). The foramen magnum is circular in outline (Fig. 5G).
The medial wall of the otic capsule is perforated by seven or eight foramina (Fig. 5H). Four foramina common to all species examined here (endo- and perilymphatic foramina, and anterior and posterior vestibulocochlear nerve foramina) are present in similar locations. Variably within the family three or four small foramina transmit the medial branch of the vestibulocochlear nerve (Maddin, 2011).
The anterior margin of the floor of the os basale comes to a rounded point in Cau. asplenia and the species of Ichthyopis (Fig. 5C), and is pointed in U. narayani (Fig. S11C). Anteriorly it extends only to the level of the main body of the sphenethmoid and does not pass beneath the nasal septum in Cau. asplenia and the species of Ichthyophis; however, it reaches to within the anterior half of the nasal septum in U. narayani. The posterior median depression is weakly developed except for U. narayani in which it is well defined. An expansion of the floor of the os basale corresponds to the position of the basicranial articulation (Fig. 5A, B), but the latter are not well developed. There is little to no constriction of the lateral margin of the floor when viewed ventrally, and there is no wing-like projection ventral to the otic capsules (Fig. 5A, C), except for U. narayani (Fig. S11A,C). The posterior margin of the floor terminates in a rounded point (Cau. asplenia and U. narayani) or arched ridge (Ichthyophis) that extends almost to the ventral margin of the foramen magnum.
The carotid artery enters the floor through a foramen just anterior to the otic capsule (Fig. 5C). It passes only a short distance anteriorly before dividing into lateral and medial canals, which terminate at foramina just anterior to the otic capsule on either side of the antotic walls.
The footplate of the stapes is elongate anteroposteriorly and its long axis is orientated roughly horizontally (Fig. 5I, J). Its anterior margin is thickened and closely applied to the anterior margin of the fenestra vestibuli. The remaining margins of the footplate closely approach the fenestra margins, except in U. narayani in which the stapes is extensively inset. In Ic. beddomei there is a dorsal process on the footplate not seen in other species (Fig. S10I). The columellar process is robust and long in ichthyophiids. It is pierced by a foramen, except for U. narayani in which the stapes is imperforate.
Indotyphlidae (Figs 6, S12–S14)
The main body accounts for roughly one third or less than the total length of the sphenethmoid (Fig. 6A). The lateral wall of the main body is short and is capped by a narrow to moderately broad dorsal sutural surface (Fig. 6B). An ossified anterolateral process is present in all indotyphlid species except for Id. russeli (Fig. S14B). The dorsomedial process is broad and long, reaching beyond the level of the antotic wall, in all species except for Id. russeli. In all species the process is not exposed dorsally (dorsal exposure in Id. russeli corresponds to the nasal septum region). The posterior margin of the lateral wall is deeply incised by the anterior margin of the oval optic foramen (Fig. 6A). The posterior margin of the floor of the sphenethmoid is shallowly concave (more so in Grandisonia alternans) and deeply incised by a slit-like opening at the midline.
The nasal region is divided by a long nasal septum that is short dorsoventrally. The nasal septum is tall near its base, but tapers in height to a relatively short profile at its tip. The dorsal sutural surface tapers from being very broad over the anterior portion of the main body to being a thin ridge near its tip, resulting in an overall very triangular shape to the surface (Fig. 6B). The ventral surface also tapers towards the tip and forms a narrow shelf below the openings for the ventral branch of the olfactory nerve (Fig. 6C). The shelf is especially broad in Gr. alternans (Fig. S12C) and no shelf is present in Id. russeli (Fig. S14C). Sola nasi are absent.
The anterior wall is perforated by the paired dorsal and ventral foramina (Fig. 6D, E). The ventral pair is located close to the midline in Id. russeli (Fig. S14E), further away from the midline in Hypogeophis rostratus (Fig. S13E) and Gegeneophis ramaswami (Fig. 6E), and even further in Gr. alternans (Fig. S12E). Both the dorsal and ventral foramina lead to short canals before exiting on the anterior surface of the sphenethmoid. The anterolateral foramen pierces the anterolateral corner (Fig. 6D).
The antotic wall of the os basale is orientated towards the midline anteriorly when viewed dorsally (Fig. 6B). It is also vertical to subvertical amongst indotyphlid species when viewed anteriorly (Fig. 6F). The dorsal sutural surface is very narrow (Id. russeli) to very broad (Hyp. rostratus), and it is continuous posteriorly with the sutural surface along the anterior margin of the otic capsule, except for Id. russeli in whichthe parietal sutural surface is lacking (Fig. S14B). A well-developed sutural surface for the quadrate is present in the posterodorsal region of the antotic wall of all indotyphlid species (Fig. 6A). The anterior margin of the antotic wall is moderately incised by the posterior margin of the oval optic foramen (Fig. 6A). Five foramina with a pattern like that of dermophiids, plus one unidentified, are present in Id. russeli (referred to here as Pattern 1; Fig. S14H). Four foramina are present in Geg. ramaswami (Fig. 6H), resembling the condition of dermophiids except for the common exit of the two trunks of the trigeminal (referred to here as Pattern 2). A single foramen for all antotic structures is present in the antotic wall of Gr. alternans (Fig. S12H) and Hyp. rostratus (referred to here as Pattern 8; Fig. S13H).
The dorsal surface of the otic-occipital complex of the os basale is strongly tilted posteroventrally, and tapers in width towards the midline (Fig. 6A). A broad portion of this surface is exposed dorsally in Gr. alternans and Hyp. rostratus (Fig. S12B). A short diagonal ridge extends anterodorsally just dorsal to the fenestra vestibuli and the fenestra is strongly anteriorly facing (Fig. 6A, G). In lateral view the occipital condyle projects well beyond the posterior limit of the otic capsule, and the large jugular foramen is visible in lateral view (Fig. 6A). The foramen magnum is circular to diamond-shaped.
The medial wall contains the four foramina in their common locations (endo- and perilymphatic foramina, anterior and posterior vestibulocochlear nerve foramina) that are present in all species examined (Fig. 6H). Variably within the family two or three additional foramina that are located along the ventral margin of the otic capsule transmit the medial branch of the vestibulocochlear nerve (Maddin, 2011).
The anterior portion of the floor of the os basale is triangular in outline (Fig. 6C). It extends roughly halfway along the length of the nasal septum, except for Id. russeli in which it extends to within the anterior half of the nasal septum (Fig. S14C). The median posterior depression is moderately well defined (Fig. 6B, F). Lateral to the antotic wall, a relatively well-developed basicranial articulation is present, complete with an ovoid sutural surface (Fig. 6A, B). In ventral view the lateral margin of the floor of the os basale is strongly constricted posterior to the basicranial articulation (arrowhead; Fig. 6C). The constriction reaches to the level of the foramen that transmits the carotid artery. A ventral wing-like projection is present on the ventral surface of the otic capsule, except for Id. russeli (Fig. S14C). The posterior margin of the floor of the os basale terminates at a sharp or rounded point that closely approaches the ventral margin of the foramen magnum (Fig. 6C).
The foramen that leads to a canal in the floor of the os basale for the carotid artery is located just anterior to the otic capsule (Fig. 6C). It bifurcates anteriorly into lateral and medial canals, which terminate in foramina located at roughly the same level, ventral to the large antotic foramen, on the lateral and medial sides of the antotic walls (Fig. 6H).
The footplate of the stapes is subcircular in Geg. ramaswami (Fig. 6I, J) and Id. russeli (Fig. S14I, J) and more elongate in Gr. alternans (Fig. S12I, J) and Hyp. rostratus (Fig. S13I, J). The long axis is roughly horizontally orientated. It is closely applied to the anterior margin of the fenestra vestibuli, and the remaining margins closely approach the margins of the fenestra vestibuli. The medial surface is strongly concave. The columellar process is very short with a lateral ridge, which is absent in Id. russeli. There is no foramen in the base of the columellar process.
Rhinatrematidae (Figs 7, S15)
The main body is relatively long, accounting for just under half the total length of the sphenethmoid (Fig. 7A). The lateral wall of the main body is long, and is capped by a broad sutural surface that receives the parietal (Fig. 7A). A broad ossified anterolateral process is present, and in E. bicolor it is connected medially to the anterior dorsal sutural surface (Fig. 7B). The dorsomedial process is thin and rod-like (Fig. 7B), and is completely covered dorsally. The posterior margin of the lateral walls is deeply incised (less so in R. bivittatum) by the anterior margin of the optic foramen (Fig. 7A). The posterior margin of the floor is also deeply concave and U-shaped in outline (Fig. 7B).
The nasal septum is moderately long, terminating posterior to the external naris. It remains uniform in height distally (Fig. 7A). The dorsal sutural surface is narrow near its base and expands towards the tip (Fig. 7A). The ventral surface also maintains a broad sutural surface as far as the tip of the nasal septum. A broad sheet of bone forms a shelf ventral to the exit points of the ventral branch of the olfactory nerve, connecting the sola nasi to the nasal septum (Fig. 7B,C).
The foramina in the anterior wall are paired, and the foramina of the ventral pair are located slightly more laterally from the midline than the dorsal pair. A single anterolateral foramen is present in the base of each expanded anterolateral process (Fig. 7E).
The wall is parallel to subparallel to the contralateral wall when viewed dorsally (Fig. 7B). In anterior view the antotic wall is vertically orientated (Fig. 7F). Posteriorly the dorsal sutural surface is expanded slightly, and is continuous with a portion of the surface on the anterior margin of the otic capsule. The anterior margin of the antotic wall is deeply incised by the posterior margin of the oval optic foramen (Fig. 7A), less so in R. bivittatum (Fig. S15A). Five foramina pierce the antotic wall (Fig. 7H). These have the same configuration, and transmit the same structures as those seen in the dermophiid species (referred to here as Pattern 1; Maddin, 2011). An additional foramen just ventral to that for the dorsal vein is present in E. bicolor. Histological data reveal that this additional foramen transmits a vein. This foramen is absent on the right side of the head in BM 1946.9.5.66 (Fig. 7H).
The dorsal surface of the otic-occipital complex is nearly horizontal, and forms the entire occipital surface (the parietal does not contribute; Fig. 7A). It tapers dramatically in width towards the midline (Fig. 7A). A sutural surface that receives the parietal is present along the anterior margin of the surface, but narrows substantially at the midline and is absent at the midline in R. bivittatum (Fig. S15A). A process extending laterally, just anterior and dorsal to the fenestra vestibuli, is present but poorly developed in E. bicolor (Fig. 7B). The region occupied by the inner ear is large and fills almost the entire extent of the otic-occipital complex in lateral view. The fenestra vestibuli is large and slightly ovoid (Fig. 7A). In posterior view the lateral edges of the otic-occipital complex are incised by the fenestra vestibuli (Fig. 7G). The occipital condyle is small, and in lateral view it is almost level with the outline of the posterior margin of the otic-occipital complex (Fig. 7A). The jugular foramen in the base of the condyle is only partially visible in lateral view. The foramen magnum is large and subcircular in outline (Fig. 7G).
The medial wall of the otic capsule is perforated by eight foramina (Fig. 7H). The four foramina common to all species are present in their usual locations (endo- and perilymphatic foramina, anterior and posterior vestibulocochlear nerve foramina). Four small foramina conducting components of the medial branch of the vestibulocochlear nerve occur along the ventral margin (Maddin, 2011).
The anterior portion of the floor of the os basale is triangular in outline. It is relatively very long, extending to the region of the anterior half of the nasal septum. A thin medial ridge that fits into a groove in the ventral surface of the sphenethmoid is present. The floor of the os basale is nearly flat and the median posterior depression is only weakly defined (Fig. 7B). A well-developed basicranial articulation is absent from rhinatrematids (Fig. 7A, C), and only a narrow shelf lateral to the antotic wall is all that represents the articulation. In ventral view there is no constriction of the lateral margin of the floor posterior to the basicranial articulation (arrowhead; Fig. 7C). A very weak ridge is present instead of a wing-like ventral projection on the ventral surface of the otic capsule (Fig. 7A). Posteriorly the ventral surface of the floor of the os basale terminates in a rounded point that closely approaches the ventral margin of the foramen magnum (Fig. 7C).
The foramen that leads to the canal carrying the carotid artery enters the ventral surface of the floor of the os basale, ventral to the anterior limit of the otic capsule (Fig. 7C). The canal terminates at lateral and medial foramina located just ventral to the trigeminal foramina (Fig. 7H).
The footplate of the stapes is large and ovoid in rhinatrematids (Fig. 7I, J), and its anterior margin is closely applied to the anterior margin of the fenestra vestibuli. The remaining margins nearly fill the fenestra. The medial surface of the footplate is strongly concave. The columellar process is short, rod-like, and extends anteriorly in roughly the horizontal plane. The base of the columellar process is pierced by a foramen (Fig. 7L). This foramen is variably present in one specimen (FMNH 152310), being present on the right and absent from the left.
Scolecomorphidae (Figs 8, S16-S17)
The main body of the sphenethmoid is long and accounts for roughly half the total length of the sphenethmoid in scolecomorphids (Fig. 8A). The wall is capped by a narrow sutural surface that receives the parietal posteriorly and frontal anteriorly (Fig. 8B). A long, ossified anterolateral process extends toward the medial surface of the prefrontal (Fig. 8B). A very small dorsomedial process is present in Cr. lamottei, but a slightly longer one is present in the species of Scolecomorphus. In all scolecomorphids the process is completely covered dorsally. The posterior margin of the long lateral wall is only slightly incised by the anterior margin of the small optic foramen (Fig. 8A). The floor of the sphenethmoid is deeply incised anteriorly, and is further incised at the midline by a thin slit (Fig. 8B).
The nasal region is divided by a tall, blade-like nasal septum (Fig. 8A) that extends anterior to the posterior limit of the external naris. The dorsal sutural surface is broad at its base, but tapers distally, producing a thin ridge-like dorsal surface along most of its length (Fig. 8B). Ventrally the nasal septum is also very thin. Long sheet-like sola nasi are present in the species of Scolecomorphus (Fig. S16B). These are weakly developed in Cr. lamottei (Fig. 8B).
Paired anterior foramina are present. The ventral pair is more laterally located from the midline in Sc. vittatus (Fig. S17E) than those of Scolecomorphus kirkii (Fig. S16E) and Cr. lamottei (Fig. 8E). A single anterolateral foramen pierces the base of each anterolateral process (Fig. 8D).
The antotic wall of the os basale is angled towards the midline anteriorly (Fig. 8B), except for Sc. vittatus in which it is roughly parallel to the contralateral wall (Fig. S17B). When viewed anteriorly the wall is dorsolaterally orientated, and is smoothly continuous with the floor of the os basale (Fig. 8F). The dorsal sutural surface is narrow to moderately broad, as in Sc. vittatus. It expands slightly posteriorly and is continuous with the sutural surface along the anterior margin of the otic capsule (Fig. 8B). The anterior margin of the antotic wall is deeply incised in Cr. lamottei (Fig. 8A), but only very weakly incised in the species of Scolecomorphus (Fig. S16A), by the posterior margin of the optic foramen. The antotic foramina of Cr. lamottei are of the pattern referred to here as Pattern 5, with an additional dorsal foramen for a vein present (Fig. 8H). Two, and variably a third, foramina are present in the antotic wall of Scolecomorphus (Fig. S16H). This is referred to here as Pattern 7.
The dorsal surface of the otic-occipital complex of the os basale is only slightly tilted posteroventrally. Only a very thin region is posteriorly exposed in dorsal view near the midline; the remainder forms the sutural surface that receives the parietal (Fig. 8B). A pointed ridge is present on the anterolateral surface of the otic capsule in Cr. lamottei (Fig. 8A), but not Scolecomorphus. In lateral view the occipital condyle only slightly projects beyond the posterior limit of the otic capsule (Fig. 8A), and the jugular foramen is partially visible in lateral view. An additional, smaller foramen is present just posterior to the jugular foramen in Cr. lamottei (Fig. 8C).
The medial wall contains the four foramina (Fig. 8H) in their common locations (endo- and perilymphatic foramina, and the anterior and posterior vestibulocochlear nerve foramina), found in all species examined here. Variably within the family two or three additional foramina transmit components of the medial branch of the vestibulocochlear nerve (Maddin, 2011).
The anterior portion of the floor of the os basale is triangular in outline (Fig. S16C), except for Cr. lamottei in which it is very thin and rod-like (Fig. 8C). In all scolecomorphids the floor reaches to contact the nasal septum. The floor is concave rather than flat. The posterior median depression is weakly defined. In lateral view the floor projects far ventral to the ventral limit of the otic capsules (Fig. 8A). The basicranial articulation is a knob-like projection capped with cartilage. In ventral view there is no constriction of the lateral margin of the floor posterior to the basicranial articulation (arrowhead; Fig. 8C). A ventral wing-like projection is absent from the ventral surface of the otic capsule. The posterior margin of the floor is represented by a weakly expressed, transverse boundary that does not approach the ventral margin of the foramen magnum (Fig. 8C).
The foramen that leads to a canal that conducts the carotid artery is located more anteriorly, relative to the otic capsule, than is the case for most other species (Fig. 8C). The canal terminates at lateral and medial foramina located anterior to the antotic foramina (Fig. 8H).
The stapes is absent from scolecomorphids.
Siphonopidae (Figs 9, S18)
The sphenethmoid is substantially more robust in Siphonops annulatus (Fig. 9A) in comparison to Microcaecilia albiceps (Fig. S18A). The main body of the sphenethmoid is relatively short and broad in siphonopids (Fig. 9A), and accounts for less than half of the total length of the sphenethmoid. An expansion is present representing the only ossified portion of the anterolateral processes, but it is weakly developed (Fig. 9B). The dorsomedial process is thin and covered dorsally in M. albiceps (Fig. S18B) and robust and exposed in Si. annulatus (Fig. 9B). In both species the process extends beyond the anterior limit of the lateral walls. The posterior margin of the lateral wall is deeply incised by the anterior margin of the optic foramen (Fig. 9A). The posterior margin of the floor is incised at the midline and concave in outline (Fig. 9B), more so in M. albiceps (Fig. S18B).
The nasal region is divided by a moderately long nasal septum, which is tall in M. albiceps (Fig. S18A) and short in Si. annulatus (Fig. 9A). It terminates as a tall process just posterior to the external naris. The dorsal sutural surface tapers towards the tip, and for the majority of its length the dorsal margin is thin and blade-like (Fig. 9B). The ventral surface is similarly thin for most of its length. A pair of well-developed, tubular sola nasi extends from the anterior surface of the main body (Fig. 9C).
The anterior wall is pierced by two pairs of foramina (Fig. 9E). The ventral pair is displaced somewhat laterally from the midline (Fig. 9E). The dorsal foramina lead to short canals before exiting onto the anterior surface of the sphenethmoid. The anterolateral foramina are incomplete laterally (Fig. 9D).
The antotic wall of the os basale is slightly angled towards the midline anteriorly when viewed dorsally (Fig. 9B), more so in M. albiceps (Fig. S18B). In anterior view the wall is vertical in Si. annulatus (Fig. 9F), but it is dorsolaterally inclined in M. albiceps (Fig. S18F). The dorsal sutural surface is very narrow; however, there is a large expansion over the antotic region in Si. annulatus (Fig. 9B). The anterior margin of the antotic wall is moderately incised by the posterior margin of the oval optic foramen (Fig. 9A). Five foramina perforate the antotic wall (Fig. 9H), and these have the same configuration and transmit the same structures as those of rhinatrematids and dermophiids (referred to here as Pattern 1; Maddin, 2011). The two large foramina that transmit the two trunks of the trigeminal nerve are incompletely differentiated in the specimens of M. albiceps examined (Fig. S18H), possibly indicating immaturity.
The dorsal surface of the otic-occipital complex is tilted posteroventrally and remains broad in Si. annulatus (Fig. 9B), but tapers in width towards the midline to a narrow surface in M. albiceps (Fig. S18B). The fenestra vestibuli is elongate anteroposteriorly (Fig. 9A), and is especially small in Si. annulatus (Fig. 9A). In posterior view the fenestra vestibuli only slightly incises the lateral margin of the otic-occipital complex (Fig. 9G). The occipital condyle projects far beyond the posterior limit of the otic capsule and the large jugular foramen is visible in lateral view (Fig. 9A). The foramen magnum is subcircular in outline (Fig. 9G).
The medial wall of the otic capsule is pierced by four foramina (endo- and perilymphatic foramina, anterior and posterior vestibulocochlear nerve foramina), in the common locations found in all species examined here (Fig. 9H). Variably within the family two or three additional foramina are present along the ventral margin of the otic capsule for the transmission of parts of the medial branch of the vestibulocochlear nerve (Maddin, 2011).
The anterior margin of the floor of the os basale tapers continuously to a point towards the tip, anterior to an abrupt constriction in Si. annulatus (Fig. 9C). The tip extends to contact the ventral surface of the nasal septum. A median posterior depression is present and well defined (Fig. 9F). Lateral to the antotic wall is the well-developed basicranial articulation (Fig. 9A). In ventral view the lateral margin of the floor of the os basale is slightly constricted medially, towards the level of the carotid foramina (arrowhead; Fig. 9C), and slightly more so in M. albiceps (Fig. S18C). A weak ventral projection is present on the ventral surface of each otic capsule, although this is very poorly developed in M. albiceps (Fig. S18C). The posterior margin of the floor of the os basale terminates at a rounded point, the apex of which closely approaches the ventral margin of the foramen magnum.
The foramen leading to the canal for the carotid artery enters the floor of the os basale anterior to the otic capsule (Fig. 9C) and bifurcates, resulting in lateral and medial canals. The canals terminate in foramina located ventral to the antotic foramina.
The footplate of the stapes is elongate anteroposteriorly, and its long axis is orientated slightly anteroventrally as in Si. annulatus (Fig. 9I, J) to horizontally (Fig. S18I, J). It is also very small and bears a large anterior articular surface in Si. annulatus. The columellar process of the stapes extends roughly horizontally from the lateral surface of the footplate. A weak ridge is present on the process of M. albiceps towards its distal tip (Fig. S18I). There is no foramen present in the base of the columellar process.
Typhlonectidae (Figs 10, S19)
The main body of the sphenethmoid is short and broad in typhlonectids (Fig. 10A). It accounts for roughly half the length of the entire sphenethmoid, which may be more the result of a very short nasal septum region. The lateral wall of the main body is short and bears a very large, broad sutural surface (Fig. 10B), less so in Typhlonectes natans (Fig. S19B). There is an expanded anterolateral corner in Chthonerpeton indistinctum (Fig. 10B) and a thin process in T. natans (Fig. S19B). The dorsomedial process is extremely truncated in Ch. indistinctum, and otherwise short and rod-like in T. natans. In both species it is covered dorsally. The posterior margin of the lateral wall is deeply incised by the anterior margin of the optic foramen (Fig. 10A). The floor of the sphenethmoid is broad and only gently concave in Ch. indistinctum but deeply so in T. natans. No medial incision is present in either species. The ventral surface of the sphenethmoid bears a groove for the palatal ramus of the facial nerve (Fig. 10C).
The nasal septum is very short in typhlonectids, terminating close to the level of the tentacular aperture, rather than at the external naris as it does in most species examined here. It is covered dorsally by a broad sutural surface in Ch. indistinctum (Fig. 10B), and by a narrower surface in T. natans (Fig. S19B). The ventral margin bears a broader sutural surface, and a pair of thin sheet-like processes extends anterolaterally from the surface, in Ch. indistinctum (Fig. 10C). No sola nasi are present in typhlonectids.
The anterior foramina of Ch. indistinctum exhibit a unique configuration amongst the species examined here. The dorsal and ventral foramina on each side have converged to form a single left and single right foramen for the branches of the olfactory nerve (Fig. 10D, E). Paired dorsal and ventral foramina are present in T. natans. There the ventral pair is large and close to the midline (Fig. S19D, E). The anterolateral foramina are present in the bases of the anterolateral corners of the main body (Fig. 10E).
The antotic wall of the os basale is angled anteromedially when viewed dorsally (Fig. 10B). In anterior view it is slightly dorsolaterally orientated in Ch. indistinctum (Fig. 10F) and more vertical in T. natans (Fig. S19F). The dorsal sutural surface is moderately broad, and is continuous with the sutural surface on the anterior margin of the otic capsule (Fig. 10B). The anterior margin of the antotic wall is slightly incised by the posterior margin of the somewhat oblique optic foramen (Fig. 10A). A single, very large foramen is present in the antotic wall of Ch. indistinctum (Fig. 10H), similar to the condition seen in some indotyphlid species (referred to here as Pattern 8; Maddin, 2011). In T. natans the pattern of foramina (Fig. S19H) is like that of caeciliids (referred to here as Pattern 6).
The dorsal surface of the otic-occipital complex of the os basale is not as steeply inclined posteroventrally as it is in caeciliids. It tapers in width towards the midline (Fig. 10B), and a broad portion of the dorsal surface is exposed dorsally at the midline. The sutural surface that receives the parietal tapers in width towards the midline. The fenestra vestibuli is small, and in one specimen (MW 23) has a unique bilobed outline. The fenestra vestibuli does not incise the lateral margin of the otic capsule when viewed posteriorly (Fig. 10G). The occipital condyle does not project far posteriorly (Fig. 10A) and the jugular foramen is visible in lateral view.
The medial wall of the otic capsule is perforated by four foramina in the common locations (endo- and perilymphatic foramina, anterior and posterior vestibulocochlear nerve foramina) found in all species examined here (Fig. 10H). Variably within the family two or three foramina transmitting components of the medial branch of the vestibulocochlear nerve are present (Fig. 10H).
The anterior portion of the floor of the os basale tapers dramatically to a very narrow point (Fig. 10C). The floor extends to reach the ventral surface of the nasal septum. The posterior depression is also weakly defined (Fig. 10B, F). The basicranial articulation is weakly developed in Ch. indistinctum (Fig. 10A), slightly more so in T. natans (Fig. S19A). In ventral view there is only a slight constriction of the lateral margin of the floor posterior to the basicranial articulation. A ventral wing-like projection is absent from the ventral surface of the otic capsule (Fig. 10A). The posterior margin of the floor terminates at a pointed transverse boundary that does not closely approach the ventral margin of the foramen magnum (Fig. 10C).
The foramen in the floor of the os basale that leads to a canal for the carotid artery enters the floor just anterior to the otic capsule and passes out almost directly into the lateral and medial foramina, both of which are located posterior to the large antotic foramen, on either side of the antotic wall (Fig. 10H).
The stapes of typhlonectids is small and gracile in comparison to that of most species. The long axis of the footplate is orientated slightly anteroventrally. The anterior margin of the footplate does not intimately articulate with the margin of the fenestra vestibuli, but it is embedded in dense connective tissue. The columellar process is depressed and anterodorsally orientated (Fig. 10K). There is no foramen in the base of the columellar process (Fig. 10L).
Morphometric analysis
Twelve linear measurements (Fig. S1), capturing variation of particular aspects of braincase morphology, were taken and regressed against skull length to explore the potential influence of size on each feature. Of the 12 regressions, four revealed outliers and r-squared values of less than 0.80, and are potentially phylogenetically informative (i.e. not autapomorphic).
Dorsomedial process length
The length of the dorsomedial process when regressed against skull length yielded an r-squared value of 0.37. A plot of the residual values of the regression versus the fitted values of the regression line reveals that three species possess a dorsomedial process that is substantially shorter than that predicted by the regression model: T. natans, Ch. indistinctum, and Cr. lamottei (Fig. 11A).

Residual plots from the regression analyses for the four measurements that had low r-squared values in their respective regression plots. Species circled represent those that deviate from the expected value of each measurement predicted by the regression plot. Data point labels correspond to the first letter of the genus followed by the first three letters of the species. A, residuals from the regression plot of dorsomedial process length against skull length, for which Typhlonectes natans (T.natans), Chthonerpeton indistinctum (C.indistinctum), and Crotaphatrema lamottei (C.lamottei) possess a process that is shorter than predicted; B, residuals from the regression plot of nasal septum length against skull length, for which T. natans and Ch. indistinctum possess a septum that is shorter than predicted; C, residuals of the regression plot of lateral wall length against skull length, for which Scolecomorphus kirkii (S.kirkii), Scolecomorphus vittatus, and Cr. lamottei possess walls that are longer than predicted; and D, residuals of the regression plot of occipital condyle protrusion against skull length, for which Epicrionops bicolor (E.bicolor), Rhinatrema bivittatum (R.bivittatum), Ichthyophis beddomei (I.beddomei), Ichthyophis kohtaoensis, Caudacaecilia asplenia (C.asplenia), and Scolecomorphus vittatus (S.vittatus) possess less protruded condyles than predicted.
Nasal septum length
The length of the nasal septum when regressed against skull length yielded an r-squared value of 0.76. Plotting the residual values from the regression versus the fitted values of the regression line reveals that two species possess a nasal septum that is substantially shorter than that predicted by the regression model: T. natans and Ch. indistinctum (Fig. 11B). This is consistent with the descriptions provided here wherein the nasal septum is noted to be relatively short, terminating much more posteriorly relative to the position of the external naris than does that of the other species examined.
Length of the lateral walls of the main body of the sphenethmoid
The length of the lateral walls of the main body of the sphenethmoid when regressed against skull length yielded an r-squared value of 0.59. A plot of the residual values of the regression versus the fitted values of the regression line reveals that three species possess lateral walls of the sphenethmoid that are substantially longer than those predicted by the regression model: Sc. kirkii, Sc. vittatus, and Cr. lamottei (Fig. 11C). This is consistent with the morphology described for these species wherein the lateral walls of the sphenethmoid appear to be relatively longer than those of the other species examined.
Degree of protrusion of the occipital condyles beyond the posterior limit of the otic capsules
The degree of protrusion of the occipital condyles beyond the posterior limit of the otic capsules when regressed against skull length yielded an r-squared value of 0.71. A plot of the residual values from the regression versus the fitted values of the regression line reveals that a number of species possess smaller, less protruding occipital condyles than would be predicted by the regression model (in order from largest to smallest residual value): E. bicolor, R. bivittatum, Ic. beddomei, Ic. kohtaoensis, Cau. asplenia, and Sc. kirkii (Fig. 11D). This result is consistent with the morphology of the occipital condyles described for these species, wherein the condyles are either continuous with the posterior margin of the otic capsule (as in the case of Epicrionops and Rhinatrema) or project only slightly beyond it (as in the case of the others). This is in strong contrast to the more well-developed to strongly protruding condyles observed in the remaining species.
The results of the morphometric analyses provide support for the development of several new morphological characters. These characters are defined quantitatively as proportions of total skull length, because they were shown to vary independently of the latter. Character state boundaries are tentative given the currently known range of variation.
Phylogenetic analysis
Phylogenetic analysis of morphology
A heuristic search of all the morphological characters (including the new braincase and stapes characters) for the original taxon sample plus the eight new species resulted in 733 most parsimonious trees of 314 steps (CI = 0.45, RI = 0.76, RC = 0.35, HI = 0.55). The 50% majority-rule consensus tree reveals a fairly well-resolved topology (CFI = 0.81; Fig. 12A). The basal portion of the tree is congruent with previous hypotheses of relationships (Nussbaum, 1977, 1979; Wilkinson, 1997; Wilkinson & Nussbaum, 2006). Rhinatrema bivittatum and the species of Epicrionops form Rhinatrematidae (Clade B). Uraeotyphlus narayani is the sister taxon to the species of Ichthyophis and Cau. asplenia (Clade F). Together these taxa form Ichthyophiidae (Clade E). At the base of Teresomata Cr. lamottei clusters with the species of Scolecomorphus (Clade I), and together they form Scolecomorphidae (Clade H). One node up He. squalostoma branches off as the sister taxon to Clade K, made up of the remaining teresomatan species. One node up again is a clade containing a sister taxon relationship between species of Caeciliidae (Clade N) and species of Typhlonectidae (Clade M).

The 50% majority-rule trees resulting from the parsimony (A–C) and 95% maximum clade credibility tree resulting from the Bayesian phylogenetic analyses of morphology (D) including the new braincase and stapes characters. A, all characters, all taxa; B, exclusion of certain eye characters (see text for details), yielding strong congruence with family level systematics; C, exclusion of certain eye characters and Sylvacaecilia grandisonae, which yields improved resolution to the family level with little discrepancy with molecular analyses (bootstrap values greater than 50% shown on the nodes); D, Bayesian analysis excluding certain eye characters and Sy. grandisonae, and the resolution of the nine families under this topology (Dermophiidae, Herpelidae, and Siphonopidae are paraphyletic). Node letters correspond to the text. 1, Rhinatrematidae; 2, Ichthyophiidae; 3, Scolecomorphidae; 4, Herpelidae; 5, Typhlonectidae; 6, Caeciliidae; 7, Indotyphlidae; 8, Siphonopidae; and 9, Dermophiidae.
The remaining assemblage consists of species of the families Dermophiidae, Siphonopidae, Indotyphlidae, and the genus Boulengerula (Fig. 12A). In this topology Indotyphlidae is paraphyletic with respect to Dermophiidae plus Siphonopidae, and Dermophiidae is paraphyletic with respect to Siphonopidae because of the position of Geo. seraphini (Fig. 12A). It was noticed that many characters driving the clustering of Boulengerula with Geg. ramaswami were those associated with reduced eyes and the exclusion of these characters (numbers 53 to 58, 60 to 64) restores monophyly to Indotyplidae [not including Sy. grandisonae, which is resolved at a polytomy at the base of Clade M (Fig. 12B)], and Boulengerula falls into a more basal position, just one node up from He. squalostoma (CFI = 0.78). Removal of Sy. grandisonae, which was not scored for braincase or stapes characters and otherwise appears to be unstable in its position, results in a polytomy between the Caeciliidae plus Typhlonectidae clade, the Dermophiidae plus Siphonopidae clade, and the Indotyphlidae clade (Fig. 12C). The results of this tree reveal that seven out of nine of the families are monophyletic, the exceptions being Herpelidae (Fig. 12C, family number 4) and Dermophiidae (Fig. 12C, family number 9). The CFI of this topology is 0.75.
The results of the Bayesian analysis of morphology were summarized as both a 95% maximum clade credibility tree (Fig. 12D) and a 50% majority-rule tree (not shown). The topology of the Bayesian analysis of morphology is overall very similar to that of the parsimony analysis; however, the 95% maximum clade credibility tree is fully resolved (Fig. 12D). The families Dermophiidae and Siphonopidae fail to be retrieved as monophyletic clades in both summary trees, and are recovered in only 4.5 and 26.6% of the post-burn-in trees, respectively. Indotyphlidae (Clade W) is identical in topology to the parsimony analysis, and this clade is retrieved as the sister taxon to the Dermophidae plus Siphonopidae assemblage (Clade Z) in the 95% maximum clade credibility tree (Fig. 12D). The clustering of members of Herpelidae fails to be retrieved in the Bayesian analysis, similarly to the parsimony analyses, and a monophyletic Herpelidae is recovered in only 7.1% of the post-burn-in trees. By contrast, the species Ichthyophiidae and Scolecomorphidae are resolved, albeit with very weak support (posterior probability < 0.36) and they are recovered in patterns not retrieved in the analysis of Zhang & Wake (2009). Caeciliidae is improved in resolution, with the species of Caecilia retrieved as a clade (Clade U) to the exclusion of Oscaecilia (Fig. 12D).
Phylogenetic analysis of combined morphological and molecular data
The results of the combined analysis were summarized as both a 95% maximum clade credibility tree and a 50% majority-rule consensus tree. As the ancestral character state reconstructions described below were performed on the 50% majority-rule consensus tree, this topology is shown here (Fig. 13). Both topologies are identical with the exception that two additional clades are resolved in the former (described below). In general, the addition of all morphological characters to the molecular matrix (excluding Caecilia sp.) results in a topology (Fig. 13) that is largely similar to that revealed by the molecular data alone (Zhang & Wake, 2009).

The 50% majority-rule tree resulting from the Bayesian analysis of the combined morphological and molecular data sets. Node letters correspond to the text, and node values are posterior probabilities.
Three species, Ic. kohtaoensis, Id. russeli, and Sy. grandisonae, lacking molecular data were included and scored for only morphology [Sy. grandisonae was only scored for the characters in Wilkinson's (1997) analysis]. The addition of these three species results in reduced resolution (Fig. 13) for the clades within which they are retrieved in comparison to the results of Zhang & Wake (2009). Ichthyophis kohtaoensis is retrieved as the sister taxon to Clade K (Ichthyophis glutinosus plus Ichthyophis orthoplicatus). With the inclusion of Ic. kohtaoensis, Ichthyophis tricolor and Clade L (Ichthyophis bannanicus plus Cau. asplenia) are reduced to a polytomy with Clade J (Ic. kohtaoensis plus Clade K). This clade represents one of the two discrepancies between the 95% maximum clade credibility tree and the 50% majority-rule consensus tree. In the maximum clade credibility tree this polytomy is resolved and Ic. tricolor forms the sister taxon to the equivalent of Clade L plus Clade J.
Idiocranium russeli and Sy. grandisonae cluster with the indotyphlid species forming Clade A' (the species of Gegeneophis, Hyp. rostratus, Gr. alternans, and Praslinia cooperi). Both are retrieved in a polytomy with Clade C' (P. cooperi and Hyp. rostratus plus Gr. alternans) and Clade B' (species of Gegeneophis). This clade represents the second of the two discrepancies between the 95% maximum clade credibility tree and the 50% majority-rule consensus tree. The maximum clade credibility tree resolves this polytomy placing Id. russeli and Sy. grandisonae as successively more closely related to the Hyp. rostratus plus Gr. alternans clade. This placement of Id. russeli differs from that obtained in the analysis of morphology alone (Fig. 12C, D).
The combined analysis retrieves monophyly for all nine families, and all, with the exception of Indotyphlidae (posterior probability 0.96), are retrieved with a posterior probability of 1.00. The species of Boulengerula and He. squalostoma form a monophyletic Herpelidae and Geo. seraphini clusters with the other dermophiid species forming a monophyletic Dermophiidae (Fig. 13). This is in contrast to the results of the morphology-based analyses (Fig. 12C, D).
Ancestral character state reconstructions and phylogenetic signal
Given the slight conflict in topology between the morphology-based and combined analyses, the 50% majority-rule consensus tree, with its lack of resolution in these ambiguous regions, was used to reconstruct character state evolution. A total of 27 new unique derived character states and 68 new non-unique derived character states was identified. These character states contribute to a body of character data that can be drawn upon to help diagnose 16 genera, eight families, and ten other clades (Table 1).
| Taxon | Unique synapomorphies | Non-unique synapomorphies |
|---|---|---|
| Rhinatrematidae (Clade B) | 84 (1) | |
| Epicrionops | 89 (1) | |
| Neocaecilia (Clade C) | 87 (1)*; 98 (1); 100 (1)†; 106 (1)‡ | |
| Ichthyophiidae (Clade D) | 104 (3) | |
| Clade H | 86 (2); 88 (1); 92 (1); 93 (1) | 84 (2); 89 (1); 105 (1); 110 (1) |
| Ichthyophis kohtaoensis | 80 (1) | |
| Uraeotyphlus | 80 (1); 93 (2); 96 (1); 102 (1); 103 (1); 107 (1) | |
| Teresomata (Clade M) | 92 (2)§; 104 (4)¶ | |
| Scolecomorphidae (Clade N) | 85 (1); 97 (1); 112 (1) | 105 (1)** |
| Crotaphatrema | 81 (1); 93 (3) | |
| Scolecomorphus | 104 (6) | 84 (2); 89 (1) |
| Clade Q | 82 (1)††; 94 (1) | 80 (1); 93 (2)‡‡; 102 (1) |
| Herpelidae (Clade R) | 96 (1); 111 (1) | |
| Boulengerula | 86 (1); 101 (1); 103 (1); 110 (1) | |
| Boulengerula boulengeri | 94 (2); 105 (1) | |
| Boulengerula taitana | 89 (1); 94 (2) | |
| Herpele | 112 (2) | |
| Clade U | 103 (1)§§ | |
| Clade V | 90 (1); 104 (5) | 86 (1)¶¶; 108 (1); 109 (1)a |
| Caeciliidae (Clade X) | 96 (1) | |
| Typhlonectidae (Clade W) | 79 (1); 100 (2) | 81 (1); 97 (2); 107 (1) |
| Chthonerpeton | 104 (7) | |
| Clade Z' | 83 (1) | |
| Indotyphlidae (Clade A') | 95 (1) | 94 (2); 101 (1); 109 (1)a |
| Clade D' | 84 (1); 89 (1); 97 (2); 104 (7) | |
| Gegeneophis | 104 (1) | 86 (1); 96 (1) |
| Grandisonia | 96 (1) | |
| Hypogeophis | 86 (1) | |
| Idiocranium | 91 (1) | |
| Clade E' | 84 (2); 89 (1) | |
| Siphonopidae (Clade F') | 91 (1) | |
| Microcaecilia | 94 (2); 111 (1) | |
| Siphonops annulatus | 97 (2); 99 (1); 110 (1) | |
| Clade K' | 86 (1); 96 (1) | |
| Clade M' | 93 (3); 99 (1) | |
| Dermophis | 109 (1) | |
| Geotrypetes | 104 (2); 112 (3) | 108 (1) |
| Schistometopum | 84 (1); 94 (2) |
- * 87 (1) reversed to state 0 in Indotyphlidae, Microcaecilia, Schistometopum thomense.
- † 100 (1) reversed to state 0 in Caudacaecilia asplenia, Gegeneophis ramaswami, Sc. thomense.
- ‡ 106 (1) reversed to state 0 in Clade U.
- § 92 (2) reversed to state 0 in Idiocranium russeli, Clade K'.
- ¶ 104 (4) reversed to state 0 in Clade Z'.
- ** 105 (1) reversed to state 0 in Scolecomorphus vitattus.
- †† 82 (1) reversed to 0 in Typhlonectidae, Microcaecilia, Idiocranium russeli.
- ‡‡ 93 (2) reversed to state 0 in Geotrypetes seraphini.
- §§ 103 (1) reversed to state 0 in Typhlonectidae, Id. russeli, Microcaecilia.
- ¶¶ 86 (1) reversed to state 0 in Typhlonectes natans.
- a 109 (1) reversed to state 0 in Oscaecilia ochrocephalus, Id. russeli.
The results of the test of phylogenetic signal revealed 84% of the braincase characters were conserved in their distribution, and therefore contain significant phylogenetic signal. This is in contrast to 98% of the traditional, and 23% of the nontraditional characters. Of note is the weak phylogenetic signal amongst the eye characters (characters 53–63), except for character 59. This supports the exclusion of these characters from the analyses, as was carried out above, because of a high likelihood of introducing homoplasy. However, given our relatively poor understanding of morphological variation and its phylogenetic significance within Gymnophiona, as well as the need for much future development of this character-taxon matrix analysed here, all characters are retained in the character list for the time being.
DISCUSSION
The influence of braincase characters on morphology-derived phylogeny
It was hypothesized that the braincase and stapes would serve as a source of characters that have the ability to resolve morphology-based phylogenetic relationships within Gymnophiona (caecilians). The identification of 34 new characters pertaining to the braincase and stapes, and their addition to the previous morphological matrix dramatically influences the hypothesis of caecilian relationships in comparison to previous analyses of morphology (Wilkinson, 1997). In total seven (parsimony) or six (Bayesian) out of nine of the families are recovered as monophyletic clades (Fig. 12C, D) and all family member's positions relative to one another are congruent with the results of molecular-based hypotheses (Roelants et al., 2007; Zhang & Wake, 2009). The similarities to the molecular analysis that were not present in the previous morphological analysis include the recovery of Scolecomorphidae at the base of Teresomata (sensu Wilkinson & Nussbaum, 2006), the clustering of the caeciliid species with the typhlonectid species, the clustering of the indotyphlid species, the clustering of the dermophiid species with siphonopid species, and the clustering of Indotyphlidae with members Dermophiidae plus Siphonopidae (Fig. 12D).
The current study also included eight species that were previously not included in the most recent morphological analyses of phylogeny (Wilkinson, 1997). In all cases, the new species are arranged in a highly congruent pattern relative to previous hypotheses of relationship, as well as the results of molecular analyses (Fig. 12A–D). The current analysis retrieves the hypothesis that Rhinatrema and Epicrionops form a clade (Rhinatrematidae) that is the sister taxon of all other caecilian species (Nussbaum, 1979). The addition of Ic. beddomei yields the expected outcome of it clustering with the other species of Ichthyophis. Caudacaecilia asplenia also forms a clade with the species of Ichthyophis; however, there is no (parsimony) to only weak support (Bayesian) for further resolution between species of Ichthyophis and Cau. asplenia. Together Ichthyophis and Caudacaecilia form a clade with Uraeotyphlus, forming Ichthyophiidae. Crotaphatrema lamottei is retrieved here as the sister taxon of Scolecomorphus, which together form a monophyletic clade (Scolecomorphidae). The position of He. squalostoma is consistent with molecular-based hypotheses in it being the sister taxon to the clade including Caeciliidae, Typhlonectidae, Indotyphlidae, Dermophiidae, and Siphonopidae (Fig. 12; Roelants et al., 2007; Zhang & Wake, 2009; Wilkinson et al., 2011). The two new species of Caecilia used here are resolved in the identical location to that of Cae. occidentalis in earlier analyses. The clustering of Caecilia with Oscaecilia retrieves the family Caeciliidae. Microcaecilia albiceps, also previously not included in morphological analyses, forms a clade with Si. annulatus, forming the family Siphonopidae.
In the first two parsimony analyses, all characters included (Fig. 12A) and eye characters excluded (Fig. 12B), there is a weakly supported relationship between the indotyphlid and dermophiid plus siphonopid clades to the exclusion of the caeciliid plus typhlonectid clade. This is consistent with hypotheses based on molecular data (Roelants et al., 2007; Zhang & Wake, 2009). However, when Sy. grandisonae was excluded in subsequent parsimony analyses (Fig. 12C), a polytomy was formed amongst these three large clades (Clades N, Q, and T; Fig. 12C). The lack of resolution is because of a conflict between a pair of characters, one of which is the shared presence of viviparity between Typhlonectidae and Dermophiidae (character 36). It has long been thought that viviparity arose multiple times in caecilians (Wilkinson et al., 2003; Gower et al., 2008a), suggesting that this hypothesis of close relationship between Clade N and Clade T (Fig. 12C), which conflicts with molecular data, is a result of homoplasy (Wake, Wake & Specht, 2011). The 95% maximum clade credibility tree from the Bayesian analysis retrieves weak support for a topology like that in molecular analyses (Fig. 12D), and the 50% majority-rule tree from the Bayesian analysis is similarly unresolved as the parsimony tree (Fig. 12C).
An unconventional pattern in the tree resulting from the parsimony analysis (Fig. 12C) is the position of Geo. seraphini on the stem of Siphonopidae, resulting in a paraphyletic Dermophiidae. In the Bayesian analysis Geo. seraphini is retrieved on the stem of a clade containing members of Dermophiidae plus Siphonopidae (Fig. 12D). This conflict appears to be because of the absence of morphological characters shared exclusively between Geo. seraphini and the other dermophiid species. Additionally, recent molecular analyses retrieve a sister taxon relationship between He. squalostoma and Boulengerula (Herpelidae). This grouping was not obtained here, and also results from a lack of synapomorphies to define this clade. In both cases, the divergence dates between the genera in question are amongst the oldest for within family splits (Roelants et al., 2007). These deep divergences may have led to changes that obscure phylogenetic information. This is evident from superficial examination of skulls, highlighting that these taxa are in need of much further detailed investigation.
At the other end of the scale, the braincase and stapes characters fail to provide additional resolution at the species level (Fig. 12C). For example, the species of Ichthyophis and the species of Scolecomorphus fail to be resolved in the parsimony analyses by the addition of the new characters incorporated here, indicating the lower limit of braincase and stapes characters for resolving relationships. In fact the vast majority of conflict resulting in the high number of MPTs can be attributed to fluctuating positions of species relative to one another within a genus. The output results were reduced from 733 MPTs to ten MPTs when the terminal taxa were reduced to the most complete species (data not shown). The topology of the consensus tree bears strong congruence amongst genera with that including all species, implicating fluctuating positions of species within genera as a major source of multiple MPTs. In the Bayesian analyses these species are resolved, but they all bear weak support and are displayed in positions that conflict with previous analyses (Gower et al., 2005; Zhang & Wake, 2009). Given that in most cases congeneric species received identical scores for the braincase and stapes characters, the resolution in the Bayesian analyses of morphology is interpreted with caution.
Interestingly, the parsimony analyses of the new braincase and stapes characters also fail to resolve the relationships between two pairs of genera: (1) Ichthyophis and Caudacaecilia, and (2) Caecilia and Oscaecilia. Both of these pairs of genera represent taxonomically and phylogenetically challenging clusters of species with historically blurry boundaries (Nussbaum & Gans, 1980; Savage & Wake, 2001). Caudacaecilia was first erected by Taylor (1968) based on specimens previously assigned to Ichthyophis asplenia (Taylor, 1965). Caudacaecilia is distinguishable from Ichthyophis only by the absence of splenial teeth (Wilkinson & Nussbaum, 2006), although splenial teeth have since been reported in putative larvae of Caudacaecilia (Matsui et al., 2006). It has been hypothesized that Ichthyophis is paraphyletic with respect to Caudacaecilia (Wilkinson & Nussbaum, 2006). The current morphological analysis is the first to include Caudacaecilia since the contribution of Nussbaum (1979), and the results of the parsimony analysis do not resolve the nature of the relationship between it and Ichthyophis. The Bayesian analysis retrieves weak support for resolution of these genera, but these are in conflict with previous studies (e.g. Gower et al., 2005). For several years, efforts focused on resolving systematic issues in the ichthyophiid cluster have been underway (e.g. Gower et al., 2005, 2008b; Gower & Wilkinson, 2007). These will undoubtedly clarify species boundaries and contribute to resolving phylogeny and taxonomy in this group.
Similarly, the genus Oscaecilia was erected by Taylor (1968) through division of the genus Caecilia. These genera remain distinguishable from one another only by the presence (Caecilia) or absence (Oscaecilia) of an open orbit (Nussbaum & Wilkinson, 1989; Wilkinson & Nussbaum, 2006). However, at least one species of Caecilia (Cae. gracilis) is known to be polymorphic in this regard. The Bayesian analysis retrieves a monophyletic Caecilia genus, but this is recovered with low support (Fig. 12D). The results concerning these pairs of genera highlight the need for low-level taxonomic work on these taxa. Similar to Ichthyophiidae, the very speciose Caeciliidae is in need of much focused attention to understand better the range and limits of variation present between species.
Combined phylogenetic analysis of morphological and molecular data
Combined approaches to phylogeny inference have the appealing attribute of being able to draw from multiple sources of character data. Until recently only parsimony approaches could be applied to combined data sets, such as those constructed from morphological and molecular data, and the benefits of a likelihood approach (e.g. more realistic substitution and rate models, statistical consistency; Felsenstein, 1978) were simply unavailable for these kinds of mixed data-type character-taxon matrices. The practical issues concerning the combination of molecular and morphological character data under a likelihood approach have only been overcome in the last decade through: (1) the development of appropriate stochastic models for morphological data (i.e. the Mk model; Lewis, 2001), and (2) the practical implementation of such models in computer programs such as MrBayes (Nylander et al., 2004).
The combined analysis of morphological and molecular character data presented here is the first analysis of its kind for Gymnophiona. The resulting topology of the combined analysis is strongly congruent with recent molecular analyses (Fig. 13; Roelants et al., 2007; Zhang & Wake, 2009). This is not surprising given the overall similarity in the topology of the trees generated by separate morphological and molecular analyses. The 50% majority-rule consensus tree from the combined analysis is slightly less well resolved in certain areas outlined above (CFI drops from 1.0 to 0.92). Idiocranium russeli, Sy. grandisonae, and Ic. kohtaoensis were absent from previous molecular phylogenetic analyses, and therefore were scored here as unknown (missing data) for the sequence characters (1883 informative sites). All of the nodes surrounding these clusters suffered decreased resolution and decreased posterior probability scores, possibly because of the large proportion of missing data owing to the absence of molecular sequences.
A monophyletic Dermophiidae and Herpelidae were retrieved in the combined analysis, consistent with the previous molecular analyses (Fig. 13), suggesting the conflicting topology obtained in the morphological analysis is only weakly supported. This is confirmed by the low bootstrap values surrounding these taxa in the morphological tree (Fig. 12C, bootstrap < 50%).
The recovery of Id. russeli (in both the morphology alone and combined analyses) and Sy. grandisonae (in the combined analysis) within a monophyletic Indotyphlidae is an interesting outcome. These two taxa were placed within Indotyphlidae by Wilkinson et al. (2011); however, this hypothesis had not been rigorously tested, and no molecular analyses including these taxa currently exist. Both taxa are the only members of the clade found in continental Africa, and the hypothesis obtained here highlights the importance of this geographical region in the radiation of this caecilian family. Unfortunately, Sy. grandisonae was not available for study; however, it is predicted that examination of the braincase and stapes of this taxon will help resolve its position within morphology-based hypotheses and contribute to understanding the phylogeography of Indotyphlidae.
Ancestral character state reconstructions and new synapomorphies
The combined approach to phylogeny inference taken here provides a test of congruence amongst all available characters (Rieppel, 1996). This permits the identification of potential synapomorphies using ACSRs.
The phylogenetic analyses conducted here incorporate several species that have never been included in computer-based analyses of phylogeny. In the case of many of these species, their inclusion in only molecular phylogenetic analyses means that clades associated with some or all of these species lack morphological synapomorphies to define them. ACSR for the nodes surrounding these species means that it is possible to identify potential new synapomorphies, and therefore contribute to taxonomic diagnoses for clades previously lacking them. In addition, ACSRs provide new characters from which to draw from to refine diagnoses for other clades as well. Table 1 lists the taxa for which new synapomorphy data were discovered and the character states forming these synapomorphies.
The clustering of Caeciliidae with Typhlonectidae is a clustering of species previously considered to be closely related (Hedges, Nussbaum & Maxson, 1993; Wilkinson & Nussbaum, 1997; Wilkinson et al., 2003). Despite this, no morphological synapomorphies have been identified for this clade. The current analysis revealed two character states that may be considered to be synapomorphies of this clade:
-
Caeciliidae + Typhlonectidae (Fig. 13; Clade V) – caecilians with a groove in the ventral surface of the sphenethmoid for passage of the palatal ramus of the facial nerve (character 90, state 1), and two antotic foramina or Pattern 6 (character 104, state 5).
A close relationship between the genera Hypogeophis and Grandisonia has been hypothesized previously. However, a morphological synapomorphy of these taxa had yet to be identified. The current analysis revealed one non-unique synapomorphy (state shared with Ch. indistinctum). This contributes to the unique character combination that can diagnose this clade:
-
Hypogeophis + Grandisonia (Fig. 13; Clade D') – indotyphlids with a single, large antotic foramen or Pattern 8 (character 104, state 7).
The genus Geotrypetes possesses relatively few diagnostic traits. It is distinguished from other dermophiid genera by an anteriorly located tentacle (Wilkinson et al., 2011). The current study identified two additional unique apomorphies and one non-unique apomorphy of this genus:
-
Geotrypetes– caecilians with three antotic foramina or Pattern 3 (character 104, state 2), and an oblique posterior margin of the optic foramen (character 112, state 3). Dermophiids with an anterodorsally orientated columellar process of the stapes (character 108, state 1).
The genus Gegeneophis also possesses relatively few diagnostic traits. It is distinguished from other indotyphlids by a closed orbit (Wilkinson et al., 2011). The current study identified one additional unique apomorphy of this genus:
-
Gegeneophis– caecilians with four antotic foramina or Pattern 2 (character 104, state 1).
The genus Herpele is distinguished from other herpelids by the presence of separate nasals and premaxillae (Wilkinson et al., 2011). The current study identified one additional unique apomorphy of this genus:
-
Herpele– caecilians with an oblique anterior margin of the optic foramen (character 112, state 2).
Several additional genus-level, non-unique apomorphies were identified (Table 1):
-
Boulengerula– herpelids with a shallowly incised floor of the sphenethmoid (character 86, state 1), an anteriorly opening fenestra vestibuli (character 101, state 1), deep wing-like ventral processes on the otic capsules (character 103, state 1), and a fossa on the anterior margin of the footplate of the stapes (character 110, state 1).
-
Crotaphatrema– scolecomorphids with a short dorsomedial process (character 81, state 1), and a rod-like distal tip of the floor of the os basale (character 93, state 1).
-
Dermophis– dermophiids with a ridge on the lateral margin of the columellar process of the stapes (character 109, state 1).
-
Epicrionops– rhinatrematids with laterally located ventral pair of olfactory foramina relative to the dorsal pair (character 89, state 1).
-
Idiocranium– indotyphlids with an incomplete lateral margin of the anterolateral foramen of the sphenethmoid (character 91, state 1).
-
Microcaecilia– siphonopids with a strong constriction of the floor of the os basale posterior to the basicranial articulations and a ridge on the distal end of the columellar process (character 94, state 2), and the presence of a ridge on the distal columellar process (character 111, state 1).
-
Schistometopum– dermophiids with sola nasi associated with a ventral sheet of bone (character 84, state 1), and a strong constriction of the floor of the os basale posterior to the basicranial articulations (character 94, state 2).
-
Scolecomorphus– Scolecomorphids with rod-like sola nasi present and laterally located ventral pair of olfactory foramina relative to the dorsal pair (character 84, state 2), and laterally located ventral pair of olfactory foramina relative to the dorsal pair (character 89, state 1).
-
Siphonops– siphonopids with a very wide dorsal exposure of the os basale (character 97, state 2), a thickened ventral margin of the fenestra vestibuli (character 99, state 1), and a large fossa on the anteroventral margin of the footplate of the stapes (character 110, state 1).
-
Uraeotyphlus– ichthyophiids with a short tip of the nasal septum (character 80, state 1), a strongly tapered anterior margin of the floor of the os basale (character 93, state 2), an abrupt right angle formed between the antotic walls and dorsal otic capsule when viewed laterally (character 96, state 1), greatly protruding occipital condyles (character 102, state 1), deep wing-like ventral processes on the otic capsules (character 103, state 1), and a small footplate of the stapes (character 107, state 1).
The reconstruction of ancestral character states permits the evaluation of morphological evolution. As such, it has been possible to observe that, amongst the characters of the braincase and stapes, Id. russeli expresses the greatest number of reversals to the plesiomorphic state of any taxon examined here (characters 82, 87, 89, 92, 103, and 109). Idiocranium russeli also represents the only taxon considered to be miniaturized amongst the caecilians examined. The morphological consequences of miniaturization, in addition to dramatic reduction in body size, have been characterized as resulting in an overall paedomorphic appearance (Alberch & Alberch, 1981; Hanken, 1983, 1984, 1985; Wake, 1991). Paedomorphs often express the plesiomorphic state of multiple traits, suggesting that miniaturization may be a driver of much homoplasy in this species. Additionally, the posterior portion of the skull, which includes much of the braincase, appears to be least affected by miniaturization (Hanken, 1983). This phenomenon may be considered to be reflected in the possession of the most well-ossified condition of the antotic wall and the resulting number of foramina, in contrast to the more open condition observed in the other indotyphlid taxa, which include representatives with the most open condition amongst all caecilians (Maddin, 2011).
Ancestral character state reconstructions additionally permit the comparison of the plesiomorphic condition for Gymnophiona to the condition observed in various fossils. Of particular significance will be comparisons with the condition observed in the fossil stem caecilian Eocaecilia micropodia (Jenkins, Walsh & Carroll, 2007). These comparisons and their implications are beyond the scope of the current study; however, these studies are in progress and the results are forthcoming.
CONCLUSIONS AND IMPLICATIONS
This study set out to test the utility of characters derived from the braincase and stapes in resolving phylogenetic relationships between low-level taxa in a non-amniote group with a highly modified morphology associated with functional adaptation and rampant homoplasy. This study revealed that the braincase and stapes harbour a significant amount of phylogenetic information that is sufficient to resolve relationships at the genus level, and in a pattern that is highly congruent with hypotheses of relationships based on molecular data. This demonstrates, as other recent studies also have (e.g. Bhullar, 2011), that morphological characters are capable of reconstructing relationships in a way that provides confidence in the accuracy in the hypothesis (i.e. through congruence with hypotheses based on alternative sources of data). Furthermore, this study provides evidence that the braincase may be the key to resolving phylogenetic relationships in taxa that are problematic for various reasons, including those that have highly modified morphology and high levels of homoplasy, which may obscure or swamp the phylogenetic signal of the morphology.
A significant outcome of this study is the generation of a vastly improved matrix of morphological characters, albeit still a work in progress, as it is certain that more phylogenetically informative variation will be revealed with additional examination. This will become increasingly important as discoveries of fossil caecilians increase (e.g. Rage & Pickford, 2011), which can only be included in discussions of caecilian evolution through the employment of morphology-based phylogenetic inference. These fossils will play a key role in understanding the evolution of morphology in this morphologically distinctive clade. Caecilians represent an excellent group for understanding the evolutionary consequences of several factors upon morphology. These include understanding the effects of diverse reproductive life history modes (e.g. oviparity, oviparity with direct development, and viviparity), the effects of strong functional constraints (e.g. fossoriality), and the effects of relaxed constraints (e.g. limb and girdle loss, reduced sensory structures). A morphology-based matrix that permits the inclusion of deep time evolutionary events found in the fossil record will be critical to advancing our knowledge of how these factors have influenced morphological evolution.
ACKNOWLEDGEMENTS
We thank M. H. Wake (MVZ), R. Brown (KU), A. Resetar (FMNH), J. Rosado (MCZ), and G. Schneider (UMMZ) for the loan of materials that formed the basis of this study. We also thank E. Sherratt, M. Wilkinson, D. Gower, and T. Kleinteich for generously sharing CT data sets of specimens while under their study, and P. Zhang and M. Wake for sharing the molecular matrix they compiled for their 2009 study. Technical support for this project was provided in part by B. Hallgrímsson and W. Liu (University of Calgary, 3D Morphometrics Laboratory). We also acknowledge M. Wake, J. Gardner, J. Vamosi, R. Cuthbertson, and J. Patterson for additional support and helpful discussion. Funding for this project was provided by the Natural Sciences and Engineering Research Council of Canada Graduate Scholarship (H. C. M.). We thank our reviewers for providing thoughtful and thorough reviews of this manuscript.
Appendix
APPENDIX 1
New characters (79–112) of the braincase and stapes developed here. It is recommended that these new characters be added to the list of previously identified characters (1–78; Wilkinson, 1997). Characters 1–78 (not listed here) are identical to those of Wilkinson (1997), with a slight modification to character 32 (see Table S2).
- 79
Length of nasal septum: (0) 20–35% of the total skull length; (1) less than 20% of the total skull length.
- 80
Nasal septum: (0) tall at tip, weakly tapered in height; (1) short at tip, strongly tapered in height.
- 81
Length of dorsomedial process of the sphenethmoid: (0) 11% or more of the total skull length; (1) 10% or less of the total skull length.
- 82
Dorsomedial process of the sphenethmoid: (0) thin and rod-like; (1) thick and broad.
- 83
Dorsal sutural surface of the sphenethmoid: (0) narrow or weakly tapered; (1) broad and triangular and strongly tapered.
- 84
Sola nasi: (0) absent; (1) present and connected to the nasal septum ventrally; (2) present, rod-like or tubular.
- 85
Length of the lateral walls of the sphenethmoid: (0) 14% or less than the total skull length; (1) 15% or greater of the total skull length.
- 86
Floor of sphenethmoid: (0) deeply incised and U-shaped in outline; (1) shallowly incised with broad coverage of os basale; (2) deeply incised and U-shaped, but with midline extension.
- 87
Thin sheet of bone anterior and ventral to ventral olfactory foramina: (0) absent; (1) present.
- 88
Dorsal olfactory nerve foramina: (0) foramina enclosed within sphenethmoid; (1) troughs that are open dorsally instead of foramina.
- 89
Ventral olfactory foramina: (0) directly ventral to the dorsal foramina; (1) located laterally relative to the dorsal foramina, away from midline.
- 90
Groove for palatal ramus of the facial nerve: (0) in the lateral walls of the main body of the sphenethmoid; (1) in the ventral surface of the main body of the sphenethmoid.
- 91
Anterolateral foramen: (0) complete; (1) incomplete laterally.
- 92
Anterior extent of the floor of the os basale: (0) extends to encroach within the region of the anterior half of nasal septum; (1) does not encroach on the nasal septum; (2) extends to encroach within the region of the posterior half of the nasal septum.
- 93
Margin of the floor of the os basale: (0) broad, tapers continuously to a point; (1) rounded anteriorly; (2) markedly tapered, resulting in bottleneck appearance; (3) rod-like distal tip.
- 94
Floor of the os basale: (0) absence of lateral constriction posterior to basicranial articulations; (1) weak constriction posterior to basicranial articulations present; (2) strong constriction posterior to basicranial articulations, reaching to the carotid foramina.
- 95
Quadrate articular fossa dorsal to antotic foramina: (0) absent; (1) present.
- 96
Angle between antotic walls and skull roof portion of os basale when viewed laterally: (0) smooth curve, angle greater than 90°; (1) abrupt, right angle.
- 97
Skull roof exposure of the os basale: (0) moderate exposure at the dorsal midline; (1) little to no exposure near the dorsal midline; (2) very wide exposure near the dorsal midline.
- 98
Occipital surface: (0) completely made up by the os basale; (1) incompletely made up by the os basale, receives contribution from parietal.
- 99
Fenestra vestibuli: (0) thickened ventral margin of fenestra vestibuli absent; (1) present.
- 100
Fenestra vestibuli: (0) large, subcircular; (1) anteroposteriorly elongate, oval; (2) very small, subcircular.
- 101
Fenestra vestibuli: (0) opening directed laterally; (1) opening directed anteriorly.
- 102
Occipital condyles: (0) in lateral view condyles are continuous with the outline of the otic-occipital complex to weakly protruding; (1) condyles protrude well beyond posterior limit of the otic-occipital complex.
- 103
Ventral muscle attachment site: (0) no ventral projections; (1) deep, wing-like processes at lateral margins of the site.
- 104
Antotic foramina: (0) Pattern 1; (1) Pattern 2; (2) Pattern 3; (3) Pattern 4; (4) Pattern 5; (5) Pattern 6; (6) Pattern 7; (7) Pattern 8. [described in text]
- 105
Canal for palatal ramus of the facial nerve in antotic region: (0) absent; (1) present.
- 106
Facial nerve and ventral vein foramina: (0) facial nerve and ventral vein exit through separate foramina; (1) both exit through a common foramen.
- 107
Footplate of stapes: (0) fills the fenestra vestibuli; (1) much smaller than the fenestra vestibuli.
- 108
Orientation of columellar process of the stapes: (0) horizontal; (1) anterodorsally orientated.
- 109
Ridge on lateral margin of columellar process: (0) absent; (1) present.
- 110
Fossa on anterior margin of footplate: (0) present and small; (1) present and very large.
- 111
Columellar process: (0) no ridge present; (1) ridge on distal columellar process.
- 112
Shape of optic foramen: (0) large, subcircular opening; (1) narrow and slit-like opening; (2) oblique anterior margin in the sphenethmoid; (3) oblique posterior margin in the os basale.




