Evolution of the brain and sensory organs in Sphenisciformes: new data from the stem penguin Paraptenodytes antarcticus
Abstract
Penguins have undergone dramatic changes associated with the evolution of underwater flight and subsequent loss of aerial flight, which are manifest and well documented in the musculoskeletal system and integument. Significant modification of neurosensory systems and endocranial spaces may also be expected along this locomotor transition. However, no investigations of the brain and sensory organs of extinct stem lineage Sphenisciformes have been carried out, and few data exist even for extant species of Spheniscidae. In order to explore neuroanatomical evolution in penguins, we generated virtual endocasts for the early Miocene stem penguin Paraptenodytes antarcticus, three extant penguin species (Pygoscelis antarctica, Aptenodytes patagonicus, Spheniscus magellanicus), and two outgroup species (the common loon Gavia immer and the Laysan albatross Phoebastria immutabilis). These endocasts yield new anatomical data and phylogenetically informative characters from the brain, carotid arteries, pneumatic recesses, and semicircular canal system. Despite having undergone over 60 million years of evolution since the loss of flight, penguins retain many attributes traditionally linked to flight. Features associated with visual acuity and proprioception, such as the sagittal eminence and flocculus, show a similar degree of development to those of volant birds in the three extant penguins and Paraptenodytes antarcticus. These features, although clearly not flight-related in penguins, are consistent with the neurological demands associated with rapid manoeuvring in complex aquatic environments. Semicircular canal orientation in penguins is similar to volant birds. Interestingly, canal radius is grossly enlarged in the fossil taxon Pa. antarcticus compared to living penguins and outgroups. In contrast to all other living birds, the contralateral anterior tympanic recesses of extant penguins do not communicate. An interaural pathway connecting these recesses is retained in Pa. antarcticus, suggesting that stem penguins may still have employed this connection, potentially to enhance directional localization of sound. Paedomorphosis, already identified as a potential factor in crown clade penguin skeletal morphology, may also be implicated in the failure of an interaural pathway to form during ontogeny in extant penguins.
© 2012 The Linnean Society of London, Zoological Journal of the Linnean Society, 2012, 166, 202–219.
INTRODUCTION
Penguins are the most highly aquatic of all living birds. The transition to a primarily aquatic existence entailed major reorganization of the integument, musculoskeletal system, and sensory organs (Watson, 1883; Simpson, 1946; Bannasch, 1994; reviewed in Ksepka & Ando, 2011). Amongst the many unique features of penguins are the modifications of the cornea, iris, and ciliary muscles of the eye for vision in two mediums (Sivak & Vrablic, 1979; Martin, 1999), development of a vascular humeral plexus counter-current heat exchanger (Frost, Siegfried & Greenwood, 1975; Thomas & Fordyce, 2008, 2012; Thomas, Ksepka & Fordyce, 2011), flattened, scale-like, and undifferentiated primary feathers (Livezey, 1989; Giannini & Bertelli, 2004), grossly enlarged feather melanosomes (Clarke et al., 2010), and markedly increased haemoglobin and myoglobin levels (Weber, Hemmingsen & Johansen, 1974; Tamburri et al., 1994). Given the major modifications of these systems accompanying the transition to an aquatic lifestyle in penguins, concomitant modifications of the brain and sensory systems can be expected. Thus far, however, few aspects of the penguin brain have been studied and most research has occurred in the context of surveys of specific features across Aves. In this contribution, we explore the evolution of penguin neuroanatomy through computed tomographic investigations of living and fossil penguin braincases.
Computed tomography (CT) is a nondestructive digital imaging tool that can be used to visualize the internal structures of extant and extinct organisms (e.g. Spoor et al., 2002; Alonso et al., 2004; Macrini, Rougier & Rowe, 2007; Sampson & Witmer, 2007; Witmer, 2007; Witmer & Ridgely, 2008; Balanoff et al., 2009; Walsh et al., 2009) and is particularly useful for the detailed study of the complex internal anatomy present in the vertebrate braincase (Carlson et al., 2003). This technology not only allows us to observe bony structures of the braincase, but also provides the opportunity to visualize more effectively soft-tissue structures of the central nervous, circulatory, and endocrine systems in three dimensions through the use of virtual endocasts created from those spaces. We focus here primarily on endocasts created from the cranial cavity in which the brain was housed during life. The morphology of this space in birds provides a close approximation to that of the actual brain (Hopson, 1979; Iwaniuk & Nelson, 2002; Striedter, 2005; Witmer et al., 2008) and allows complex measurements to be obtained.
METHODS
Sampling
Virtual endocasts were rendered for three extant penguin species (Aptenodytes patagonicus, Spheniscus humboldti, and Pygoscelis antarctica), the fossil penguin Paraptenodytes antarcticus, and two outgroups (Gavia immer and Phoebastria immutabilis) (Table 1).
| Taxon | Specimen | Type | Data set | Z spacing (mm) | X, Y spacing (mm) |
|---|---|---|---|---|---|
| Aptenodytes patagonicus | AMNH 1623 | Extant | Slices, virtual endocast | 0.100 | 0.1875 |
| Gavia immer | TCWC 13300 | Extant | Slices, virtual endocast | 0.109 | 0.0508 |
| Phoebastria immutabilis | FMNH 313780 | Extant | Slices, virtual endocast | 0.138 | 0.0846 |
| Paraptenodytes antarcticus | AMNH 3338 | Fossil | Slices, virtual endocast | 0.100 | 0.1875 |
| Pygoscelis adeliae | Uncatalogued | Extant | Dissected brain | NA | NA |
| Pygoscelis antarctica | AMNH 26121 | Extant | Slices, virtual endocast | 0.100 | 0.1875 |
| Spheniscus humboldti | AMNH 6169 | Extant | Slices, virtual endocast | 0.100 | 0.1875 |
- NA, not applicable.
Paraptenodytes antarcticus occurred in the early Miocene of Patagonia (Ameghino, 1891; Simpson, 1946). Phylogenetic analyses (Bertelli, Giannini & Ksepka, 2006; Ksepka, Bertelli & Giannini, 2006) place Pa. antarcticus as a stem member of Sphenisciformes (Fig. 1A). Thus, this taxon has the potential to reveal neurological transformations occurring during the later stages of the transition from the volant ancestor of penguins to extant forms. The specimen selected for scanning (AMNH 3338: Fig. 1B) includes a well-preserved skull as well as a large portion of the postcranial skeleton. This specimen was first described by Simpson (1946) and later revisited by Bertelli et al. (2006). The braincase is preserved in its entirety and exhibits no deformation. Contrast between bone and matrix is easily observable in the CT images. A metal rod had been inserted into the skull some time after its initial description, presumably as a support for displaying the fossil. Although typically such objects result in oversaturation of greyscale values, which can cast streaks across the CT images, the rod caused very few artefacts and does not obscure any critical features.

A, simplified phylogeny of penguins after Ksepka et al. (2006) showing relationships of outgroup taxa, the stem penguin Paraptenodytes antarcticus, and crown clade (Spheniscidae) penguins studied here. B, the fossil skull of Pa. antarcticus (AMNH 3338) reconstructed and rendered from computed tomography scan data. Abbreviation: r, metal rod inserted to display specimen.
Extant taxon sampling for this study was designed to phylogenetically bracket the crown clade Spheniscidae. Morphology-based analyses place Spheniscus as part of the basal divergence within extant penguins, whereas molecular and combined analyses place Aptenodytes as the basal-most divergence (Bertelli & Giannini, 2005; Baker et al., 2006; Ksepka et al., 2006; Ksepka & Clarke, 2010). Our taxon sampling strategy ensured that the entire crown clade is bracketed, regardless of whether the morphology-based or molecular phylogeny is preferred. Therefore, endocast features shared by all three extant penguins examined here can safely be inferred to have been present in the most recent common ancestor of the crown clade Spheniscidae.
In order to test the correspondence of endocast features to anatomical structures, we dissected out the brain of one specimen of Pygoscelis adeliae. The symmetry of the brain of Py. adeliae and endocast of the closely related Py. antarctica confirms that the morphology of the endocast closely resembles the anatomy of the brain in Pygoscelis.
In order to establish polarity of characters, we constructed virtual endocasts from representatives of Procellariiformes (Ph. immutabilis) and Gaviiformes (G. immer). Recent phylogenetic studies suggest Procellariiformes are the sister taxon of penguins whereas Gaviiformes are closely related but outside the Procellariiformes−Sphenisciformes clade (Cracraft et al., 2004; Livezey & Zusi, 2007; Hackett et al., 2008) or at least support the inclusion of these three clades in part of a larger waterbird assemblage (Ericson et al., 2006; Smith, 2010).
Scanning and endocast rendering
Penguin specimens were scanned in air using a GE Lightspeed 16 medical scanner at Stony Brook University Hospital, and outgroup specimens were scanned at the High-Resolution X-ray Computed Tomography Facility at The University of Texas at Austin. Negative internal spaces were isolated in the program AVIZO to create virtual endocasts of the brain, semicircular canals and vascular structures, following the methods outlined in Balanoff & Rowe (2007). Original CT imagery as well as animations of the three-dimensional reconstructions are archived on the digital library Digimorph.org (http://www.digimorph.org/specimens/Paraptenodytes_antarcticus). Raw scan data are also archived at MorphoBank (http://www.morphobank.org– Project 695 Penguin Endocasts).
Regressions
We investigated the relationships between estimated body mass/total endocranial volume, estimated body mass/cerebrum volume, and total endocranial volume/forebrain volume amongst volant birds, crown penguins, and Pa. antarcticus (Table 2). All volumetric data were obtained from endocranial casts so as to maintain consistency between fossil and extant taxa. Volume of the forebrain was determined by excluding the olfactory tracts rostrally and using the crista tentorialis as an osteological marker to signify the posterior limit of the cerebrum. Body size means for extant species were taken from Dunning (2008). Paraptenodytes antarcticus specimen AMNH 3338 falls within the range of A. patagonicus and so was assigned the same body mass. All data were log-transformed in order to account for the large disparity in body sizes.
| Taxon | Endocast length (mm) | Endocast volume (mm3) | Cerebrum volume (mm3) | Body mass estimate (g) | Semicircular canals diameter (mm) | Osseous labyrinth (mm) | ||
|---|---|---|---|---|---|---|---|---|
| Length | Height (w/cochlear canal) | Height (w/o cochlear canal) | ||||||
| Gavia immer | 44.4 | 9935 | 5503 | 5460 | 0.95 | 8.51 | 14.97 | 7.70 |
| Phoebastria immutabilis | 42.9 | 14383 | 9446 | 3310 | 1.10 | 11.92 | 13.57 | 12.34 |
| Aptenodytes patagonicus | 46.2 | 26204 | 19315 | 12435 | 0.82 | 9.76 | 17.18 | 8.47 |
| Paraptenodytes antarcticus | 56.3 | 29355 | 19908 | 12435 | 1.87 | 11.24 | 18.72 | 12.27 |
| Pygoscelis antarctica | 37.1 | 15183 | 11544 | 4435 | 0.89 | 8.70 | 14.95 | 8.12 |
| Spheniscus humboldti | 43.4 | 15904 | 10907 | 4470 | 0.85 | 7.61 | 15.35 | 7.80 |
DESCRIPTION OF ENDOCASTS
General morphology
This study focused on the endocranial anatomy of Pa. antarcticus. Therefore, a detailed description of this taxon is given and extant taxa are described in relation to Pa. antarcticus. The endocranial morphology of extant penguins is fairly conservative, so those taxa are not referenced explicitly for features that do not depart from the morphology seen in Pa. antarcticus or show variation within Spheniscidae. As this study focused on endocasts, it should be made clear that all descriptions of brain structures refer to the external morphology of the brain as preserved by the endocranial surface unless explicitly noted as an observation from dissected brains.
A large amount of variation in the orientation of the brain with respect to the bones of the braincase is seen amongst birds. At one end of the spectrum, the brain may be almost parallel to the long axis of the skull in taxa such as Phalacrocoracidae (cormorants), whereas at the other extreme the brain can be orientated nearly perpendicular to the long axis of the skull as in Scolopax (woodcocks) (Portmann & Stingelin, 1961). Paraptenodytes antarcticus and extant penguins exhibit an orientation that is essentially parallel to the long axis of the skull. A similar parallel orientation characterizes Gavia, whereas in Phoebastria the brain is more rostrally inclined.
Telencephalon
The olfactory bulbs of birds are continuous with the cerebral hemispheres, making it difficult to extrapolate their exact size from endocasts. No noticeable variation occurs in the size of the olfactory bulb region in Pa. antarcticus or the extant penguins. Thin olfactory filaments extend from the olfactory bulbs and expand as they exit into the narial cavity in extant penguins. Unfortunately, the delicate walls of the narial cavity are not preserved in AMNH 3338. A condition similar to that in extant penguins is observed in G. immer, but in Ph. immutabilis the olfactory bulbs are significantly larger and more widely separated upon exiting the braincase. Observations on the size of the olfactory bulbs are in concordance with those of Zelenitsky et al. (2011), who reported high olfactory ratios (greatest linear dimension of the olfactory bulb divided by the greatest linear dimension of the cerebral hemispheres) in Procellariiformes and low olfactory ratios in Py. adeliae and G. immer.
The cerebrum of Pa. antarcticus is expanded laterally relative to the rest of the brain, as in most extant birds (Pearson, 1972). In dorsal view, the cerebral hemispheres of all sampled penguins have a ‘heart-shaped’ appearance (Fig. 3). This shape is exaggerated in Py. antarctica because of the greater lateral projection of the cerebral hemispheres at the level of the boundary with the cerebellum. The skull of Pa. antarcticus bears an internal midline ridge with a triangular cross section that would have sharply divided the cerebral hemispheres in life. The dorsal surface of the cerebral hemispheres shows a prominent ridge-like projection referred to as the sagittal eminence or wulst. This structure houses a series of cell layers that are primarily associated with complex visual functions (Northcutt, 1981), although some regions may also be associated with migration (e.g. magnetic Cluster N; Heyers et al., 2007). In Pa. antarcticus, the sagittal eminence extends to the caudal margin of the cerebral hemisphere and is essentially identical in shape, position, and projection to that in extant penguins (Fig. 3).
Diencephalon
At the base of the anterior wall of the braincase, a single large opening indicates the exit of cranial nerve II (optic nerve). This corresponds to optic foramen Type 3 of Hall, Iwaniuk & Gutiérrez-Ibáñez (2009), which is considered unreliable for estimating the width of the nerve bundle because the opening is larger than the bundle. In extant penguins, the interorbital septum divides the exit of the optic nerve into two openings. This region is not fully intact in AMNH 3338, but the preserved base of the interorbital septum suggests that the exit was also divided in Pa. antarcticus. In Aptenodytes, small fonticuli orbitocraniales perforate the rostral wall of the braincase dorsal to the exit of cranial nerve II (Bertelli & Giannini, 2005). No such openings are present in Pa. antarcticus (see Bertelli et al., 2006).
The pineal gland lies at the intersection of the cerebral and cerebellar hemispheres in birds. However, no impression indicating the location of this gland is visible in any of the penguin endocasts, and the pineal gland could not be readily discerned on the dorsal surface of the dissected brain of Py. adeliae.
The pituitary fossa sits dorsal to the parasphenoid lamina. In AMNH 3338, this fossa appears relatively larger and more asymmetrical than that of extant penguins in sagittal section. However, this difference is largely an effect of the anastomosis of the cranial carotid arteries immediately posterior to the pituitary fossa. At this point, the pituitary fossa and carotid anastomosis are confluent and difficult to differentiate on the endocast. Edinger (1942) found that the pituitary gland is enlarged relative to overall endocast size in gigantic vertebrate species relative to smaller species belonging to the same lineage. However, some evidence suggests that ocular musculature also affects pituitary size (Säve-Söderbergh, 1952). Aptenodytes patagonicus and Pa. antarcticus were the largest taxa sampled here, but there is no appreciable increase in the pituitary to brain size ratio compared to the two smaller penguin taxa. However, given the relatively small range of size spanned by the four penguins examined, it would be worthwhile to obtain CT scans of the braincase of giant penguin taxa such as Inkayacu paracasensis (Clarke et al., 2010) in order to test this relationship.
Mesencephalon
Large optic lobes are located ventrolateral to the cerebral hemispheres in Paraptenodytes, as in all extant birds (Fig. 2). The optic lobe is more distinctly separated from the cerebrum and more ventrally projected in Pa. antarcticus than in S. humboldti and Py. antarctica. Aptenodytes patagonicus shows an intermediate state. Although notable, the amount of variation in the form of the optic lobe within Sphenisciformes is small compared to that between penguins and other groups.

Virtual endocasts of A, Gavia immer (common loon); B, Phoebastria immutabilis (Laysan albatross); C, Paraptenodytes antarcticus (fossil stem penguin); D, Spheniscus humboldti (Humboldt penguin); E, Aptenodytes patagonicus (king penguin); and F, Pygoscelis antarctica (chinstrap penguin) in rostral aspect. Olfactory bulbs are slightly truncated in E and F because of scan length. Abbreviations: c, cerebrum; cca, cranial carotid artery; mo, medulla oblongata; ob, olfactory bulb; ol, optic lobe; pb, pituitary body; se, sagittal eminence; II−XI, cranial nerves II−XI.
Metencephalon
In all penguins examined, there is a sharp distinction between the cerebrum and cerebellum on the dorsal surface of the endocast. The cerebellum appears to project relatively further posteriorly in Pa. antarcticus and S. humboldti than in other penguins, although whether the cerebellum is proportionally larger in volume remains uncertain because the depth of the cellular layers of the cerebrum overlapping those of the cerebellum cannot be ascertained from the endocast. In Spheniscus a narrow occipital sinus is weakly developed on the dorsal cerebellar surface of the endocast. Cerebellar folds cannot be detected on the endocast of AMNH 3338 or any of the endocasts from extant penguins or G. immer, indicating that the occipital sinus or other sinusoidal/meningial tissue was thick enough to prevent the cerebellar surface from contacting the endocranium in these taxa. In contrast, individual cerebellar folds can be distinguished on the Ph. immutabilis endocast (3, 4). Greater protection for the cerebellum is presumably afforded by a thicker development of the subarachnoid space, and Elzanowski & Galton (1991) proposed that this morphology may be related to diving habits. Cerebellar folds, however, are also indiscernible on endocasts of some nondiving birds (S. Walsh, pers. observ.), suggesting that more data are needed to substantiate a link between endocast foliation and ecology.

Virtual endocasts of A, Gavia immer (common loon); B, Phoebastria immutabilis (Laysan albatross); C, Paraptenodytes antarcticus (fossil stem penguin); D, Spheniscus humboldti (Humboldt penguin); E, Aptenodytes patagonicus (king penguin); and F, Pygoscelis antarctica (chinstrap penguin) in dorsal aspect. Abbreviations: c, cerebrum; cb, cerebellum; fl, floccular lobe; mcv, middle cerebral vein; ob, olfactory bulb; ol, optic lobe; os, occipital sinus; se, sagittal eminence.
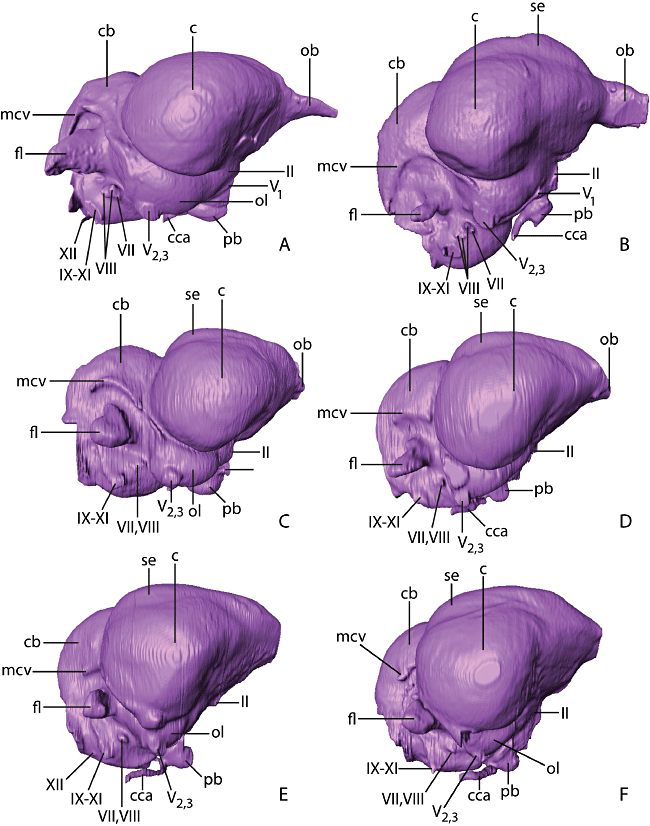
Virtual endocasts of A, Gavia immer (common loon); B, Phoebastria immutabilis (Laysan albatross); C, Paraptenodytes antarcticus (fossil stem penguin); D, Spheniscus humboldti (Humboldt penguin); E, Aptenodytes patagonicus (king penguin); and F, Pygoscelis antarctica (chinstrap penguin) in lateral aspect. Abbreviations: c, cerebrum; cb, cerebellum; cca, cranial carotid artery; fl, floccular lobe; mcv, middle cerebral vein; ob, olfactory bulb; ol, optic lobe; pb, pituitary body; se, sagittal eminence; II−XI, cranial nerves II−XI.
In Pa. antarcticus, the floccular lobes are strongly laterally projected from the lateral surface of the cerebellum. The distal ends of the floccular lobes are less caudally deflected than in extant penguins (Fig. 3C). The flocculus in Paraptenodytes is particularly large relative to overall endocast size, and there is little evidence of the strong rostrocaudal compression present in many other seabirds, such as Gavia (Fig. 3A) and Phaethon (see Milner & Walsh, 2009: fig. 8). The floccular lobes of Pa. antarcticus and S. humboldti appear much more strongly developed in dorsal view than those of Py. antarctica and A. patagonicus (Fig. 3C). However, upon closer examination the degree of lateral projection of the floccular lobes is actually quite similar for all taxa. Differences in appearance are largely because of the fact that the cerebral hemispheres overlie more of the cerebellum in Py. antarctica and A. patagonicus, which obscures the floccular lobes in dorsal view. Clear separation of the cerebral hemispheres and floccular lobes appears to be correlated to the development of the temporal fossae. Paraptenodytes antarcticus and S. humboldti have deep, dorsally extensive temporal fossae that incise the skull to such a degree as to displace the cerebral hemispheres rostrally. In Py. antarctica and A. patagonicus, the temporal fossae are shallow and restricted to the lateral part of the skull, and the cerebrum obscures the endosseous flocculae in dorsal view.
Semicircular canals
In the endocast of the bony labyrinth for Paraptenodytes, all three canals are intact on both sides, and the sacculus and cochlear duct are also observable (Fig. 7). The anterior, horizontal, and posterior canals are not orientated at right angles to one another, but are instead skewed. In addition, the anterior canal is strongly tilted posteriorly, a feature typical of birds and some non-avian theropods and hypothesized to enhance sensitivity to pitch movements (Gray, 1955). In Paraptenodytes, the horizontal canal projects laterally so as surpass the floccular lobe in dorsal view, whereas in the extant penguins the horizontal canal does not project as far laterally as the floccular lobe. However, the origin of the caudal horizontal canal lies on the caudal surface of the crus commune, and the canal therefore lacks some of the expansion that occurs in avian species in which the caudal origin of the canal lies on the medial surface of the crus commune. The cochlear duct is orientated anteromedially and projects ventrally beyond the level of the medulla oblongata. Interestingly, the semicircular canal system is larger (relative to brain size) in Paraptenodytes than in extant penguins. This is true both in terms of individual canal circumference and the height/width of the system as a whole (Table 2).
Eustachian canal
The Eustachian canal (tuba auditiva) is fully ossified throughout its course in Pa. antarcticus and in all extant penguins examined. The posterior opening occurs in the tympanic cavity, from which the canals run anteromedially to exit at the base of the parasphenoid rostrum. The paths of the Eustachian canals are directly ventral to those of the carotid canals for much of their length. An ossified Eustachian canal was reported in extant penguins by Saiff (1976), which agrees with observations from the endocasts. In some individuals of Py. antarctica, the anterior portion of the canal wall fails to ossify (Ksepka et al., 2006), although in our scanned specimen the wall is complete.
Carotid arteries
The cranial carotid arteries enter the braincase at the carotid foramina and travel anteromedially, each in a separate ossified canal (Fig. 5). The posterior end of each canal is formed by a thin and delicate sheet of bone in extant penguins. Broken edges indicate that abrasion has destroyed the posterior portions of the canals in AMNH 3338. The left and right cranial carotid canals anastomose immediately posterior to the pituitary fossa. Baumel & Gerchman (1968) recognized three major types of anastomosis in birds: H-type, in which the anastomosis is an elongate transverse vessel; X-type, in which the left and right carotid converge to a short anastomosis; and I-type, in which the left and right carotids merge into a single midline vessel for a considerable length. Following this terminology, the cranial carotid arteries of Pa. antarcticus form an X-type anastomosis (Fig. 5C). Baumel & Gerchman (1968) found an H-type anastomosis in Py. adeliae and an X-type anastomosis in Spheniscus demersus. The present study adds to these observations the identification of an H-type anastomosis in Py. antarctica (Fig. 5F) and A. patagonicus (Fig. 5E), and an X-type anastomosis in S. humboldti (Fig. 5D). Gavia immer (Fig. 5A) and Ph. immutabilis (Fig. 5B) also exhibit an X-type anastomosis (Baumel & Gerchman, 1968).
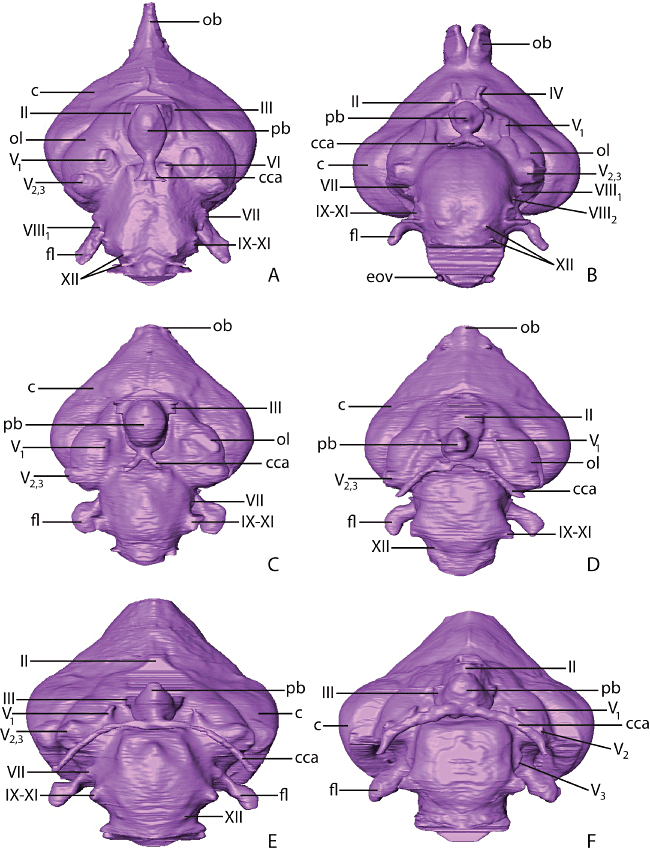
Virtual endocasts of A, Gavia immer (common loon); B, Phoebastria immutabilis (Laysan albatross); C, Paraptenodytes antarcticus (fossil stem penguin); D, Spheniscus humboldti (Humboldt penguin); E, Aptenodytes patagonicus (king penguin); and F, Pygoscelis antarctica (chinstrap penguin) in ventral aspect. Abbreviations: c, cerebrum; cca, cranial carotid artery; fl, floccular lobe; ob, olfactory bulb; ol, optic lobe; pb, pituitary body; II−XI, cranial nerves II−XI.
Cranial sinuses
Dramatic differences in the extent of the anterior tympanic recess are visible 6between 7Pa. antarcticus and extant penguins. In extant penguins, the anterior tympanic recess has a smaller volume and its anterior limit is restricted to near the level of the quadrate. In Pa. antarcticus, the sinus is large and continues further anteromedially 8to inflate the parasphenoid rostrum (Fig. 9). The left and right anterior tympanic recesses meet at a level slightly posterior to the cranial carotid anastomosis and form a large chamber ventral to the pituitary fossa (Fig. 9). The left and right anterior tympanic recesses in extant penguins end far posterior to this point (Fig. 9), and as a consequence of this arrangement do not meet.

Virtual endocasts of A, Gavia immer (common loon); B, Phoebastria immutabilis (Laysan albatross); C, Paraptenodytes antarcticus (fossil stem penguin); D, Spheniscus humboldti (Humboldt penguin); E, Aptenodytes patagonicus (king penguin); and F, Pygoscelis antarctica (chinstrap penguin) in posterior aspect. Abbreviations: c, cerebrum; cb, cerebellum; cca, cranial carotid artery; eov, external occipital vein; fl, floccular lobe; mcv, middle cerebral vein; os, occipital sinus; se, sagittal eminence; II−XI, cranial nerves II−XI.

Virtual endocasts of the labyrinth of A, Gavia immer (common loon); B, Phoebastria immutabilis (Laysan albatross); C, Paraptenodytes antarcticus (fossil stem penguin); D, Spheniscus humboldti (Humboldt penguin); E, Aptenodytes patagonicus (king penguin); and F, Pygoscelis antarctica (chinstrap penguin) in posterior aspect. Abbreviations: aa, ampulla of anterior semicircular canal; asc, anterior semicircular canal; cc, common crus; ed, endolymphatic duct; ha, ampulla of horizontal semicircular canal; hsc, horizontal semicircular canal; lc, lagenar canal; pa, ampulla of posterior semicircular canal; psc, posterior semicircular canal; vf, vestibular foramen.
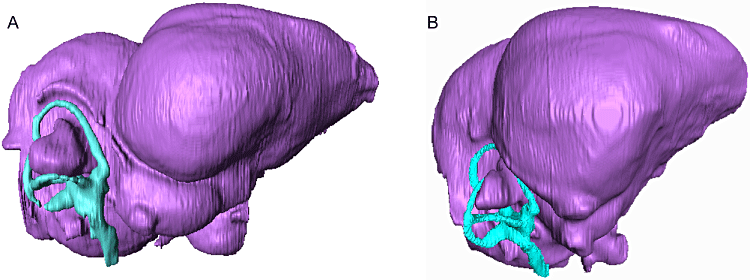
Virtual endocasts of the brain and labyrinth of A, Paraptenodytes antarcticus (fossil stem penguin) and B, Aptenodytes patagonicus (king penguin) in lateral aspect, illustrating relative size of the labyrinth.
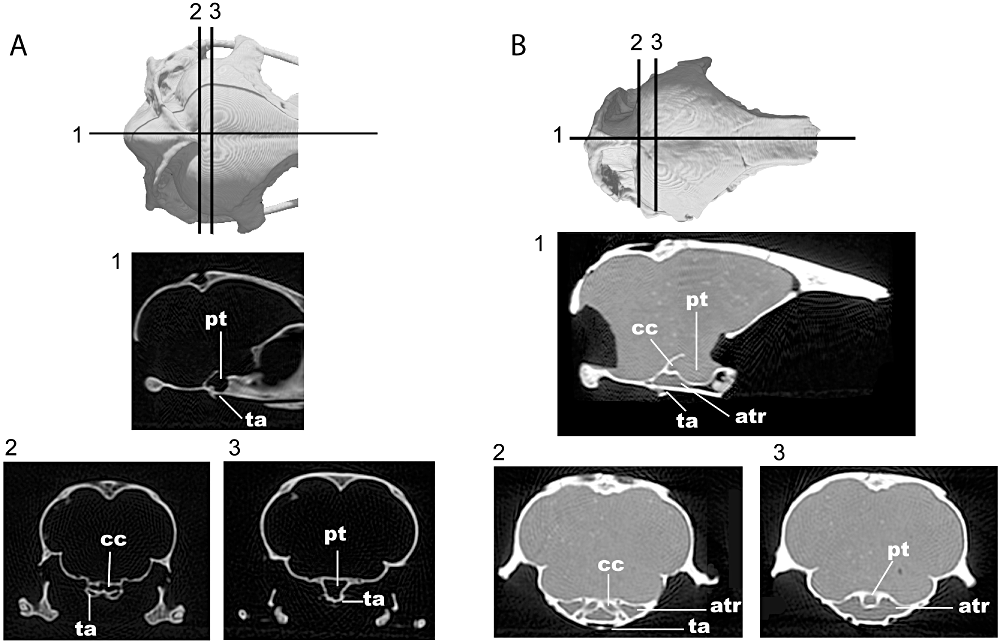
Computed tomography slices of the skull of A, Spheniscus humboldti and B, Paraptenodytes antarcticus in 1, sagittal and 2–3, coronal planes. Abbreviations: atr, anterior tympanic recess; cc, cranial carotid artery; pt, pituitary space; ta, tuba auditiva.
The individual bones of the skull roof exhibit a denser construction in penguins than most other birds. For example, the diploe of the bones forming the skull roof is pneumatized in most birds (G. immer being one exception), whereas in penguins there is no discernible air space between the inner and outer surface of these bones. Likewise, the postorbital process, which shows significant pneumatization in G. immer and Ph. immutabilis, is essentially solid in most extant penguins (although Aptenodytes forsteri shows a small amount of pneumatization). Spheniscus departs slightly from other extant penguins in terms of cranial bone structure, showing a more cancellous internal structure.
Reduction of skeletal pneumaticity occurs in many aquatic avian lineages, including Anatidae, Rallidae, and Gaviidae, and correlation between reduced pneumaticity and diving ecology has been demonstrated (Smith, 2011). Extant penguins have eliminated most cranial pneumaticity and essentially all postcranial skeletal pneumaticity. The reduction of pneumaticity in penguins may stem from selective pressure for negative buoyancy and avoidance of problems associated with decompression of gases. However, it should be noted that advantages gained in these directions by reducing cranial pneumaticity are probably minor, as extant penguins retain significant pneumaticity in the skull and the difference in buoyancy gained by eliminating cranial pneumaticity is negligible compared to the effects of density increase in the limb bones and large increases in the size of the pectoral musculature in penguins.
Volumetric data
Volumetric analyses indicate that the endocranial volumes of penguins relative to body size fall within the range of modern volant birds (Fig. 10). Even considering the increased skeletal density observed in flightless birds, extant penguins are at the upper range for crown birds and values fall above the best-fit line for all observed taxa. Paraptenodytes antarcticus had a body mass approximately equal to that of A. patagonicus based on limb bone dimensions. Using the average mass for A. patagonicus as an estimate for the mass of Pa. antarcticus suggests that this fossil species had a larger endocranial volume for its body size than the extant penguins (Fig. 10A). It is interesting to note that penguins are much heavier than similarly sized volant birds because of their dense bones, thick fat layers, and large ‘flight’ muscles. If an alternate measure of size such as body length were to be used instead of mass, it would result in penguins moving further above the regression line.
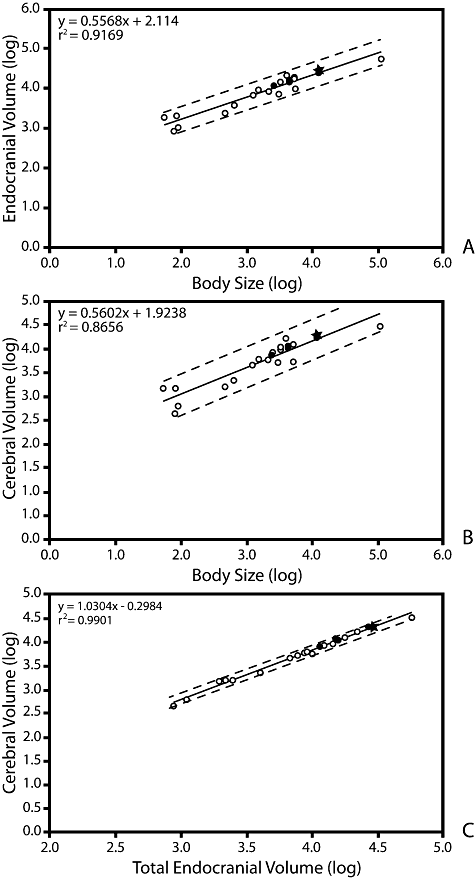
Log-log plots of A, total endocranial volume and body size; B, cerebral volume and body size; C, cerebral volume and total endocranial volume. Closed circles indicate penguin taxa. Paraptenodytes is shown as a closed star. All other avian taxa are indicated by open circles. Dashed lines indicate 95% confidence intervals.
The positions of penguin taxa within the charts of isolated cerebrum relative to body size do not diverge greatly from those of total endocranial volume relative to body size (Fig. 10B). This result is not surprising given that these two variables tend to scale proportionally with each other. Penguins again are positioned near the top of the range for extant birds, with Pa. antarcticus and A. patagonicus having the largest relative cerebral volumes for their body size (Table 2).
DISCUSSION
Extant penguins are capable of rapid manoeuvring in three-dimensional space. The underwater environment inhabited by penguins is arguably more spatially complex than that navigated by volant birds. Although aquatic, penguins locate their prey primarily by sight. Several endocast features observed in Pa. antarcticus confirm that neuroanatomical adaptations compatible with fast and manoeuvrable aquatic ‘flight’ capabilities were present in stem lineage penguins. First, the unusually large size of the cerebellar flocculus relative to the rest of the endocast suggests that Pa. antarcticus was especially adapted for pursuit diving. This region is mostly responsible for integrating afferent stimuli from the retina and vestibular system to ensure the occulomotor system stabilizes a focal image on the retina despite movements of the head and body during locomotion (vestibulo-occular reflex; Witmer et al., 2003). The horizontal semicircular canal is expanded, although less so than in the most manoeuvrable extant aerial fliers, which are characterized by a caudal origin of the canal on the medial surface of the crus commune (Sipla, 2007). The expansion of the horizontal canal suggests an increased sensitivity to yaw movement, which may represent an adaptation for rapid sideways turns during pursuit diving, possibly indicating a preference for capturing isolated prey items rather than feeding on densely shoaling planktonic species.
Importance of the sagittal eminence in visual field signal processing is well documented, and the notable development of this region in Pa. antarcticus might also be expected to relate to sensory adaptations for underwater flight. The sagittal eminence is typically much larger in species with forward facing eyes that are capable of partial stereopsis (e.g. owls) than in species with laterally facing eyes (e.g. pigeons; Dubbeldam, 1998; Iwaniuk & Wylie, 2006). As the binocular field of extant penguins falls within the Type 1 (20°–30°) visual field type of Martin (2007), stereopsis seems unlikely to have been present in Pa. antarcticus. However, stereopsis is not a prerequisite for accurate interpretation of fast-flowing visual fields (Martin, 2007), and the large size of the sagittal eminence in Pa. antarcticus may instead relate to some other visual adaptation such as visual perception in low light conditions during deep diving. Although little is known of the dive depths of extinct penguins, maximum dive depth is tightly correlated to body size in extant penguins (Williams, 1995) and other marine birds (Piatt & Nettleship, 1985). Many extant penguin species are capable of reaching depths where light levels fall below those measured under terrestrial starlight and tracking nonbioluminescent prey visually becomes difficult (Williams, 1995; Martin, 1999). Given the large estimated body mass and derived flipper structure of Pa. antarcticus, this fossil species may have been capable of deep diving as well.
One complication to interpretations of palaeoecology based on properties of the sagittal eminence is that, as a mesopallial and hyperpallial outgrowth of the telencephalon, the large size of the sagittal eminence region in some clades also appears to be related to higher cognitive functions such as problem solving, tool use, and behavioural flexibility (Lefebvre et al., 1997, 2004; Cnotka et al., 2008), migrational navigation (Heyers et al., 2007), and even self awareness (Butler & Cotterill, 2006; Prior, Schwarz & Güntürkün, 2008). Presently, any correlation of this structure to complex flight or diving capabilities remains far from clear. For instance, the sagittal eminence is extremely large in some flightless birds such as the elephant bird Aepyornis hildebrandti (Wiman & Edinger, 1942), Struthio camelus (A. M. Balanoff, unpubl. data), and Dinornis spp. (Ashwell & Scofield, 2008). It seems implausible that such metabolically expensive tissue would be maintained in these taxa if the cell layers of the sagittal eminence were primarily concerned with dealing with the visual demands of aerial locomotion (Walsh & Milner, 2011a). It therefore seems likely that the development of the sagittal eminence in Pa. antarcticus and extant Spheniscidae may relate to a variety of both visual and nonvisual functions.
At present, the phylogenetic pattern of shifts in sagittal eminence development remains poorly understood for birds. This structure is absent in non-avian dinosaurs (Franzosa, 2004; Sanders & Smith, 2005) as well as early avialans such as Archaeopteryx (Milner & Walsh, 2009). It was also reported absent in a supposed endocast of an avialan used to erect the Cretaceous taxon ‘Cerebavis cenomanica’ (Kurochkin et al., 2006, 2007). However, this specimen was later recognized to represent an abraded skull rather than a natural endocast and details of the internal anatomy are unknown at present (Walsh & Milner, 2011a). Amongst extant birds, distribution varies somewhat, but the sagittal eminence appears to be well developed in nearly all taxa (Stingelin, 1957). In Palaeognathae, the sagittal eminence is extremely poorly projected in the kiwi Apteryx (Franzosa, 2004; Martin et al., 2007), but is well developed in other surveyed taxa including the tinamou Crypturellus cinnamomeus and the ratites Struthio camelus, Aepyornis hildebrandti, and Dinornis novaezealandiae (Wiman & Edinger, 1942; Franzosa, 2004; Ashwell & Scofield, 2007; A. M. Balanoff, unpubl. data). In Neognathae, the sagittal eminence is well developed in most surveyed species, including for example the anseriforms Anas platyrhynchos and Chauna chavaria (Franzosa, 2004), the bucerotiform Bucorvus abyssinicus (A. M. Balanoff, unpubl. data), the columbiform Columba livia (Proctor & Lynch, 1993), and Sphenisciformes (this study). This distribution suggests that a well-developed sagittal eminence is synapomorphic at the level of crown Aves or a more inclusive clade and reduction of this structure has occurred in multiple lineages.
Consideration of Palaeogene fossil taxa complicates the picture. Interestingly, the Eocene seabirds Prophaethon shrubsolei (Prophaethonidae: stem tropicbirds) and Odontopteryx toliapica (Pelagornithidae: Anseriformes or stem Galloanserae) both possess a very weakly developed sagittal eminence (Milner & Walsh, 2009). In contrast, the contemporary Halcyornis toliapicus (Halcyornithidae), tentatively identified as a stem parrot (Mayr, 2007), exhibits a strongly projected sagittal eminence (Walsh & Milner, 2011b). As Pr. shrubsolei and O. toliapica are both clearly nested within Neognathae (Bourdon, 2005; Bourdon, Bouya & Iarochene, 2005; Smith, 2010; Mayr, 2011), the weak development of the structure in these taxa requires additional evolutionary transitions. A very weakly developed sagittal eminence may be the result of secondary reduction in the fossil seabird taxa. Alternatively, weak development may represent a primitive condition, supporting multiple independent increases in sagittal eminence development in Aves. Only denser sampling of brain morphology across stem members of avian clades can provide the data needed to test whether parallel trends in enlargement occurred across multiple clades during the Tertiary. Additionally, data from Mesozoic Avialae are needed to properly polarize shifts in cerebral development. Unfortunately, endocasts from basal birds other than Archaeopteryx remain essentially non-existent, because the Cretaceous fossil record of birds is dominated by flattened specimens preserved on slabs and most three-dimensional specimens comprise only postcranial elements.
Absence of the interaural pathway in extant penguins is clearly a derived feature. An interaural pathway was observed in Gaviiformes and Procellariiformes in this study, and appears to be present in all extant birds other than penguins (see Starck, 1995). Furthermore, the interaural pathway has been reported in basal birds such as Hesperornis, Parahesperornis, and Enaliornis (Witmer, 1990) and some non-avian theropods (e.g. Currie, 1985). Loss of this pathway is currently documented only in extant penguins, although some stem fossil taxa that fall closer to the crown clade than Paraptenodytes have not yet been sampled (e.g. Marplesornis).
The interaural pathway of birds is believed to assist in directional localization of sound via acoustic interaction across this pathway (Calford & Piddington, 1988). How differences in sound transmission underwater affect the functionality of the interaural pathway has not been studied. Thus, whether loss of the interaural pathway has any effect on underwater hearing is unclear. Directional localization of sound may be less important to penguins because they locate their prey visually. However, localization may still be useful for penguins in terrestrial settings for locating chicks or nesting mates as well as predators such as skuas. The question of why the interaural pathway was lost in penguins still remains. Selective pressure for diving seems unlikely, as the positive buoyancy created by such a small space is negligible. Furthermore, extant penguins still retain the anterior tympanic recesses and other cranial sinuses, and so can clearly handle changes in gas volume associated with deep diving.
We propose that the separation of the contralateral anterior tympanic recesses can also be interpreted as evidence for paedomorphosis within crown penguins. These recesses have been described as separate in juvenile mousebirds (Goldschmid, 1972) and juveniles of the rail Fulica atra (Macke, 1969). In these and other groups of extant birds, the contralateral anterior tympanic recesses attain a connection only during posthatching ontogeny. Failure of the anterior tympanic recesses to join in adult extant penguins may thus represent a truncation of the normal ontogenetic trajectory of this recess. Several additional morphological features of penguins have been attributed to paedomorphosis (Livezey, 1989; Mayr, 2005) relative to outgroup waterbirds and/or stem Sphenisciformes. The shortened bill of living penguins relative to stem taxa mirrors the proportionally shortened bill of hatchlings relative to adults. Likewise, weak fusion of the metatarsals in extant penguins contrasts with the more completely fused metatarsals in many stem fossil penguins and other waterbirds. In order to evaluate these hypotheses quantitatively, a growth series from a stem penguin taxon would be necessary. Unfortunately, hatchling and juvenile penguin fossils are extremely rare and aside from a single, nearly complete subadult skeleton of the stem taxon Kairuku (Ksepka et al., 2012) are limited to isolated bones (Olson, 1985; Walsh & Hume, 2001; Jadwiszczak, 2006; Ksepka & Thomas, 2012)
CONCLUSIONS
Two major events define penguin evolution: the acquisition of wing-propelled diving and subsequent loss of aerial flight. The fossil record remains silent on when the first event occurred and on the morphology of early, volant stem penguins. Although penguins probably diverged from other extant avian lineages in the Cretaceous, no volant basal members of the penguin lineage have been recognized in the fossil record and there is no record of any member of the clade until the Palaeocene (Slack et al., 2006). Flightless stem penguins are well represented in the Cenozoic record and provide an opportunity to explore the later phases of adaptation to an aquatic lifestyle. Although the most profound changes in neuroanatomy might be expected to occur at these two major locomotor transitions, a surprising suite of differences were noted in this study between the endocranial anatomy of the Miocene stem penguin Pa. antarcticus and extant penguins, indicating that significant modification of the neuroanatomy and cranial sinuses occurred tens of millions of years after the initial loss of aerial flight in penguins. Expanding taxonomic sampling to include more basal fossil penguins may reveal earlier phases in the evolution of penguin neuroanatomy. Comparisons to other avian taxa such as Alcidae and Pelecanoididae are also desirable, to help determine whether convergences in the brain and sensory organs occur as birds evolve a wing-propelled diving ecology.
ACKNOWLEDGEMENTS
This research was supported by NSF DEB grant 0949899. We thank Justin Sipla and Justin Georgi for scanning penguin specimens used in this study, and Tim Rowe and Digimorph.org for providing access to outgroup scans. Comments from two anonymous reviewers also helped improve this manuscript.




