Morphological and molecular characterization of the problematic whip black coral genus Stichopathes (Hexacorallia: Antipatharia) from Indonesia (North Sulawesi, Celebes Sea)
Abstract
This study represents a preliminary systematic reorganization of the critical whip black coral genus Stichopathes from Indonesia, and a validation test of its principal morphological features as suitable taxonomic characters. A phylogenetic analysis based on rDNA internal transcribed spacer sequences ITS1 and ITS2 was performed on several specimens coming from different areas of the Indonesian Archipelago. Within the family Antipathidae, these analyses confirmed the separation of the three traditional genera of whip black corals (Stichopathes, Cirrhipathes, and Pseudocirrhipathes). Additionally, the analyses identified five clades for the studied Stichopathes specimens. In each clade, the wire specimens were well characterized by a distinctive set of morphological features, including: the shape of the corallum, the size and arrangement of the polyps, and the shape of the spines. The molecular data obtained, combined with other sequences available in the literature, indicate that the traditional genus Stichopathes is a polyphyletic taxon. In three clades, unbranched Stichopathes-like specimens group together with branched specimens morphologically belonging to the genus Antipathes. This evidence suggests caution when using the corallum branching pattern in the taxonomy of the order, as this character may have evolved separately in different taxa, thus suggesting that an extensive taxonomic revision of the whip black coral genera is required.
© 2012 The Linnean Society of London, Zoological Journal of the Linnean Society, 2012, 166, 1–13.
INTRODUCTION
The order Antipatharia Milne-Edwards & Haime, 1857 (Cnidaria, Anthozoa) is a small taxon of hexacorallians with a worldwide distribution, mainly at depths exceeding 100 m (Opresko & Försterra, 2004). In tropical and subtropical regions, such as the Indonesian Archipelago or the Caribbean Sea, these corals are particularly abundant in shallow water coral reefs where they create multi-specific communities occupying various types of rocky habitat (Sánchez, Díaz & Zea, 1998; Sánchez, 1999; Tazioli et al., 2007).
Morphological characters (such as the general structure of the corallum, the pattern of ramification, and the shape of spines and polyps) are traditionally used as systematic features to discriminate the species within the order (Opresko, 1972). Modern microscopy techniques have much improved the observation of these distinguishing features and their ultrastructural variations, leading to the revision of many groups (Opresko, 2001, 2002, 2003, 2004, 2006). However, the separation of the nominal species of shallow water antipatharians still appears to be confused, and their number may be overestimated. The species characterization is complicated by the phenotypic plasticity induced by the variable environment, particularly in shallow water rich communities, (Warner, 1977, 1981; Lapian et al., 2007; Wagner et al., 2010).
Whip black corals, formally described as unbranched and unpinnulated antipatharians, constitute a morphological category of species characterized by a simple, single-stem corallum. They are found worldwide, with the exception of the Mediterranean Sea where existing records are considered doubtful (Pallas, 1766; Brook, 1889). Several species are found in deep habitats (Genin et al., 1986; Molodtsova, 2006), but the highest diversity is recorded in the Caribbean and Indo-Pacific shallow water coral reefs (Brook, 1889; Schultze, 1896; van Pesch, 1914; Grigg & Opresko, 1977; Sánchez et al., 1998; Echeverria, 2002; Opresko & Sánchez, 2005; Tazioli et al., 2007). Their adult size ranges from a few centimetres to several metres in length (Pax, van-Praët & Doumenc, 1987), and in unusually strong currents they can be recorded aggregated in dense meadows (Genin et al., 1986; Sánchez et al., 1998; Opresko & Sánchez, 2005; Tazioli et al., 2007; Bo et al., 2009a). Their general morphology is distinguished into three major subgroups on the basis of the convolution of the stem: straight, helicospiral, and contorted corallum.
From the systematic point of view, three genera are considered to be representative of the whip morphology: StichopathesBrook, 1889, Cirrhipathes (Blainville, 1857), and the recently described Pseudocirrhipathes Bo & Bavestrello, 2009 (Bo et al., 2009b). Their separation is mainly based on the arrangement of polyps along the stem: in Cirrhipathes the polyps are present all around the axis; in Stichopathes they form a unique line on one side (Brook, 1889; van Pesch, 1914); whereas in Pseudocirrhipathes an intermediate situation is observed, with polyps irregularly distributed but only on one side of the stem (Bo et al., 2009b). Among the three genera, Stichopathes shares the greatest similarity with the genus Antipathes, with both being characterized by polyps regularly distributed in one line along the ramifications. Nevertheless, the latter taxon groups a wide range of very different species, always showing ramified colonies, with various types of spines and transversally elongated polyps.
The Indonesian community is especially rich in terms of species diversity, and recent ecological investigations have highlighted the bathymetric distribution, growth rates and strategies, feeding behaviours, symbioses, the in vivo appearance of polyps, and biological characteristics of some of the Indonesian whip black coral species (Tazioli et al., 2007; Gaino et al., 2008; Gaino & Scoccia, 2008; Bo et al., 2009a, b, 2010).
In total, 54 nominal species of whip black corals are known in the literature: 35 Stichopathes, 18 Cirrhipathes, and one Pseudocirrhipathes. These species are distributed in a wide bathymetric range, even if generally they are found within the first 100 m of the water column. Although these organisms are quite common on tropical shallow water reefs, especially those of the Indo-Pacific Ocean, their taxonomy is not completely clear. Major sources of uncertainty in the species determination are hypothesized to be the ecologically induced variations in corallum morphology (Warner, 1981; Bavestrello et al., 2012), which may lead to a high subjectivity in choosing the taxonomic characters, and the status of the preservation of type specimens. The latter, when still available, often lack polyps or are constituted only by fragments that do not allow consideration of the spine variability along the stem.
Nucleotide sequences are an obvious source of additional evidence on the systematic relationships of these corals. This approach has already been used in combination with morphological analyses to solve the taxonomy of this problematic group (Brugler & France, 2007; Lapian et al., 2007; Bo et al., 2009b; Wagner et al., 2010). As black coral mitochondrial DNA is scarcely variable, and therefore poorly informative, the rDNA internal transcribed spacer sequences ITS1 and ITS2, which have commonly been used for intra- and interspecific studies, were considered (Odorico & Miller, 1997; Takabayashi et al., 1998; van Oppen et al., 2000; Diekmann et al., 2001; van Oppen, Wörheide & Takabayashi, 2002; Rodriguez-Lanetty & Hoegh-Guldberg, 2002; Lam & Morton, 2003; Chen et al., 2004; Lapian et al., 2007; Bo et al., 2009b). The ITS sequences have been demonstrated to be suitable for solving the phylogenetic relationships of some closely related taxa in anthozoans, such as the scleractinians (Takabayashi et al., 1998). Recently, Lapian et al. (2007) reported a divergence of 17.38% within the seven considered Indonesian species of the family Antipathidae, confirming the reliability of these molecular markers for this taxon. Concerning whip black corals, ITS sequences clearly separated the three genera, which share single-stem morphology, probably as a result of evolutive convergence (Lapian et al., 2007; Bo et al., 2009b, 2011; Wagner et al., 2010).
The main goal of this article is to phylogenetically analyse 14 new specimens of whip antipatharians taken from several Indonesian coral reefs, which have tentatively been attributed to the genus Stichopathes on a morphological basis. The strength of the taxonomic characters defining the genus Stichopathes is considered, and the position of this genus in the family Antipathidae is stated.
MATERIAL AND METHODS
Sample collection and morphological analysis
Fourteen specimens of whip coral were photographed underwater then collected by SCUBA diving on the shallow water reefs of five localities of the Indonesian Archipelago, within a depth range of 10–50 m (Fig. 1): Bunaken Marine Park (North Sulawesi) (three samples); Raja Ampat Marine Park (Irian Jaya) (four samples); Ambon Island (Moluccas Sea) (three samples); Bali Island (Flores Sea) (two samples); and Mentawai Islands (Indian Ocean) (two samples). Two specimens of Rhipidipathes reticulata (Esper, 1795) (RHIP2, RIPSIL) and a juvenile colony of the genus Antipathes (INDO20), collected in the Bunaken Marine Park, were also analysed.
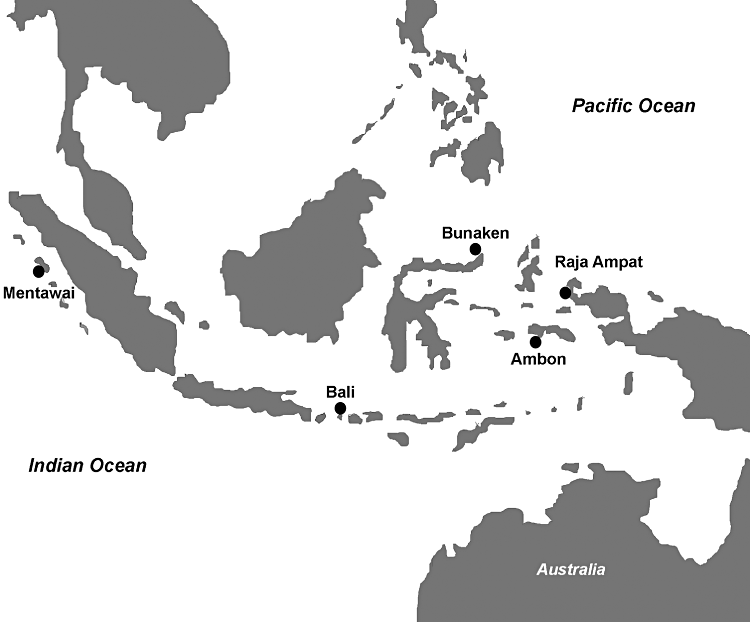
Map of the sampling localities in the Indonesian Archipelago.
A portion of each sample was preserved in buffered 4% formaldehyde for morphological analysis of polyps and spines (Bo et al., 2009b), under both optical and scanning electron microscopy (SEM). For the SEM analysis of the spines, fragments of stems (apical and central portions of the colonies) were coated with gold-palladium in a Balzer Union evaporator and examined using a Philips XL20 SEM. The remaining portion of each collected specimen was preserved in 95% ethanol and used for phylogenetic analyses.
Phylogenetic analyses
The genomic DNA was extracted by a Qiamp tissue kit (Qiagen, Hilden, Germany), and then PCR-amplified using primer RA2 and primer ITS 2.2 (Wörheide, 1998) for ITS1 and ITS2, using the HotStar Taq Master Mix Kit (Qiagen) under the following conditions: 94 °C for 30 s; 52 °C for 30 s; and 72 °C for 60 s (for 30 cycles). Cycle sequencing reactions were performed using the Big Dye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Warrington, UK) according to the protocol provided by the manufacturer, with the same primers used in the PCR. To obtain the sequences in both directions, a forward primer and a reverse primer designed on the 5.8S rDNA (Lapian et al., 2007) were used. The sequencing reaction product was sequenced on an automated DNA sequencer (ABI PRISM 310; Applied Biosystems).
The reference sequences used to delimit the different markers included in the analyses (partial 18S rDNA, full-length ITS1, 5.8S rDNA, ITS2, and partial 28S rDNA) were HM060618 and HM060625 (Wagner et al., 2010). In a few specimens ITS1 and ITS2 highlighted an intra-individual variability, but this was lower than 0.2%.
The complete sequences of 17 collected specimens were aligned by ClustalW2 (using the default settings; Larkin et al., 2007) with 28 other sequences of previously analysed species (Lapian et al., 2007; Bo et al., 2009b; Wagner et al., 2010). The scleractinian coral Porites lutea Milne-Edwards & Haime, 1860 was used as an out-group. The complete list of the species analysed and the relative sequence accession numbers (deposited in GenBank) are reported in Table 1.
| Family and species | Code | Locality | Accession number | References |
|---|---|---|---|---|
| Antipathidae | ||||
| Allopathes desbonni | USNM88327 | Louisiana, USA | FM882171* | Bo et al., 2009b |
| Antipathes atlantica | na | Hawai'i | HM060621/HM060624 | Wagner et al., 2010 |
| Antipathes caribbeana | USNM1122627 | Netherlands Antilles | GU296498/GU296486 | Wagner et al., 2010 |
| Antipathes curvata | USNM1015453 | South China Sea | GU296497/GU296485 | Wagner et al., 2010 |
| Antipathes elegans | BUNA6 | Bunaken, Indonesia | AM404317 | Lapian et al., 2007 |
| Antipathes furcata | na | Hawai'i | HM060620/HM060623 | Wagner et al., 2010 |
| Antipathes grandis | BUNA2 | Bunaken, Indonesia | AM404316 | Lapian et al., 2007 |
| Antipathes grandis | na | Hawai'i | GU296493/GU296480 | Wagner et al., 2010 |
| Antipathes griggi | na | Hawai'i | GU296496/GU296484 | Wagner et al., 2010 |
| Antipathes sp. 1 | BUNA25 | Bunaken, Indonesia | AM404315 | Lapian et al., 2007 |
| Antipathes? sp. 2 | ANT10 | Bunaken, Indonesia | AM404321 | Lapian et al., 2007 |
| Antipathes sp. 3 | INDO20 | Bunaken, Indonesia | HE600716 | Present study |
| Cirrhipathes spiralis | BUNA23 | Bunaken, Indonesia | AM404320* | Lapian et al., 2007 |
| Cirrhipathes sp. | ANT2b | Bunaken, Indonesia | AM404319 | Lapian et al., 2007 |
| Pseudocirrhipathes mapia | ANT5 | Bunaken, Indonesia | FM882167 | Bo et al., 2009b |
| Pseudocirrhipathes mapia | CMLIK | Bunaken, Indonesia | FM882168 | Bo et al., 2009b |
| Stichopathes cf. occidentalis | SED804 | NW Atlantic Ocean | HM060618/HM060625 | Wagner et al., 2010 |
| Stichopathes clade A | BALA48 | Bali, Indonesia | HE600722 | Present study |
| Stichopathes clade A | AMBA5 | Ambon, Indonesia | HE600721 | Present study |
| Stichopathes clade B | BUK11 | Bunaken, Indonesia | HE600719 | Present study |
| Stichopathes clade B | INDO19 | Bunaken, Indonesia | HE600720 | Present study |
| Stichopathes clade C | AMBA6 | Ambon, Indonesia | HE600717 | Present study |
| Stichopathes clade C | MENT52 | Mentawai, Indonesia | HE600718 | Present study |
| Stichopathes clade D | AMBA45 | Ambon, Indonesia | HE600710 | Present study |
| Stichopathes clade D | BALA46 | Bali, Indonesia | HE600711 | Present study |
| Stichopathes clade D | BUK14b | Bunaken, Indonesia | HE600713 | Present study |
| Stichopathes clade D | BUNA14 | Bunaken, Indonesia | AM404318 | Lapian et al., 2007 |
| Stichopathes clade D | MENT8b | Mentawai, Indonesia | HE600715 | Present study |
| Stichopathes clade D | RJ15 | Raja Ampat, Indonesia | HE600712 | Present study |
| Stichopathes clade D | RJ16 | Raja Ampat, Indonesia | HE600708 | Present study |
| Stichopathes clade D | RJ22 | Raja Ampat, Indonesia | HE600709 | Present study |
| Stichopathes clade D | RJ35 | Raja Ampat, Indonesia | HE600714 | Present study |
| Aphanipathidae | ||||
| Aphanipathes cf. sarothamnoides | USNM1007094 | Palau, N Pacific | FM882166 | Bo et al., 2009b |
| Aphanipathes pedata | USNM74819 | Florida, USA | FM882170* | Bo et al., 2009b |
| Rhipidipathes reticulata | BUNA17 | Bunaken, Indonesia | AM404322 | Lapian et al., 2007 |
| Rhipidipathes reticulata | RHIP2 | Bunaken, Indonesia | HE600723 | Present study |
| Rhipidipathes reticulata | RIPSIL | Bunaken, Indonesia | HE600724 | Present study |
| Phanopathes rigida | USNM88335 | Louisiana, USA | FM882169 | Bo et al., 2009b |
| Myriopathidae | ||||
| Antipathella subpinnata | ANTSUB04 | Messina Strait, Italy | AM404329 | Lapian et al., 2007 |
| Cupressopathes abies | BUNA28 | Bunaken, Indonesia | AM404324 | Lapian et al., 2007 |
| Cupressopathes pumila | BUNA7 | Bunaken, Indonesia | AM404326 | Lapian et al., 2007 |
| Cupressopathes sp. 1 | ANT14 | Bunaken, Indonesia | AM404325 | Lapian et al., 2007 |
| Cupressopathes sp. 2 | BUNA3 | Bunaken, Indonesia | AM404323 | Lapian et al., 2007 |
| Myriopathes myriophylla | ANT15 | Bunaken, Indonesia | AM404328 | Lapian et al., 2007 |
| Myriopathes sp. | BUNA4 | Bunaken, Indonesia | AM404327 | Lapian et al., 2007 |
| Poritidae (out-group) | ||||
| Porites lutea | na | Taiwan: Penghu Island | AY722786 | Chen et al., 2004 |
- * For these species, only ITS1 was analysed.
The alignment was 1047 positions long and contained 340 parsimony-informative sites. To ensure the reliability of data, different ClustalW2 alignments were obtained by changing gap open penalty, gap extension, and gap distance, which were calculated using values ranging from 2 to 25, 2.5 to 7.5, and 2 to 6, respectively. All parameter combinations resulted in an identical topology of the phylogenetic trees. The distance matrix (Table S1) was obtained by MEGA4 (Tamura et al., 2007) using the Kimura two-parameter method (Kimura, 1980), with the same correction parameter as was used by Wagner et al. (2010) for antipatharians. The trees were constructed by maximum parsimony (MP) method using the beta version of PAUP 4.8 (Swofford, 1998) and Bayesian inference with MrBayes 3.1 (Huelsenbeck & Ronquist, 2001). Gaps were considered as missing data. The MP tree was constructed following a heuristic search with tree bisection and reconnection (TBR) branch swapping, and using random stepwise additions with 100 replications and 1000 bootstrap replicates. Only minimal trees were retained. For the Bayesian analysis, the optimum substitution model (TVMef + I + G; Rodríguez et al., 1990) was determined using ModelTest 3.7 (Posada & Crandall, 1998). The analysis was performed using all parameter values provided by ModelTest [proportion of invariable sites = 0.2417; gamma distribution shape parameter = 0.4725; substitution model, (A–C) = 1.6573; (A–G) = 3.9637; (A–T) = 1.2081; (C–G) = 1.5572; (C–T) = 3.9637; (G–T) = 1.0000; base frequencies, equal). The Markov chain Monte Carlo (MCMC) was run for 2 000 000 generations, sampling every 100 steps (burn-in 25%). Stationarity was defined as when the standard deviation of split frequencies reached 0.005. The reliability of the nodes was expressed by posterior probabilities.
RESULTS
The sequences obtained of partial 18S rDNA (from 161 to 166 nt), full-length ITS1 (from 223 to 247 nt), 5.8S rDNA (158 nt), ITS2 (from 228 to 288 nt), and partial 28S rDNA (30 nt) for the analysed specimens of the genus Stichopathes have a total length ranging from 827 nt (AMBA6) to 879 nt (BALA48), with the maximum variability for ITS2 (40 nt between AMBA6 and BALA48). The average base composition is: A, 22.3 ± 0.61; C, 27.8 ± 0.29; G, 28.7 ± 0.8; and T, 21.2 ± 0.33. The length of the two Rhipidipathes reticulata (Esper, 1795) sequences is 812 nt (A, 23.0 ± 0.07; C, 26.1 ± 0.14; G, 28.3 ± 0.07; and T, 22.7 ± 0.0), whereas that of Antipathes sp. 3 is 842 nt (A, 22.2; C, 28.0; G, 28.4; and T, 21.4).
Both the Stichopathes sequences and the three new sequences not belonging to the Stichopathes group were compared with the 21 sequences previously studied by Lapian et al. (2007) and Bo et al. (2009b), and with the seven sequences attributed to the genera Stichopathes and Antipathes by Wagner et al. (2010) (Table 1). The phylogenetic trees were obtained by Bayesian inference and MP methods. The reliability of the analyses was improved by constructing phylogenetic trees based on different alignments obtained with different gap penalty values. Results showed differences only at the level of the posterior probabilities and the bootstrap values.
The phylogenetic tree (Fig. 2) clearly separates three main groups of specimens. The first group includes the genera belonging to the family Myriopathidae (Cupressopathes, Myriopathes, and Antipathella). The second group includes the three analysed species belonging to the family Aphanipathidae, namely two species of Aphanipathes[Aphanipathes pedata (Gray, 1857) and Aphanipathes sarothamnoides Brook, 1889], together with Phanopathes rigida (Pourtalès, 1880).

Phylogenetic tree obtained by Bayesian inference, based on internal transcribed spacer rDNA of 45 antipatharian specimens, and with the scleractinian coral Porites lutea as the out-group. The numbers on the left represent the posterior probabilities (> 95). The maximum parsimony tree (length, 975 steps; consistency index, CI, 0.711; and retention index, RI. 0.874) shows identical topology, and the numbers on the right represent the bootstrap estimated values (> 50).
The external cluster of the third group includes the specimens of Pseudocirrhipathes mapia (Bo & Bavestrello, 2009) together with Allopathes desbonni (Duchassaing & Michelotti, 1864), whereas all the species belonging to the genera Antipathes, Stichopathes, Cirrhipathes, and Rhipidipathes form a second cluster.
In the third cluster, besides the separation of the Rhipidipathes clade, two other groups are evident. One comprises all the analysed Cirrhipathes species, three Antipathes species (Antipathes curvatavan Pesch, 1914, Antipathes atlanticaGray, 1857, and Antipathes furcataGray, 1857), and a species of Indonesian Antipathes. The remaining species belonging to the genera Stichopathes and Antipathes are distributed among various groups in the last clade.
The Indonesian Stichopathes specimens, all characterized by a single-stem corallum, with the exception of a branched sample (MENT8b), are divided into four clades, each one associated with clearly distinct morphological characteristics (Fig. 3). Two specimens (AMBA5 and BALA48) form the most distinct and separated group (clade A): they are characterized by a spiral or contorted, thin corallum (less than 1 mm in diameter in the apical portion, and up to 50 cm long) that is elliptical in transverse section (Fig. 3A). Their triangular spines, pointed and laterally compressed, are taller on the polypar side (0.2–0.25 mm high) than on the abpolypar side (0.05–0.1 mm high) along the curved portions. Papillae, at times in the form of marked rounded ridges, are homogeneously distributed on the surface of their spines. Secondary spines are not present (3, 4). The colour of the living coenenchyme is light brown and polyps are small (0.5–0.6 mm in transverse diameter), distributed (0.3–0.4 mm apart, with a density of 7–8 polyps cm–1), and slightly sagittally compressed (Fig. 3D). These specimens are grouped together with the Hawaiian Antipathes grandis Verrill, 1928.
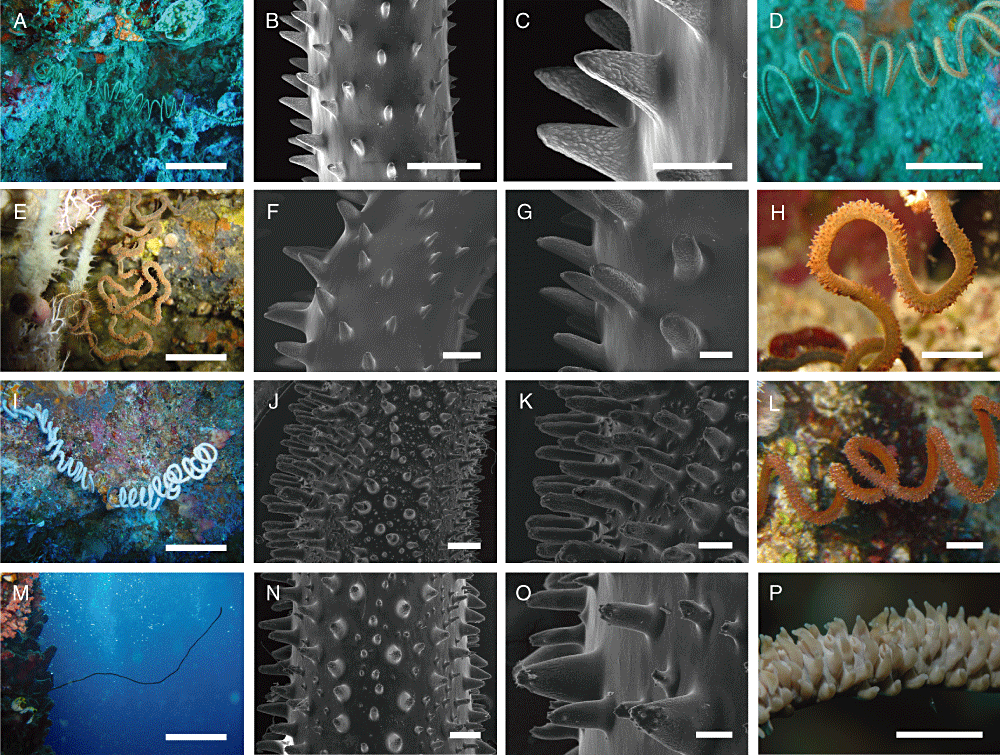
Morphological characteristics of the four Indonesian clades. A–D, clade A. A, spiral or contorted, thin stem. B, C, triangular, laterally compressed, papillose spines. D, light-brown polyps. E, H, clade B. E, contorted, thick stem, with flat and meandritic convolutions. F, G, triangular–conical spines, with few, small apical tubercles, and, at times, basal papillae. H, brown polyps, sagittally compressed. I, L, clade C. I, spiral, thick stem. J, K, triangular–conical spines, with numerous, small apical tubercles and sparse secondary spines. L, dense brown or white polyps, sagittally compressed. M, P, clade D. M, straight, thick stem, several metres long, and often with one or a few lateral branches. N, O, triangular–conical spines, with numerous, small apical tubercles and sparse secondary spines. P, dense brown or white polyps, sagittally compressed. Scale bars: M, 20 cm; A, E, I, 10 cm; D, 5 cm; H, L, P, 0.5 cm; B, F, J, N, 400 µm; C, G, K, O, 200 µm.
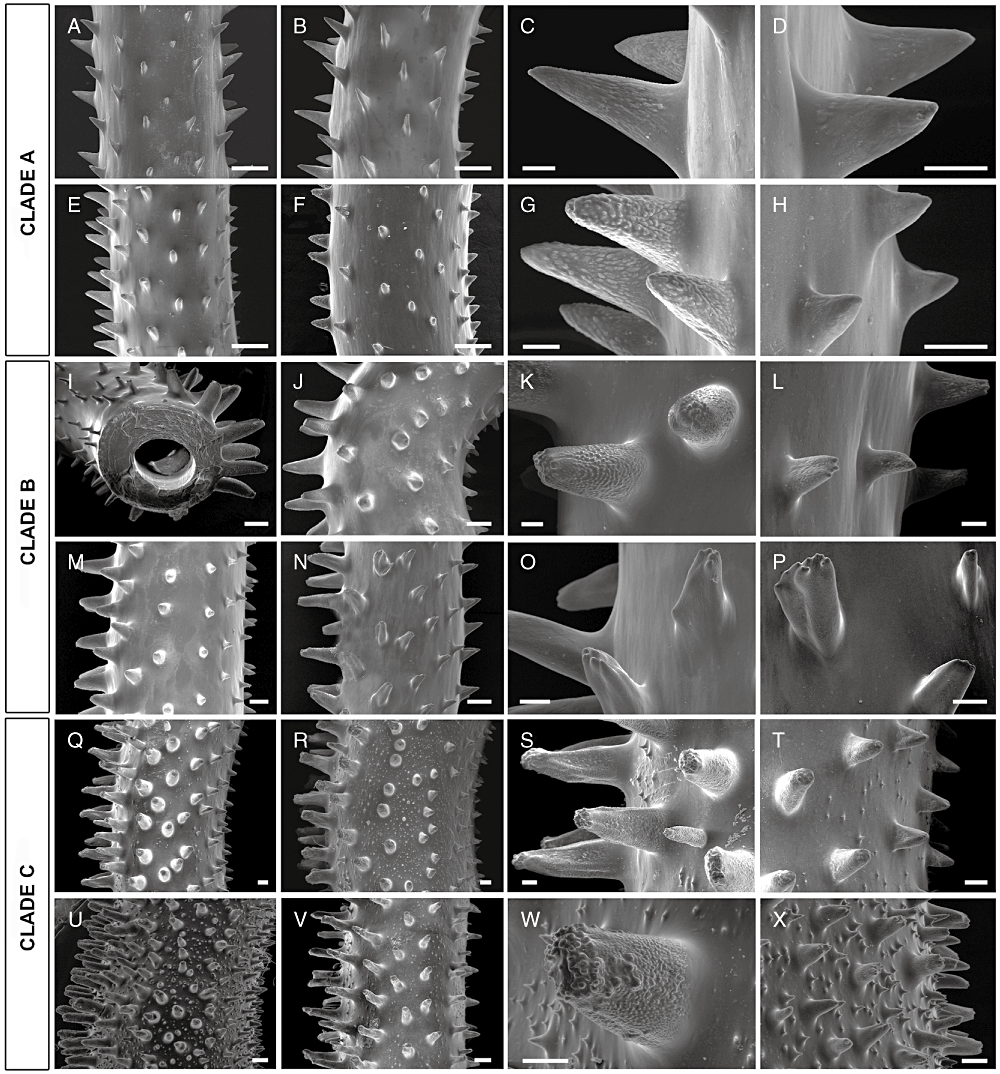
SEM photographs showing the variability of the spines in clade A (A–H), clade B (I–P), and clade C (Q–X) (central portions shown). A, E, straight portions of the stem. B, F, curved portions of the stem. C, G, polypar spines. D, H, abpolypar spines. I–J, M–N, curved portions of the stem. K, O, polypar spines. L, M, abpolypar spines. Q–R, U–V, curved portions of the stem. S, W, polypar spines. T, X, abpolypar spines. Scale bar: A, C, E, F, I, J, M, N, Q, R, U, and V, 250 µm; K, L, O, P, S, T, W, and X, 100 µm; C, D, G, and H, 50 µm.
All the other Stichopathes specimens, including the Caribbean one, are characterized by thick stems (more than 1 mm in diameter in the apical portion) covered by triangular–conical spines, with small apical papillae or tubercles, and, in some cases, by small secondary spines. Polyps are larger, more sagittally compressed, and closer together in comparison with specimens of clade A. Within this group, the phylogenetic analyses separate BUK11 and INDO19 (clade B), showing a contorted, thick corallum, up to 50 cm long, with convolutions mostly flat and meandritic (Fig. 3E). The spine pattern of these specimens is characterized by the absence of a significant number of secondary spines. The primary ones, showing a great size difference between polypar (0.4–0.42 mm high) and abpolypar sides (0.16–0.2 mm high) in the coiled portions, show fewer apical tubercles and basal papillae, which at times may be completely lacking (3, 4). The polyps are brown and are sagittally compressed (1.0–1.4 mm in transverse diameter), and slightly spaced apart (0.1–0.2 mm apart, with a density of 6–7 polyps cm–1).
All the remaining undescribed specimens have primary spines with large, mainly apical tubercles and interspersed secondary spines. Within this group, the polyps reach their largest size, are more compressed sagittally, and no significant interpolypar space was observed. Within these samples, the phylogenetic analyses separate two other clades. One of these (clade C) includes two unbranched specimens (AMBA6 and MENT52), and is grouped together with the Antipathes species showing instead a wide variety of branching and spine patterns. Specimens of clade C are characterized by a white or brown spiral corallum (up to 50 cm long), with tight helicoidal coils and a thick stem (more than 3 mm in diameter in the apical portion) (Fig. 3I, L). Polyps (0.8–1.2 mm in transverse diameter) are closely arranged on the external side of the coils (density of 7–9 polyps cm–1) along the polypar side of the stem bearing high primary spines (0.45–0.6 mm high), with small tubercles mainly aggregated on the apex and papillae underneath. The abpolypar side is characterized by smaller primary spines (0.15–0.18 mm high), is more triangular in shape, and is less tuberculated. Triangular or subcylindrical secondary spines are sparse on the skeletal surface, with varying density depending on the portion considered (3, 4).
Finally, clade D includes eight unbranched specimens and one branched specimen from different regions of Indonesia. All these specimens show a straight, thick stem (more than 3 mm in diameter in the apical portion), up to 4–5 m long, often showing swellings along its length (Fig. 3M). Both MENT8b and numerous underwater observations of similar specimens (not included in the phylogenetic analyses) show that these corals may have one or more lateral branches, especially in conditions of strong current. The typical coloration pattern of these specimens is brown (RJ16, RJ22, BUK14b, BUNA14, BALA46, and MENT8b) or white (RJ15, RJ35, and AMBA45), but no genetic differences were evident between the two phenotypes (Fig. 3P). Polyps (0.8–2.2 mm in transverse diameter), much compressed sagittally (density of 4–6 polyps cm–1), especially in the tallest colonies, are arranged along the polypar side of the stem bearing higher primary spines (0.4–0.5 mm high), with small apical tubercles and papillae underneath. In the tallest and more conical spines, tubercles are not only present at the apex, but are also found along the entire surface of the spine. The abpolypar side is characterized by slightly smaller primary spines (0.15–0.3 mm high), which are more triangular in shape and are less tuberculated. In addition, triangular or subcylindrical secondary spines are sparse on the skeletal surface, with a variable density depending on the portion considered (generally less abundant than in clade C). In particular, the most apical portions generally show more compressed primary spines and few or no secondary spines (3, 5). On the basis of underwater observations, this clade is the most represented in terms of abundance in the entire Indonesian Archipelago.
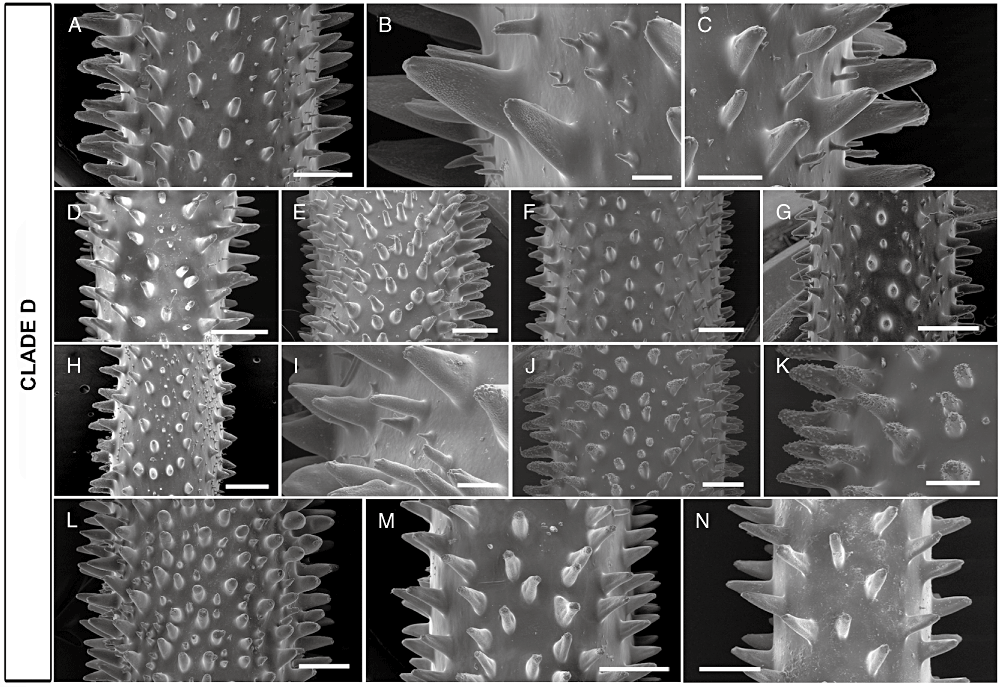
SEM photographs showing the variability of the spines in clade D. A–L, central portions of several specimens, with close-up views of the spines. M, N, apical portions of two specimens. Scale bars: A, D, E, F, G, H, J, L, and M, 1 mm; C, K, and N, 500 µm; I, 200 µm; B, 100 µm.
The Caribbean Stichopathes species groups together with Antipathes caribbeana Opresko, 1996, forming a fifth clade. Stichopathes cf. occidentalisBrook, 1889 is characterized by tall, straight colonies, which occasionally spiral on top in large specimens. The group shows only primary spines, with the polypar ones being taller (0.14 mm tall) than the abpolypar ones (0.07 mm tall), and markedly papillose. Polyps, 1.4 mm in transverse diameter, are distributed very closely together (Opresko & Sánchez, 2005).
The five clades can be characterized on the basis of the genetic distances (see matrix of distances, Table S1). Clade D shows a maximum divergence of 1.20%. The divergence between the two specimens of clade C is 0.36%, which also shows a maximum divergence of 2.10% with the species of the genus Antipathes with which they group. Specimens of clade B show a divergence of 0.48%. The maximum divergence of the specimens of clade A is 3.10%. The divergence in the Caribbean clade is 1.30%.
From the matrix, it is possible to observe that the maximum divergences between clades are: 3.46% between clades C and D; 3.11% between clades B and D; and 3.89% between clades B and C. The maximum divergences between the Caribbean and the Indonesian clades are 7.19, 8.38, and 5.83% with clades D, C, and B, respectively. The maximum value of divergence is that of clade A with respect to other clades: specifically 8.57% with clade B; 11.13% with clade C; 10.34% with clade D; and 8.39% with the Caribbean clade. These values are partially comparable with the maximum divergence that clade A shows with the specimens of the clade including Cirrhipathes spp. and Antipathes curvata (14.75%).
DISCUSSION
The main purpose of this research was to understand the phylogenetic relationships among the unbranched and unpinnulated Indonesian black corals characterized by a monoserial arrangement of polyps, traditionally attributed to the genus Stichopathes.
The phylogeny of the Stichopathes specimens is closely related with that of the Antipathes species. The molecular data indicate, based on the ITS sequences, that the traditional genus Stichopathes is a polyphyletic taxon. In various cases, specimens morphologically belonging to the genus Stichopathes cluster with some of the examined Antipathes species. The recorded high degree of similarity (Table S1) between the genera Stichopathes and Antipathes was already highlighted on the basis of ITS and mitochondrial sequence comparisons of both Indonesian and Caribbean species (Lapian et al., 2007; Wagner et al., 2010). The contemporaneous occurrence of branched (Antipathes-like) and unbranched (Stichopathes-like) specimens in three of the five clades identified by the phylogenetic analyses allows the tentative consideration of the entire group as a single genus (maximum internal divergence 11.13%), including both branched and unbranched species. Within this group, the phylogenetic and morphological homogeneity of clades B and D support the existence of two distinct species. In this context, the family Antipathidae contains at least five genera: Pseudocirrhipathes, Rhipidipathes, a putative Antipathes?, Cirrhipathes, and Stichopathes–Antipathes.
The phylogenetic separation of the studied specimens into five main clades is well supported by the morphological analyses, particularly by the structure of the corallum and the characteristics of the spines and the polyps. As already noted in previous works (Lapian et al., 2007; Bo et al., 2009b), the shape of the spines and, in this case, the presence/absence of secondary spines, seem to be the most important characteristics, explaining most of the differences between the clades. Moreover, the shape, the thickness of the stem, and the size of the polyps are useful in the identification of possible taxa. A taxonomic role of the thickness of the stem was already stated in the description of the whip-like antipatharian Pseudocirrhipathes mapia (Bo et al., 2009b). The relationship that exists between morphological characteristics and phylogeny is also partially evident for the other species examined so far. For example, similarities in spine morphology are evident both between Antipathes caribbeana and Stichopathes cf. occidentalis (showing tuberculated spines) (Wagner et al., 2010), and between Antipathes? sp. 2 and two Hawaiian Antipathes species (all showing sparse smooth, triangular spines) (Gray, 1857; Lapian et al., 2007).
This evidence suggests caution in the use of the corallum branching pattern in the taxonomy of the order: the single-stem morphology may have evolved separately in different taxa, as is strongly suggested by the clade comprising both Cirrhipathes and Antipathes species. The branching pattern may represent an ambiguous character: in some cases it appears to be an unreliable feature. The existence of branched whip black corals, for example, has always been interpreted as an important taxonomic character (Brook, 1889), but recent field observations have shown that Stichopathes corals that are living under strong current conditions may undergo breakages of their apical portions. These situations may give rise to anomalous growth, with bifurcations of the stem, indicating that a certain aptitude to branching is also present in wire specimens (Bo et al., 2009a). In support of this idea, the phylogenetic analyses include the branched colony MENT8b within clade D, which is mainly made up of unbranched colonies. Moreover, besides the lateral branch, the specimen does not show any morphological variation from the single-stem colonies. In addition to this branching behaviour, the shape of the corallum should also be considered controversial. Clade A, for example, includes specimens showing highly variable corallum, which are either perfectly spiral or more contorted, probably depending on the environmental growth conditions. In the same reefs, all the studied specimens of clade C were always perfectly spiral: this suggests that in some groups this character is more stable. In this context, it is interesting that specimens of clades C and D are very similar in terms of spine pattern, and are easily distinguishable by their corallum morphology: spiral and straight, respectively.
Our data do not indicate any taxonomic value for the colour of living coenenchyme in this group of black corals. Specimens belonging to clade D are widely diffused throughout the entire Indonesian Archipelago, from the Indian Ocean (Mentawai Islands) to Iryan Jaya (New Guinea), with two chromatic phenotypes; however, the phylogenetic analyses do not indicate significant differences between the two colour groups. Moreover, no geographically based difference is evident within the specimens of clade D. Currently there are 35 nominal species classified within the genus Stichopathes. The various combinations of morphological features highlighted in this study can represent a useful base for a future rational rearrangement of the group at generic and specific levels. Of all the known Stichopathes species reported in the literature, about 17 have been collected in the Indo-Pacific area, which includes the Indonesian Archipelago, Indian Ocean, Japan, and Australia. The present taxonomic knowledge on whip black corals does not allow a correct systematic identification of the specimens; however, we could tentatively identify clade D, the most diffuse species, as Stichopathes cf. maldivensis (Bo et al., 2009a).
With respect to the data presented by Lapian et al. (2007) and Bo et al. (2009b), in this work we have also analysed a juvenile colony of Antipathes sp. 3, placed in one Antipathes group, and two additional samples of Rhipidipathes reticulata. These latter specimens show a small genetic distance between each other (0.62%) and with the specimens already analysed by Lapian et al. (2007) (0.25% and 0.37% respectively), confirming the position of this genus as a separate taxon in the phylogenetic tree. This suggests that considering its genetic distance from the other genera of the family Aphanipathidae, it would probably be more correct to regard Rhipidipathes as belonging to a different family.
ACKNOWLEDGEMENTS
This study was funded by MIUR, the Italian Ministry for University and Research, and by MAE, the Ministry for Foreign Affairs. Samples for scientific analyses have been transported with regular CITES permissions (export, 08583/IV/SATS-LN/2010 released by the Ministry of Forestry of the Republic of Indonesia – Directorate General of Forest Protection and Nature Conservation, Jakarta; import, IT/IM/2010/MCE/03224, released by the Ministry of Economic Development – Directorate General for International Trade Policy, Rome). The authors are thankful to Dr Jon Collett for his kind revision.




