Phylogenetic analysis of Micrathena and Chaetacis spiders (Araneae: Araneidae) reveals multiple origins of extreme sexual size dimorphism and long abdominal spines
Abstract
The phylogenetic relationships amongst the New World spiny orb-weaving spiders Micrathena and Chaetacis were assessed through parsimony and Bayesian analyses of morphological characters. A total of 146 characters was scored for ten outgroup taxa and 37 Micrathena and four Chaetacis species. The results indicate that Chaetacis nests within Micrathena and we propose Chaetacis as a junior synonym of Micrathena. Twelve subgeneric species groups of Micrathena are recognized and diagnosed. Species with extremely long spines evolved at least eight times in the genus and we suggest that this may be related to antipredator defences. Micrathena is primitively sexually monomorphic and extreme sexual size dimorphism has arisen at least six times in the genus. Most of these events are because of enlargement of the female in relation to the ancestral size, although in two cases sexual dimorphism was attained through male reduction, adding more data to the ‘giant females’ vs. ‘dwarf males’ controversy. The genus is probably of South American origin and has repeatedly invaded Central and North America.
© 2012 The Linnean Society of London, Zoological Journal of the Linnean Society, 2012, 166, 14–53.
INTRODUCTION
The spider genera MicrathenaSundevall, 1833, and ChaetacisSimon, 1895, are composed of 105 and ten species, respectively (Platnick, 2011). Both have a predominantly Neotropical distribution, with Chaetacis restricted to tropical South America and southern Central America, whereas Micrathena is distributed all over the Americas, from northern Argentina to the north-eastern USA (Levi, 1985; Platnick, 2011). Members of both genera are well known for their abdominal spines, eye-catching coloration, and marked sexual dimorphism. They occur primarily in the understory of woodland habitats and are diurnal, building relatively small orb-webs with an open hub (Levi, 1985; I.L.F. Magalhães & A.J. Santos, pers. observ.). Species of Micrathena are often used as models in ecological, behavioural, and natural history studies (e.g. Robinson & Robinson, 1980; Shelly, 1984; Carvalho Jr., 1992; Díaz-Fleischer, 2005; Meling-López, Martínez-Camacho & Duarte-Fuentes, 2008; Moya et al., 2010; Gálvez, 2011), and Micrathena gracilis (Walckenaer, 1805) is the most widely studied species (Hinton & Wilson, 1970; Uetz & Biere, 1980; Biere & Uetz, 1981; Hodge, 1987a, b; Uetz & Hartsock, 1987; Bukowski & Christenson, 1997a, b, 2000; Vanderhoff, Byers & Hanna, 2008; Opell, Schwend & Vito, 2011). The taxonomy of the two genera is well resolved because of exhaustive revisions made by Levi (1978, 1985). Since these, the systematics of the two genera has changed little (reviewed by Argañaraz & Rubio, 2011; Magalhães & Santos, 2011).
Since the 19th century, Micrathena and Chaetacis have been considered closely related to each other and to other spiders with rigid and/or spiny abdomens. Simon (1895), the first author to propose a formal subfamilial classification for the Araneidae, included both genera in Argiopinae, a subfamily that corresponds largely to the present-day Araneidae. Within this subfamily, he erected three tribes for genera with spiny abdomens: Gastheracantheae (GasteracanthaSundevall, 1833, EncyosaccusSimon, 1895, Isoxya Simon, 1885, Augusta O. P.-Cambridge, 1877, and other Palaeotropical genera), Xylethreae (XylethrusSimon, 1895), and Micratheneae (Micrathena– including species that are today placed in EnacrosomaMello-Leitão, 1932–Chaetacis and Pronous Keyserling, 1881). All the members of these tribes, with the exception of Pronous and, perhaps, Enacrosoma, were thought to be closely related until recently (Levi, 1985, 1996; Scharff & Coddington, 1997), when more molecular evidence became available and indicated a more distant relationship between Micrathena and Gasteracantha (Álvarez-Padilla et al., 2009; Dimitrov et al., 2012).
Levi (1985) postulated a sister-group relationship between Micrathena and Chaetacis based on several morphological features, such as the presence of a stridulating surface on the booklung covers, a glabrous, sculptured carapace with dimples and a high thoracic region, an elongated fourth femur, and a large and modified paracymbium in the male palpus. The cephalic tubercles, posterior to the lateral eyes, were considered as a synapomorphy for Chaetacis only. However, Levi (1985) did not define a characteristic exclusive to Micrathena, stating that the modified carapace with dimples and a high thorax would be synapomorphic for the genus. This is, in fact, a characteristic shared with Chaetacis. Hence, Micrathena was not objectively defined in its taxonomic review and is still diagnosed by the absence of cephalic tubercles, a plesiomorphic feature. Levi (1985, 1996) included these two genera in Gasteracanthinae, along with Xylethrus, Encyosaccus, Enacrosoma, Gasteracantha, and other Palaeotropical genera, based on the elongated fourth femora and a sclerotized ring around the spinnerets.
Finally, in the only formal and comprehensive cladistic analysis of Araneidae, Scharff & Coddington (1997) recovered Micrathena and Chaetacis as sister taxa. This relationship was supported by the stridulating files on the booklung covers and loss of extreme sexual size dimorphism, amongst other characters. Contrary to the classification proposed by Levi (1985), Scharff & Coddington (1997) found no evidence of a close relationship between Micrathena plus Chaetacis and Gasteracantha. Thus, they included the former genera in Simon's Micratheninae, along with Xylethrus and Encyosaccus, and maintained Gasteracantha and closely related Palaeotropical genera in Gasteracanthinae. However, in that study the monophyly of all these genera remained untested because the terminal taxa used were the genera themselves, coded from one or two exemplar species.
The relationships within Micrathena and Chaetacis are more controversial. Traditionally, Micrathena has been divided into subgenera (e.g. Micrathena, AcrosomaPerty, 1833, and MeganoplaSimon, 1864) or species groups (see Simon, 1895; Mello-Leitão, 1932; Levi, 1985), which reflect the great morphological heterogeneity of the genus. It is noticeable that there is little agreement amongst authors on the matter of species composition of each group. In the most recent generic revision, Levi (1985) divided Micrathena into eight species groups defined by somatic features of the females and genitalic features of both sexes. Two morphologically peculiar species, Micrathena pungens (Walckenaer, 1842) and Micrathena funebris (Marx in Banks, 1898), were not allocated to any of the groups. Additionally, Levi (1985) proposed a speculative tree representing the internal phylogeny of Micrathena, including some of the characters that were supposed to support his groupings. The tree was mostly nonresolved, placing the Micrathena kirbyi and Micrathena guerini groups as the most basal within the genus and indicating a close relationship between the Micrathena militaris and Micrathena spinosa groups, Micrathena lepidoptera and Micrathena triangularispinosa groups, and the Micrathena gracilis group and M. funebris. As yet, these relationships still have not been assessed by means of a formal phylogenetic analysis. In fact, despite the phylogenetic analysis at family level (Scharff & Coddington, 1997), few araneid genera have had their monophyly and internal genealogy tested through this kind of procedure (e.g. Singafrotypa Benoit, 1962, in Kuntner & Hormiga, 2002; Demadiana Strand, 1929, in Framenau, Scharff & Harvey, 2010), none of them Neotropical.
The sharp spines that adorn the abdomens of females are amongst the most distinctive characteristics of Micrathena and Chaetacis, and inspired the name of the first described Micrathena species, M. spinosa (Linnaeus, 1758). Despite being very conspicuous, little is known about abdominal spine evolution and function, if any. There have been frequent suggestions that they are defensive structures (Peckham, 1889; Edmunds & Edmunds, 1986; Cloudsley-Thompson, 1995; Gonzaga, 2007), but this has not been tested empirically. The size, number, and position of abdominal spines vary considerably amongst species of Micrathena (Levi, 1985), but it is still not clear how this variation evolved. The proposition of homologies amongst spines of different species and the identification of patterns in their evolution could help in the proposition of hypotheses on their possible functions.
Another distinguishing characteristic of Micrathena and Chaetacis is their sexual dimorphism. Males and females differ greatly in several traits, such as total size, leg lengths, carapace and abdomen shapes, and possession of abdominal spines. Both genera have been included in studies concerning sexual dimorphism (Elgar, Ghaffar & Read, 1990; Hormiga, Scharff & Coddington, 2000). As with abdominal spines, the degree of sexual size dimorphism in Micrathena is extremely variable, with species in which males and females are about the same size and others in which females may be up to 2.5 times larger than males in respect to carapace length (not considering differences in abdomen size, which are related to egg production and fecundity in females; Prenter, Elwood & Montgomery, 1999; Higgins, 2002). In their study of the evolution of sexual size dimorphism in orb-weavers, which included Micrathena and Chaetacis, Hormiga et al. (2000) used median values for male and female sizes of each genus, despite the great intrageneric variation of these traits. The authors themselves stated that using the ancestral values of each genus for these traits, instead of median values, would be a better option. However, this is only possible if phylogenies for the groups under study are available. Hence, a cladistic analysis of Micrathena and Chaetacis would contribute to an understanding of how sexual size dimorphism evolved within these genera, as well as providing a basis for comparative studies on the evolution of this trait.
As Micrathena and Chaetacis are genera with great potential as models in evolutionary biology, concerning both sexual dimorphism and antipredator defences, a robust phylogeny will be valuable for future studies of ecology, behaviour, and evolutionary biology. Hence, in this paper we present a morphology-based phylogenetic analysis of the two genera to: (1) test the monophyly of both of them; (2) resolve the relationships amongst Micrathena species groups; and (3) explore the evolution of characters of interest, especially sexual size dimorphism and length of abdominal spines.
MATERIAL AND METHODS
Choice of terminal taxa
At least two species of each Micrathena species group and four species of Chaetacis were chosen so that the monophyly of these could be tested. The M. militaris and M. kirbyi groups were represented by more species as they are morphologically more diverse. Genera of Araneidae that are closely related to Micrathena and Chaetacis, according to Levi (1985, 1996) and Scharff & Coddington (1997), were chosen as outgroups. Terminal taxa were chosen to cover the morphological and geographical variation of the genera and restricted to species known from both sexes. The following species were selected: outgroups: Actinosoma pentacanthum (Walckenaer, 1842), Argiope argentata (Fabricius, 1775), Araneus venatrix (C.L. Koch, 1838), Aspidolasius branicki (Taczanowski, 1879), Cyclosa fililineata Hingston, 1932, Enacrosoma anomalum (Taczanoswki, 1873), Gasteracantha cancriformis (Linnaeus, 1758), Hypognatha belemLevi, 1996, Wagneriana dimastophora (Mello-Leitão, 1940), and Xylethrus superbusSimon, 1895. Chaetacis: Chaetacis aureola (C.L. Koch, 1836), Chaetacis cornuta (Taczanowski, 1873), Chaetacis picta (C.L. Koch, 1836), Chaetacis bandeiranteMagalhães & Santos, 2011. Micrathena species groups: M. gracilis group: M. gracilis, Micrathena horrida (Taczanoswki, 1873); M. guerini group: M. bifida (Taczanowski, 1879), M. guerini (Keyserling, 1864), Micrathena nigrichelis Strand, 1908; M. kirbyi group: Micrathena clypeata (Walckenaer, 1805), Micrathena digitata (C.L. Koch, 1839), Micrathena excavata (C.L. Koch, 1836), Micrathena fissispina (C.L. Koch, 1836), Micrathena furcula (O.P.-Cambridge, 1890), Micrathena gaujoni Simon, 1897, Micrathena guanabara Levi, 1895, M. kirbyi (Perty, 1833), Micrathena patruelis (C.L. Koch, 1839), Micrathena plana (C.L. Koch, 1836), Micrathena triserrataF.O.P.-Cambridge, 1904; M. lepidoptera group: Micrathena decorata Chickering, 1960, Micrathena lepidoptera Mello-Leitão, 1941; M. militaris group: Micrathena cyanospina (Lucas, 1835), Micrathena furcata (Hahn, 1822), Micrathena lata Chickering, 1960, Micrathena sagittata (Walckenaer, 1842), Micrathena swainsoni (Perty, 1833); Micrathena schreibersi group: Micrathena balzapambaLevi, 1985, M. schreibersi (Perty, 1833), Micrathena spitziMello-Leitão, 1932, Micrathena vigorsiPerty, 1833; Micrathena spinosa group: Micrathena brevipes (O.P.-Cambridge, 1890), Micrathena pichinchaLevi, 1985, M. spinosa; M. triangularispinosa group: Micrathena acuta (Walckenaer, 1842), Micrathena evansi Chickering, 1960, Micrathena jundiaiLevi, 1985, Micrathena schenkeli Mello-Leitão, 1939; incertae sedis: M. funebris, M. pungens.
Argiope argentata was chosen to root the tree as this genus was considered a basal araneid by Levi (1983) and a close relative of the spiny orb-weavers by Scharff & Coddington (1997).
Studied specimens (Appendix 2) come from the following institutions (names of curators in parentheses): AMNH, American Museum of Natural History, New York (N.I. Platnick); IBSP, Instituto Butantan, São Paulo (I. Knysak); INPA, Instituto Nacional de Pesquisas da Amazônia, Manaus (A.L. Henriques); MCZ, Museum of Comparative Zoology, Harvard University, Cambridge (G. Giribet); MNHN, Muséum National d'Histoire Naturelle, Paris (C. Rollard); MPEG, Museu Paraense Emílio Goeldi, Belém (A.B. Bonaldo); MNRJ, Museu Nacional do Rio de Janeiro, Rio de Janeiro (A.B. Kury); MZSP, Museu de Zoologia da Universidade de São Paulo, São Paulo (R. Pinto-da-Rocha); SINMNH, Smithsonian Institution, National Museum of Natural History, Washington, (J.A. Coddington); NRM, Naturhistoriska Riksmuseet, Stockholm (G. Lindberg); UFMG, Coleções Taxonômicas da Universidade Federal de Minas Gerais, Belo Horizonte (A.J. Santos).
Character survey
Many characters were taken from Levi (1985), especially the ones used to define Micrathena species groups, and Scharff & Coddington (1997). Besides this, several new characters are here proposed (see character list in Appendix 1). Autapomorphic characters were included only in the Bayesian analyses because they are not decisive in parsimony analyses (Bryant, 1995), and all characters were treated as unordered. Coloration character scoring follows the Pantone (http://www.pantone.com) colour palette and colour codes are cited every first time a colour is mentioned in a character description. Besides discrete characters, continuous characters were also included in the analysis using the technique described in Goloboff, Mattoni & Quinteros (2006). Continuous characters are especially suitable for studying traits that cannot be sorted into discrete states, such as sexual size dimorphism and length of abdominal spines. They can also be used to infer the phylogeny itself (Wiens, 2001; Goloboff et al., 2006; de Bivort, Clouse & Giribet, 2010 and references therein), and have been used in chelicerate phylogenetic analysis recently (see Hendrixson & Bond, 2009; de Bivort et al., 2010; Lopardo, Giribet & Hormiga, 2011). We took measurements from one to 18 males (mean = 4.43) and from one to 19 females (mean = 7.94) per species depending on material available. Carapace length was expressed in millimetres and all other continuous characters were expressed as ratios to carapace length (see de Bivort et al., 2010; Lopardo et al., 2011). This correction is necessary to remove the effect of size, avoiding spurious groupings containing similar sized species, which would be a violation of the principle of independence amongst characters. Whenever a structure was absent in the taxon being measured, the character was scored as 0 for the continuous characters associated with it. We took this approach because we believe that in cases where a species has a structure very reduced in length (e.g. a very short spine or carapace rim), this character state is more closely related to the absence of the structure than to the fully developed structure. Nevertheless, we also tested data matrices with this character state scored as inapplicable, with no changes in the topologies obtained.
Only adult specimens were considered in the analysis. They were examined and measured immersed in 75% ethanol under an Olympus SZ40 stereoscopic microscope. For examination of male genitalia, especially in the cases where the terminal apophysis covers part of the palpus in mesal view, palpal bulbs of some species were expanded after a few minutes of immersion in a saturated KOH solution followed by a wash in distilled water. For observation of internal structures of females and booklung covers, these parts were removed with entomological pins and treated with a pancreatin solution (prepared as described in Álvarez-Padilla & Hormiga, 2008). Booklung covers were mounted in temporary slides, in 75% ethanol and without coverslip, and observed under an Olympus CX40 light microscope in search of stridulatory files. Genitalia were examined in the standard position for Araneidae (Levi, 1985, 2002) except where stated otherwise. Male specimens of M. decorata could not be obtained and the scoring of masculine characters for this terminal was carried out from Levi's (1985) measurements and illustrations. However, because of the limitations in this approach, some structures could not be observed and the corresponding characters were scored as missing entries in the matrix.
Illustrations were created either on a Motic K400 or on a Leica M205c stereoscopic microscopes, each equipped with a camera lucida. Specimen photographs were taken on a DFC295 digital camera in a Leica M205c stereoscopic microscope and composed as a single, multifocal image using Leica Application Suite. Specimens for scanning electron microscopy (SEM) were dried under a bulb lamp, fixed to aluminium stubs with conductive adhesive copper tape, sputter coated with 10 nm of gold, and examined and photographed in a Quanta 2000 SEM microscope in Centro de Microscopia da UFMG.
Parsimony analysis
The discrete character matrix was assembled in the Character Matrix Editor module of MESQUITE 2.74 (Maddison & Maddison, 2010) and exported in the .tnt format. Continuous characters were inserted manually in this file using Microsoft NOTEPAD. Searches for most parsimonious trees (MPTs) were carried out in TNT 1.1 (Goloboff, Farris & Nixon, 2008), using heuristic searches with 10 000 random addition sequences (RAS) followed by rounds of tree bisection and reconnection (TBR), retaining 100 trees per replication. Searches for maximum fit trees (MFTs) were carried out using the implied weighing method (Goloboff, 1993, 1995). As the k constant affects the resulting trees and the choice of this value is arbitrary, we used a script described in Mirande (2009) to search for MFTs in 11 different weighting schemes. Then, a majority-rule consensus was inferred from all the obtained trees and the lowest entire k-value that yielded the most similar tree to this consensus tree was chosen to perform the character optimization. Retention and consistency indexes were calculated using the wstats.run script, which is part of the TNT package. Branch supports were calculated using the Bremer decay index (Bremer, 1994) and symmetric resampling (P = 33; Goloboff et al., 2003). For the latter, calculations were carried out based on 1000 pseudoreplicates with 1000 RAS followed by TBR and saving 1000 trees per replication, and support values are given in frequency differences (GC; Goloboff et al., 2003).
Bayesian analysis
Apart from the parsimony analyses, we analysed our matrix using Bayesian inference of phylogeny using a likelihood model suitable for morphological data (Lewis, 2001). We decided to do so for two main reasons. The first is that this approach permits branch lengths to be estimated, an outcome not possible with parsimony analyses alone. The second is that the likelihood approach takes these branch lengths in consideration and helps in the resolution of clades in the presence of high levels of homoplasy (Lewis, 2001). Thus, by combining both approaches we could test whether our parsimony analysis could be under the influence of long-branch attraction. The likelihood model for morphology only handles discrete data (Lewis, 2001). Thus, eight of the 18 continuous characters were recoded as discrete (see details in the character list, Appendix 1). The remaining continuous characters were not considered in this analysis because they could not be coded in unambiguous discrete states. Two analyses were performed in MrBayes 3.1.2 (Ronquist & Huelsenbeck, 2003), one under the Mkv model (Lewis, 2001) and one under the MkvΓ model (Nylander et al., 2004). The Γ parameter allows rate variation amongst characters to follow a gamma distribution, which may lead to homoplasy-penalizing effects that are analogous to those of implied-weighted parsimony (Nylander et al., 2004). For each of these analyses, two independent runs with four chains (one cold and three heated) each were run for five million generations, sampling trees every 250 generations. We checked for stationarity of the chains using the standard deviation of the split frequencies and plotting the log-likelihood of the trees against generation using TRACER 1.5 (Rambaut & Drummond, 2009). Twenty-five per cent of the trees were discarded as burn-in and the remaining were used to calculate the posterior probabilities. The consensus trees containing branch lengths and node posterior probabilities were plotted in FigTree 1.3.1 (Rambaut, 2009).
Character optimization and ancestral states reconstruction
Character optimization was carried out in TNT and in WinClada 1.00.08 (Nixon 1999–2002). We reconstructed ancestral states of the continuous characters corresponding to posterior spine lengths and male and female carapace lengths using the Trace Character History and Reconstruct Ancestral States modules from MESQUITE 2.74 (Maddison & Maddison, 2010) under a squared-change parsimony model.
RESULTS
Spine and apodeme homology
The term ‘spine’ in this paper will usually refer to the pointed, sclerotized structures that project from the abdomen of females. The pointed, rigid, movable setae in the legs will be termed ‘macrosetae’. We grouped Micrathena spines in anterior, lateral, and posterior spines [a classification similar to the one used by Simon, (1895: 849–850), which included épines antérieures, dorsales, angulares, and postérieures]. The anterior pair of spines lies in the anterior edge of the abdomen and is anteriorly directed (Figs 1, 2, AS). All pairs of lateral spines are usually laterally directed. The first pair of lateral spines lies in the same line or anteriorly in relation to the first pair of primary apodemes (Figs 1, 2, LS1), the second pair of lateral spines is located between the first and second pairs of primary apodemes (Figs 1, 2, LS2), and the third pair of lateral spines is located at the same line or posterior to the second pair of primary apodemes (Figs 1, 2, LS3). The fourth pair of lateral spines is located at the base of the first pair of posterior spines. The first pair of posterior spines (Figs 1, 2, PS1) is usually dorsolaterally directed and always the largest, except in the M. spinosa group, in which the second pair of posterior spines is the largest. The second and third (respectively PS2 and PS3 in Fig. 2) pairs of posterior spines are posteriorly directed.

Abdominal spine homology and measurements taken for this study. Fig. 1. Chaetacis bandeirante, female habitus, dorsal. Arrows indicate primary apodemes. Fig. 2. Chaetacis bandeirante, female habitus, lateral. Abbreviations: AS, anterior spine; AW, abdomen width; CL, carapace length; CW, carapace width; ES, eye interdistance; FL, femur length; FSL, first posterior spine length; LS1, first lateral spine; LS2, second lateral spine; LS3, third lateral spine; PS1, first posterior spine; PS2, second posterior spine; PS3, third posterior spine; RW, rim width; SCL, spinneret cone length. Scale bars = 1 mm.
The primary apodemes (indicated by arrows in Fig. 1) lie in the centre of the abdominal dorsum and consist of two or three pairs, being present in most araneid spiders examined. Secondary apodemes are here defined as the sclerotized disks that surround the primary apodemes [they correspond to what Scharff & Coddington (1997) called ‘sigillae’, char. 56].
Phylogenetic analyses
For each of the 51 taxa, up to 146 characters were scored, of which 18 are continuous, 111 are binary, and 17 are multistate. The character matrix covers mostly the female body (67 characters) and male genitalia (42), but also includes 14 female genitalic and 23 male somatic characters. All characters are described in Appendix 1. Additionally, the matrixes in .tnt and .nex files, used as inputs to TNT and MrBayes, respectively, are available as supporting information (Appendices S1, S2) or upon request to the first author.
A single MPT [length = 575.3; consistency index (CI) = 0.305; retention index (RI) = 0.693; Fig. 3] was obtained through the equal weights parsimony analysis. Following the procedure described above, the value of k for the implied weighted parsimony analysis of this data set was 5. This analysis yielded a single MFT (fit = 105; length = 579.718; CI = 0.303; RI = 0.689; Fig. 4). The Bayesian analyses yielded trees that are very similar to the ones obtained through parsimony analyses (Figs 5, 6). The only differences are the position of M. kirbyi in the Mkv run (Fig. 5), C. bandeirante being sister to C. picta + C. aureola in the MkvΓ run (Fig. 6) and Micrathena ruschii being sister to M. excavata in both runs (Figs 5, 6). Overall nodal support was also similar between Bayesian and parsimony analyses. The Mkv model yielded a tree that is slightly better resolved within the outgroups although it has some polytomies within Micrathena (Fig. 5), whereas the MkvΓ model shows the inverse pattern (Fig. 6).

Optimal trees obtained under parsimony analyses. Fig. 3. Unweighted analysis [length = 575.3; consistency index (CI) = 0.305; retention index (RI) = 0.693]. Fig. 4. Implied weighted analysis (k = 5; length = 579.718; fit = 105; CI = 0.303; RI = 0.689). Bremer supports and symmetric resampling values are indicated below and above branches, respectively. Symmetric resampling values are given in frequency differences (GC; Goloboff et al., 2003).
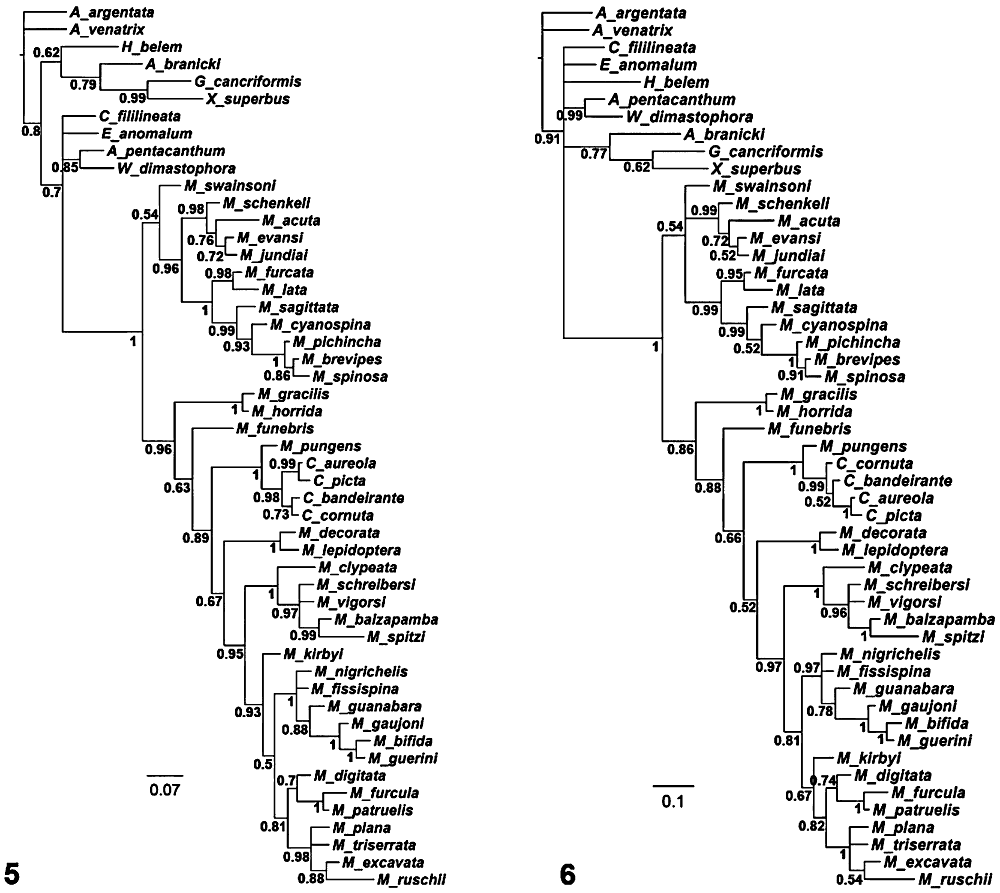
Optimal trees obtained under Bayesian analyses. Fig. 5. Mkv model. Fig. 6. MkvΓ model. Posterior probabilities values are indicated below branches.
As all the topologies obtained were very similar, character optimizations were performed on the trees obtained through weighted parsimony analysis only, except when stated otherwise. In all of the four trees, Micrathena plus Chaetacis form a monophyletic, well-supported group. In the weighted analysis, this clade is supported by 12 non-ambiguous synapomorphies (Fig. 7): second posterior pair of spines (char. 10), a projecting cone surrounding the spinnerets (char. 11), projected femoral setal bases (char. 42: 1), third pair of lateral spines (char. 53: 1), sclerotized ring around the spinnerets (char. 65: 1), two median ventral apodemes in the abdomen [a small, anterior (char. 67: 1), and a large, median one (char. 68: 1)], oval male carapace (char. 89: 1), rectangular male abdomen (char. 101: 1), conductor lobe (char. 125: 1), basal projection of the median apophysis (char. 135: 1) and a well-developed paracymbium (char. 139: 1). Micrathena, however, is paraphyletic in relation to Chaetacis, which was recovered as the sister-group of M. pungens. Chaetacis is monophyletic and supported by four non-ambiguous synapomorphies (Fig. 7): curved post-ocular macrosetae absent (char. 22: 1), cephalic tubercles or spines (char. 23: 1), carapace dimples modified into sulci (char. 34: 1), and basal projection of the median apophysis tooth-shaped (char. 136: 3). Based on these results, we here propose that Chaetacis be considered a junior synonym of Micrathena (see Discussion and Taxonomy below).
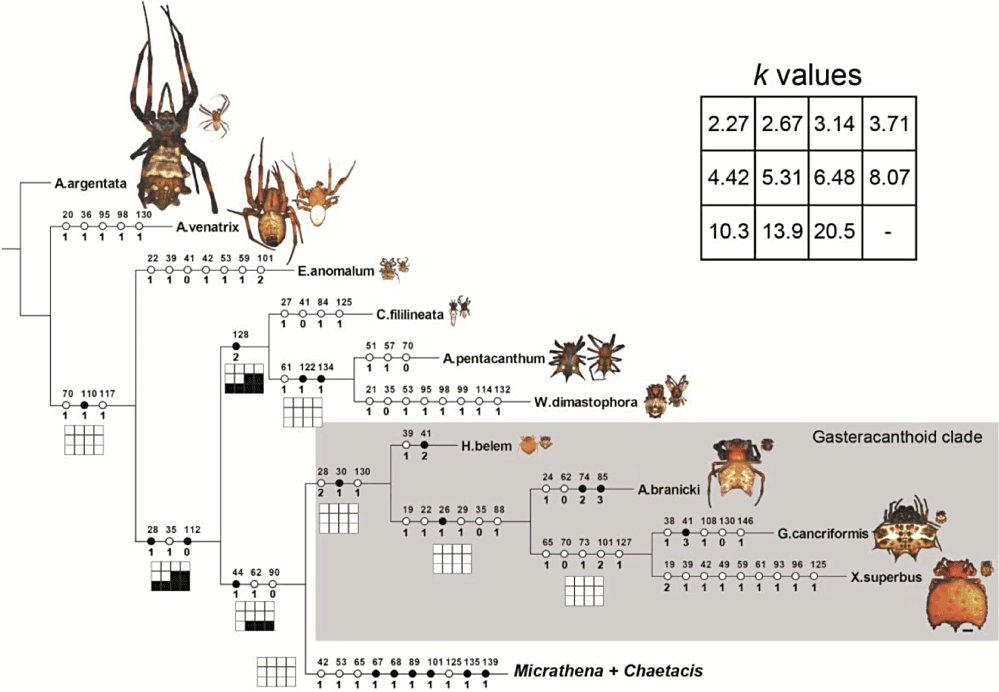
Character optimizations of the discrete data set in the tree obtained through implied-weighted parsimony (k = 5). Character numbers are indicated above circles and character states are indicated below circles. Filled circles indicate convergence-free apomorphies, open circles represent homoplasious synapomorphies. For character descriptions, see Appendix 1. The grids near the nodes represent a sensitivity analysis using different values of k; open cells represent presence of the corresponding clade in a given weighting scheme, whereas filled cells represent its absence. Different Micrathena species groups are indicated by the alternating background colour. Scale bar = 2.0 mm. All spiders (females at left, males at right) drawn to scale and printed approximately at natural sizes. Continued in 8, 9.
The two analyses yield very different results when the outgroups are considered. In both of them, Gasteracantha, Xylethrus, Aspidolasius, and Hypognatha form a monophyletic group, hereafter denominated the ‘gasteracanthoid clade’. In the equal weights analysis, Cyclosa is sister to Micrathena + Chaetacis, supported by the high thoracic region (char. 27: 1), glabrous pleura (char. 35: 1), male palpus with a tegular projection (char. 108: 1), and a conductor lobe (char. 125: 1). These three genera are closer to Enacrosoma and Wagneriana + Actinosoma than to the gasteracanthoid clade. In the implied weights analysis, Micrathena + Chaetacis was recovered as sister to the gasteracanthoid clade. The group is supported by three non-ambiguous synapomorphies (Fig. 7): sclerotized female abdomen (char. 44: 1), wide and modified setal bases in the abdomen (char. 62: 1), and loss of the male endite tooth (char. 90: 0).
Micrathenaspecies groups
Considering the internal phylogeny of Micrathena, two great subdivisions were recovered (Fig. 8). The first group, hereafter named clade M. swainsoni, is formed by the M. triangularispinosa, M. militaris, and M. spinosa groups (sensuLevi, 1985) and is supported by the loss of the tapetum in the posterior median eyes (char. 25 : 1), a sclerotized plate anterior to the epigynum (char. 73: 1), and by a laterally projected paracymbium (char. 140: 1) (Fig. 8). The second, hereafter denominated the M. clypeata clade, is formed by Chaetacis and the remaining Micrathena species groups (M. gracilis, M. funebris, M. pungens, M. lepidoptera, M. kirbyi, and M. guerini, sensuLevi, 1985) and is supported by four non-ambiguous synapomorphies (Fig. 8): wrinkled booklung covers (which are also modified into stridulatory files; char. 70: 0; char. 71: 1), epigynum with a pair of anterior apodemes (char. 75: 1), copulatory openings small and concealed beneath lateral plates (char. 84: 1), and terminal apophysis fused to the embolus (char 114: 1). Most species groups proposed by Levi (1985) are monophyletic, excluding the M. militaris group (paraphyletic in relation to the M. spinosa group) and the M. kirbyi and M. guerini groups (which are not mutually monophyletic). Therefore, to increase agreement between taxonomy and phylogeny, we here propose a new, natural classification scheme for Micrathena groups (7-9; see Discussion and Taxonomy below).

Fig. 7 continued. Scale bar = 2.0 mm.
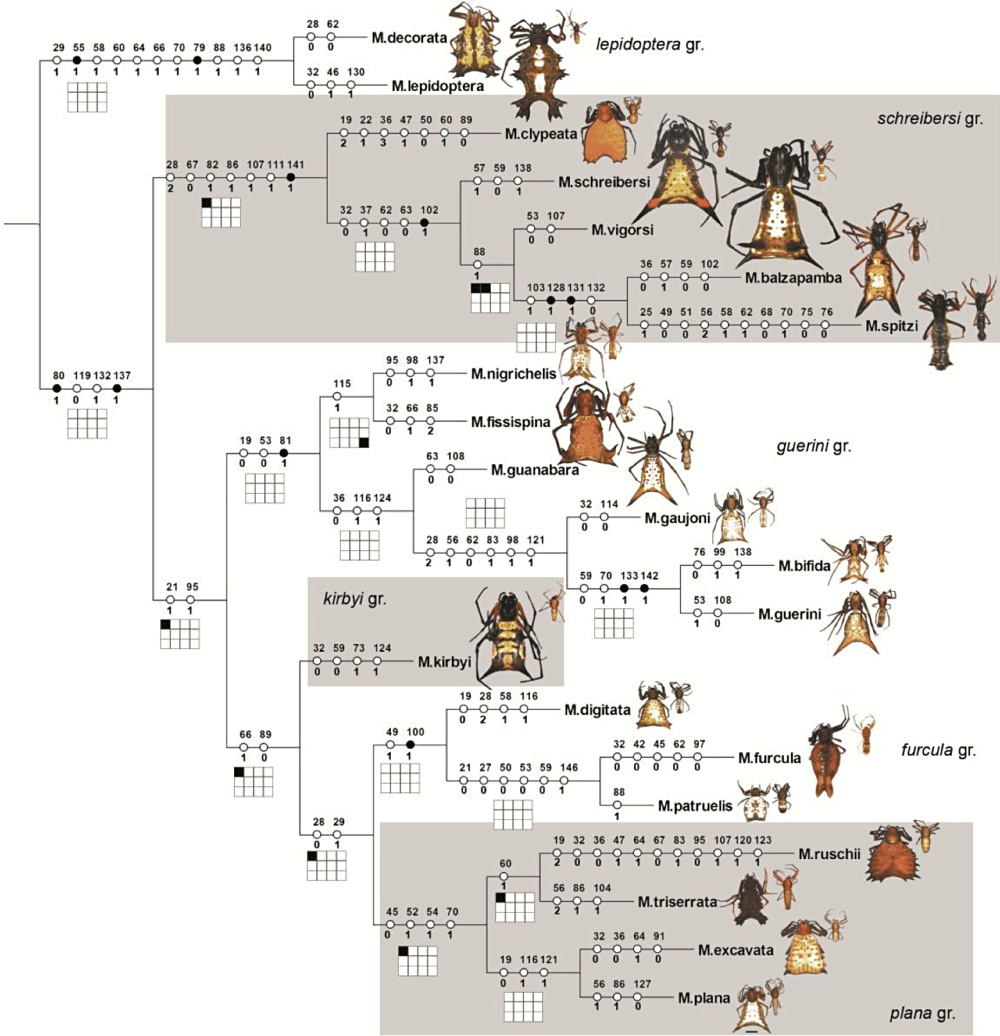
Fig. 8 continued. Scale bar = 2.0 mm.
Abdominal spines
The abdominal spines of females have a complex evolution within Micrathena. The anterior pair of spines appears at the base of the M. clypeata clade and is lost at least three times. The first pair of lateral spines is highly homoplastic, and appears at least four times in the outgroups. In Micrathena, it is lost three times, and one of its losses is synapomorphic for the M. furcula group. The second pair of lateral spines appears three times in Micrathena, supporting the M. cornuta, M. gracilis, and M. plana groups (although these are three independent gains). The third pair of lateral spines is a synapomorphy of Micrathena + Chaetacis, being lost at least five times. The fourth pair of lateral spines is a synapomorphy of the M. plana group, appearing independently in M. pungens. The first pair of posterior spines appears at the base of the cladogram (Fig. 10) and is lost twice, in Cyclosa and Hypognatha, being present in all Micrathena and Chaetacis species. Additionally, there are at least eight independent events of extreme elongation of this pair, in which the spines are longer than the carapace. Extreme elongated spines appear in the M. triangularispinosa group, in M. cyanospina, M. pungens, M. kirbyi, M. schreibersi, M. balzapamba, M. guanabara, and M. guerini (Fig. 10). The second pair of posterior spines is one of the synapomorphies of Micrathena + Chaetacis (appearing convergently in Gasteracantha + Xylethrus) and is lost at least four times (Fig. 10). The extreme elongation of the second pair of spines occurred a single time and is a synapomorphy of the M. spinosa group. The third posterior spine is gained at the base of the M. clypeata clade and is lost at least eight times.
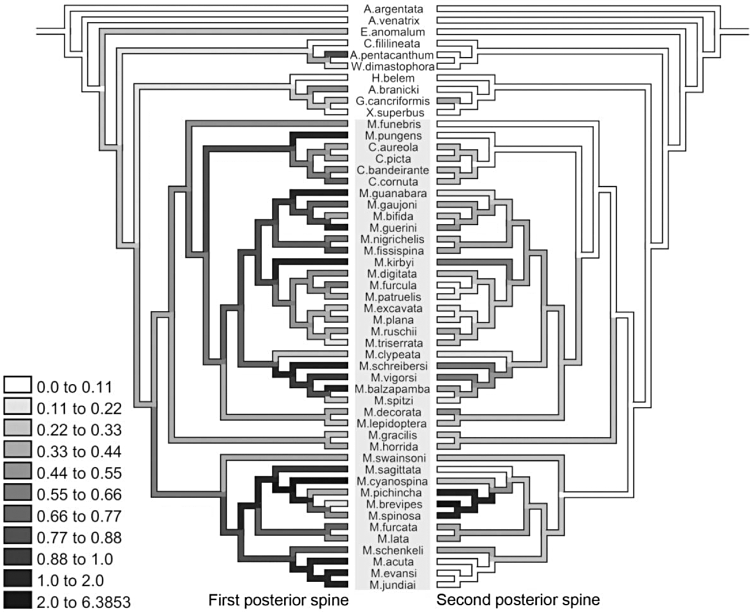
Optimization of the lengths of the first (left) and second (right) pairs of posterior spines under a squared-change parsimony model. Values are expressed as a proportion of carapace length. Darker values indicate greater lengths and the scale is the same for both traits. Spines are considered to be extremely elongated if longer than the carapace. Micrathena spiders are highlighted by the grey background.
Sexual dimorphism
The extreme sexual size dimorphism in the group is here defined as when the female carapace is at least two times larger than in males (following Scharff & Coddington, 1997; Hormiga et al., 2000). Sexually dimorphic lineages appear seven times in the phylogeny (Fig. 11): in Aspidolasius + (Gasteracantha + Xylethrus), in M. cyanospina, M. spinosa, M. schreibersi, M. vigorsi, M. lepidoptera, and M. horrida + M. gracilis. In four of these cases, sexual size dimorphism was attained through enlargement of females in relation to the ancestral size, rather than male diminishment. However, in two lineages males are smaller than the estimated ancestral size (M. lepidoptera and the M. gracilis group; Fig. 11), showing that in these lineages sexual dimorphism was attained through male reduction. In some other cases (e.g. M. spitzi + M. balzapamba, M. fissispina, M. swainsoni), enlargement of females was also observed, but the male became larger as well, and hence these lineages have remained monomorphic with respect to size.
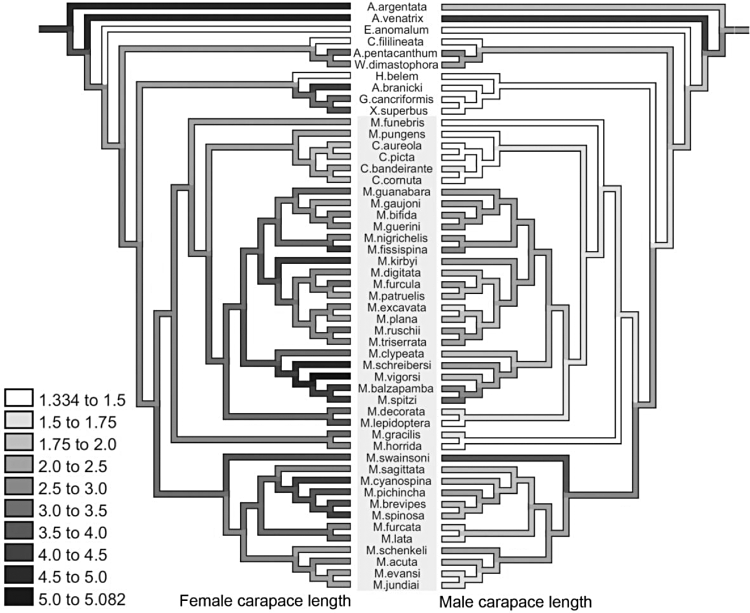
Optimization of female (left) and male (right) carapace lengths under a squared-change parsimony model. Values are expressed in millimetres. Darker values indicate greater lengths and the scale is the same for both sexes. The greater the difference of tone between males and females, the higher the sexual size dimorphism for a given species. Micrathena spiders are highlighted by the grey background.
DISCUSSION
Our results show that Micrathena and Chaetacis form a monophyletic group, although its sister group could not be reliably recovered. The internal relationships recovered for the two genera demonstrate the need for taxonomical rearrangements in the subgeneric groups of Micrathena. Micrathena should also be regarded as a senior synonym of Chaetacis. These relationships also allow us to infer that abdominal spines have a very labile evolution, and, more importantly, that long abdominal spines have evolved independently several times in the evolutionary history of the genus. The evolution of sexual size dimorphism in Micrathena is also remarkable because of its multiple, independent origins that can be explained by male reduction or female enlargement. Finally, we argue that the genus is probably South American in origin and has invaded Central and North Americas several times.
Phylogenetic analyses
The highly supported monophyly of Micrathena + Chaetacis confirms previous ideas of a close relationship between these two genera (Simon, 1895; Levi, 1985; Scharff & Coddington, 1997). The analysis, however, indicates that the first is paraphyletic in relation to the latter, which was recovered as sister group to M. pungens. Micrathena pungens males are astonishingly similar to those of Chaetacis, whereas females do not have most of the diagnostic characters of this genus. This led Levi (1985) to suggest the possibility of a mismatch between males and females of M. pungens. Specifically, he thought the males could belong to Chaetacis abrahami Mello-Leitão, 1948. However, males and females attributable to M. pungens, based on Levi's (1985) diagnosis, have broadly overlapping distributions and are frequently found together in museum collections. Additionally, a male has been collected in a supporting thread of a female's web in Belém, Pará, Brazil by the first author. Moreover, there are derived characters shared between Chaetacis and M. pungens females, such as spiny fourth coxae and reniform spermathecae. Hence, the present evidence supports the male−female match in this species and thus, there are no strong reasons to doubt the sister-group relationship between M. pungens and Chaetacis. As Micrathena is an older name than Chaetacis, we here propose that the latter be considered a junior synonym of the first (see Taxonomy section below).
The relationships amongst the outgroups differ between this study and the topologies obtained by Scharff & Coddington (1997), where (1) Enacrosoma and Cyclosa (as well as Alpaida, closely related to Wagneriana and Actinosoma; Levi, 1988) were recovered closer to Araneus than to Gasteracanthinae genera; ( 2) Xylethrus is recovered as sister group to Micrathena + Chaetacis; and (3) these latter three genera are closer to Hypognatha than to Gasteracantha. These differences probably reflect the lower sampling of genera in the present study. However, it should be noted that Scharff & Coddington (1997) did not provide supporting measures of any kind for the clades recovered in their analysis and thus it is not immediately possible to know how well supported are their groupings. Additionally, the authors themselves stated that their topology is very unstable. Our results are congruent with other studies (Scharff & Coddington, 1997; Álvarez-Padilla et al., 2009; Sensenig, Agnarsson & Blackledge, 2010) in refuting the monophyly of the spiny orb-weavers (Micrathena + Chaetacis and Gasteracantha + related Palaeotropical genera).
Micrathenaspecies groups
Most Micrathena species groups proposed by Levi (1985) are monophyletic in our analyses, with some important exceptions. Levi (1985) suggested that the M. spinosa group might be a subgroup of the M. militaris group, a view confirmed by our results. The M. guerini and M. kirbyi groups were not clearly defined by Levi (1985). As a matter of fact, the author did not provide a single characteristic to tell males from these two groups apart, providing a single key for the males of both groups. This is reflected in the fact that, in our analyses, they were recovered as not mutually monophyletic. We propose some nomenclatural changes, especially concerning Chaetacis and Micrathena of the M. kirbyi, M. spinosa, and M. guerini groups. These are discussed in detail in the Taxonomy section (see below).
Abdominal spines
The results of our analyses suggest multiple gains and losses of abdominal spines in Micrathena, and multiple events of spine elongation throughout the phylogeny. Abdominal spines are large, sclerotized structures, probably energetically costly. Their convergent evolution in different Micrathena species is intriguing and may suggest that they play a functional role. An explanation based on sexual selection seems unlikely, as abdominal spines are almost exclusively feminine structures; males are the competing sex and courtship in araneid spiders is primarily tactile, not visual (Robinson & Robinson, 1980; Bukowski & Christenson, 1997b). Several authors have advocated that they may represent antipredator devices (Peckham, 1889; Edmunds & Edmunds, 1986; Cloudsley-Thompson, 1995; Gonzaga, 2007) because spines play this role in several animal and plant groups (e.g. Milewski, Young & Madden, 1991; Arnqvist & Johansson, 1998; Gilbert, 2001; Bollache et al., 2006). Most orb-weavers are nocturnal and hide themselves during the day or, if diurnal, build retreats outside the web, where they remain most of the time (Blackledge & Wenzel, 2000). Spiders that remain in the hub of their webs during the day, such as Micrathena, frequently make web stabilimenta, which are possibly defensive structures (e.g. Argiope, Blackledge & Wenzel, 2000; Cyclosa, Chou et al., 2005; Gonzaga & Vasconcellos-Neto, 2005a; see Herberstein et al., 2000 and Blackledge, Kuntner & Agnarsson, 2011 for reviews on web stabilimenta, with suggestions of other possible functions). Despite some records of stabilimenta in Micrathena webs (Robinson & Robinson, 1980; Nentwig & Heimer, 1987; Herberstein et al., 2000), this behaviour does not seem to be widespread in the genus and the structure is usually inconspicuous (I. L. F. Magalhães, pers. observ.). Furthermore, there is evidence that the stabilimenta built by Micrathena sexspinosa are prey-attracting structures (Gálvez, 2011; but see Blackledge et al., 2011), although they have not been tested as a defensive strategy. Hence, in the absence of other obvious defensive structures, we suggest that the abdominal spines may be antipredator devices.
There is evidence both that Micrathena may be avoided by some kinds of orb-weaver predators and that other genera of spiny orb-weavers may use their spines as defences. Wasps of the families Sphecidae and Crabronidae are important predators of orb-weaving spiders, which are captured to serve as food to their developing larvae inside tubular mud nests built specially for this purpose (Coville, 1987; Gonzaga, 2007; see references below). Several studies show that wasps capture spiders of many different araneid genera, but not Micrathena, despite the availability of species of the genus in the areas studied (Genaro & Alayón, 1994; Genaro, 1996; Camillo & Brescovit, 1999a, b, 2000; Gonzaga & Vasconcellos-Neto, 2005b; Araújo & Gonzaga, 2007; Buschini, Borba & Brescovit, 2008; Buschini et al., 2010). Other studies, however, show that Micrathena are occasionally captured, and at least some indicate that these spiders are the preferred prey in some cases (Obin, 1982; Landes et al., 1987; Gonzales-Bustamante, 1994; Camillo, 2002). In fact, Levi (1985) stated that large numbers of individuals of some species [Micrathena mitrata (Hentz, 1850), M. furcula, M. swainsoni, M. sexspinosa (Hahn, 1822)] were obtained from wasp nests. However, none of these species would be classified in the present study as having extremely long spines, except M. sexspinosa, which has the second posterior pair elongated (but running in parallel to the main body axis). In fact, M. mitrata and M. furcula belong to the M. furcula group (see below), whose species frequently possess only one or two pairs of very short spines. Other important spider predators are hummingbirds (Aves: Trochilidae) (Peckham, 1889; Hoffmaster, 1982). It is possible that long spines are a hindrance to predation by wasps and hummingbirds, as they would make it more difficult to insert the spider through a narrow tubular nest or a bird's beak because of the mechanical impediment. Additionally, long abdominal spines could reduce wasp attacks on Micrathena species, as they would occupy too much space inside mud nests, making the nest construction and provisioning more costly for female wasps. If this is confirmed, the emergence of several lineages with long spines should reflect similar selective pressures exerted by predators on different species. There is some evidence that wasps and other predators avoid spiny orb-weavers. Edmunds & Edmunds (1986) observed that birds attack individuals of Nephilengys cruentata (Fabricius, 1775), but not of the spiny spider Gasteracantha curvispina (Guérin, 1837). Additionally, only small individuals of the latter have been found in the nests of predatory wasps, possibly because mature females are too large to fit into the nests (Edmunds & Edmunds, 1986). The absence of spines in males supports the hypothesis of defence against predators because adult males are less likely to be captured by wasps (Buschini et al., 2008, 2010). Males possibly suffer selective pressures against the evolution of long spines as they would make their movement outside webs, in search for females, more difficult. Another explanation for the apparent bias against capture of Micrathena by predatory wasps could be the sclerotized abdomen of these spiders. Elgar & Jebb (1999) observed that wasps do not provision their first-instar larvae with Thelacantha brevispina (Doleschall, 1857) and suggested that this could be because of the hard integument of the spiders. However, the possibility of spines functioning as antipredator devices against wasps, birds, and other predators is not incompatible with this hypothesis and should not be ruled out.
It is important to stress that our suggestions that Micrathena spines are adaptations against predators are tentative suppositions based on their multiple independent origins, and should be addressed by specific experiments in the future. Studies of adaptation certainly are more accurate when carried out in a phylogenetic context (Coddington, 1988, 1994). However, our study only accounts for the pattern of spine evolution in Micrathena, and we can say nothing directly about the process that leads to it (see de Pinna & Salles, 1990; Grandcolas & D'Haese, 2003). Nevertheless, it is important to note that the current study indicates that there are several clades in which one of the species is short-spined whereas its sister group is long-spined. These should be of great value in comparative studies of spine functions, and this field surely deserves more empirical attention in the future.
Sexual dimorphism
The current study indicates that Micrathena is primitively monomorphic in respect to size and that at least six of its lineages became secondarily dimorphic independently. The primitively monomorphic condition suggested here conflicts with Scharff & Coddington (1997), who suggested that a return to a monomorphic condition is a synapomorphy for Micrathena + Chaetacis. Despite receiving attention from biologists at least since Darwin (1871: 314), sexual dimorphism in spiders still does not have its causes entirely understood. According to Hormiga et al. (2000), dimorphism is subject to two relatively independent traits: male size and female size. Some studies (Prenter, Elwood & Montgomery, 1998, Prenter et al., 1999; Hormiga et al., 2000) have suggested that, in orb-weavers, dimorphism usually arises when females are larger in relation to the ancestral size – the ‘giant females’ hypothesis. One of the possible explanations for this pattern is that larger females can produce larger clutches of eggs, and thus are to be positively selected given that all other conditions are equal (Blackledge, Coddington & Agnarsson, 2009). There is some empirical support for larger sizes leading to increased fecundity (Prenter et al., 1999; Higgins, 2002). The alternative hypothesis is that, in dimorphic lineages, males are reduced in relation to ancestral size – the ‘dwarf males’ hypothesis, suggested by Vollrath & Parker (1992). In this case the mechanisms are much more unclear and could include differential mortality, protandry because of scramble competition, the ‘gravity hypothesis’, and copulatory and postcopulatory processes (Foellmer & Moya-Laraño, 2007 and references therein). Our study suggests that, at least in Micrathena, extreme sexual size dimorphism evolves primarily because of enlargement of females. Nevertheless, male diminishment has also occurred in at least two lineages. We demonstrate that new sexually dimorphic lineages can arise within genera, something that could not be detected by Hormiga et al. (2000) because they used genera as terminal taxa in their analysis. More importantly, our results show that dimorphism can evolve by either female enlargement or male reduction even amongst closely related species. The fact that Micrathena exhibits several dimorphic lineages that have arisen independently, and by different mechanisms, makes it an excellent model for studies on the evolution of sexual dimorphism in spiders.
Biogeography
The results of the phylogenetic analyses suggest that Micrathena originated in South America. All Micrathena lineages include at least one South American species, and the M. guerini, M. schreibersi, M. kirbyi, M. triangularispinosa, and M. lepidoptera groups are endemic to this subcontinent. Nevertheless, several clades include species from Central or North America, including many species endemic to Caribbean islands, suggesting that these areas have been repeatedly colonized from South America. For example, Micrathena osa (Levi, 1985) (M. cornuta group), M. brevipes and M. sexspinosa (M. spinosa group), Micrathena molesta Chickering, 1961, and M. triserrata (M. plana group), and M. furcula and Micrathena bimucronata (O.P.-Cambridge, 1899) (M. furcula group) occur in Central America and Mexico. Three lineages (M. militaris, M. furcula, and M. gracilis groups) reach as far as the north-eastern USA (M. sagittata, M. mitrata, and M. gracilis) and some Caribbean islands, such as Cuba [Micrathena banksiLevi, 1985, and M. militaris (Fabricius, 1775), Micrathena cubana (Banks, 1909), M. horrida, and Micrathena forcipata (Thorell, 1859)], Hispaniola (M. militaris, Micrathena similis Bryant, 1945, M. forcipata) and Jamaica [Micrathena rufopunctata (Butler, 1873), M. horrida]. The M. gracilis group is of special interest because five out of seven described species are from Central America. In this case, the group main diversification probably took place in Central America with later invasions of South America (M. horrida) and North America (M. gracilis). All of these distribution patterns suggest that Micrathena originated in South America and subsequently invaded the continents to the north. As each of the groups is thought to have invaded Central America independently, our results suggest at least six different invasions of this subcontinent. Following the same line of thought, both the Caribbean islands and North America have been independently colonized three times each, probably by species from Central America. The routes used in these invasion are unclear, however, as there is no fossil record for the genus (see Dunlop, Penney & Jekel, 2011), but include the Panama Isthmus, the Caribbean islands, and overseas dispersal before closure of the former.
Although most Micrathena species and species groups have wide distribution areas, some clades are endemic to certain regions, such as the M. lepidoptera group, which occurs in the Sierras Nevadas of northern Colombia; M. gaujoni + (M. bifida + M. guerini), native to Andean cloud forests of eastern Colombia and Ecuador; and M. cyanospina + the M. spinosa group, which occur predominantly in the Amazon and southern Central America. Interestingly, some clades have disjunct geographical distributions, such as M. spitzi (south-eastern Brazil) + M. balzapamba (Ecuador) and M. guanabara (south-eastern Brazil) + (M. gaujoni + (M. bifida + M. guerini) (Ecuador and Colombia). Additionally, several species also have disjunct distributions and occur in both the Atlantic forest of eastern Brazil and the Amazon forest of northern South America, such as M. fissispina (Levi, 1985: map 3), Micrathena macfarlanei Chickering, 1961 (Levi, 1985: map 5), M. excavata (Levi, 1985: map 6), Micrathena triangularis (C.L. Koch, 1836) (Levi, 1985: map 6), Micrathena brevispina (Keyserling, 1864) (Levi, 1985: map 6), M. schreibersi (Levi, 1985: map 9), and M. lata (Levi, 1985: map 10). This pattern could be the result of vicariance (in this case, this would support the hypothesis that the Amazon and Atlantic forest have been continuous in the past) or long-range dispersal through the open, dry biomes of central South America.
The results of this study raise several interesting questions regarding Micrathena evolution and natural history. The availability of a phylogenetic hypothesis for the group opens new opportunities for the use of this genus as a model for biogeography and evolutionary biology. For instance, the existence of lineages that independently acquired long spines makes it possible to test hypotheses about the functions of these spines using phylogenetically independent replicates. Additionally, the genus would be an interesting model for studies of sexual size dimorphism evolution, as the extreme size difference between the sexes has evolved several times independently in the genus, both through female enlargement and male diminishment. Finally, the geographical distribution is restricted to the New World, with localized cases of endemism, making this genus an interesting model to study the evolution of the American Biota.
TAXONOMY
Family Araneidae Clerck, 1757
GenusMicrathena Sundevall, 1833
Micrathena Sundevall, 1833: 14. Read in April 1833 (F.O.P.-Cambridge, 1904: 525); publication date uncertain [F.O.P.-Cambridge (1904) states that the work was publicly read in April 1833, therefore the actual publication date must be earlier. Thus, the name Micrathena Sundevall, 1833 has priority over Acrosoma Perty, 1833.]. Type species Epeira clypeata, only species listed in section one of the genus, designated by Simon (1895: 848).
Acrosoma Perty, 1833: 193. Published in December 1833 (F.O.P.-Cambridge, 1904: 525). Type species Acrosoma swainsoni, designated by F.O.P.-Cambridge, 1904: 525. First synonymized with Micrathena by F.O.P.-Cambridge (1904).
Meganopla Simon, 1864: 292. Type species Meganopla cyanospina, designated by Bonnet, 1957: 2752. First synonymized with Acrosoma by Butler (1873).
Keyserlingia O.P.-Cambridge, 1890. Type species by monotypy Keyserlingia cornigeraO.P.-Cambridge, 1890 (= M. sexspinosa); preoccupied by Pander, 1861 (Brachiopoda) (Levi, 1985). First synonymized with Micrathena by Simon (1895).
Ildibaha Keyserling, 1892: 31. Type species by monotypy I. albomaculataKeyserling, 1892 (= M. flaveola (C.L. Koch, 1839)). First synonymized with Micrathena by Levi (1985).
Chaetacis Simon, 1895: 863. Type species by original designation Acrosoma affinis C. L. Koch, 1839 (= C. aureola). syn. nov.
Thaumastobella Mello-Leitão, 1945. Type species by monotypy Thaumastobella moureiMello-Leitão, 1945[= M. saccata (C.L. Koch, 1836)]. First synonymized with Micrathena by Scharff (1991).
Diagnosis: Micrathena females have a circular, excavated thoracic fovea (Magalhães & Santos, 2011: figs 15, 18, 21, 33) and a ventral, median line of up to three apodemes in the abdomen (Fig. 12, LMA, SpA). The carapace is glabrous or has reduced setae (but is hirsute in the M. plana and M. furcula groups) and is modified in shape, having a high thoracic region and often a pair of lateral rims and up to three pairs of dimples. The abdomen is usually longer than wide, heavily sclerotized with a ring around the spinnerets (which is found on the tip of a ventrally projected cone; Fig. 2, SCL), often has the dorsum glabrous (hirsute in the M. plana group) and has from one to nine pairs of spines (usually three to seven), but no median posterior tubercle (Figs 1, 2).

Scanning electron microscopy (SEM) images of species included in the current study. Fig. 12. Micrathena nigrichelis, female abdomen, ventral view, showing median line of apodemes. Fig. 13. Micrathena evansi, cymbium, retrolateral. Fig. 14. Micrathena spinosa, male copulatory bulb, submesal. Fig. 15. Micrathena horrida, male copulatory bulb, mesal. Fig. 16. Micrathena evansi, male copulatory bulb, submesal. Terminal apophysis accidently torn off during the process of preparation for SEM. Fig. 17. Micrathena plana, male copulatory bulb, mesal. Abbreviations: BP, basal projection of the median apophysis; C, conductor; CL, conductor lobe; Cy, cymbium; DP, digitiform projection of the median apophysis; E, embolus; EF, epigastric furrow; LMA, large median apodeme; MA, median apophysis rim; MAL, median apophysis lobe; P, paracymbium; R, radix; S, spiracle; SpA, spinnerets apodeme; SR, spinnerets ring; ST, spinnerets tubercle; TA, terminal apophysis; TAP, terminal apophysis projection; TP, tegular projection.
Micrathena males also present a circular thoracic fovea. They have an elongated abdomen that is longer than wide and rectangular to subtrapezoidal in form (Levi, 1985: figs 15, 487, 561; Magalhães & Santos, 2011: figs 15, 18, 21). The paracymbium of the palpus is large (Fig. 13, P) and often has extra lobes or is modified otherwise (Levi, 1985: figs 541, 711; Magalhães & Santos, 2011: fig. 40). The median apophysis has a basal projection that is partially fused to the frontal base of the radix (Figs 14–16, BP; Magalhães & Santos, 2011: figs 16, 22, 39, BP; frequently small and not fused to the radix in the M. plana group, Fig. 17; Magalhães & Santos, 2011: fig. 19).
Description: See descriptions of Micrathena and Chaetacis provided by Levi (1985: 441, 600).
Natural history: See Robinson & Robinson (1980), Uetz & Biere (1980), Biere & Uetz (1981), Shelly (1984), Levi (1985), Hodge (1987a, b), Uetz & Hartsock (1987), Carvalho Jr. (1992), Bukowski & Christenson (1997a, b, 2000), Díaz-Fleischer (2005), Meling-López et al. (2008), Vanderhoff et al. (2008), Moya et al. (2010), Opell et al. (2011), and Gálvez (2011).
Genus subdivisions
We have rediagnosed all of Levi's (1985) original Micrathena species groups, proposing new groups and species transfers whenever necessary. We have proposed taxonomic rearrangements for both species in the phylogenetic analysis and species not included in it. For these species not included in the analysis, we examined specimens whenever possible to assure correct assignment to a group. When specimens were unavailable, we assigned specimens based on Levi's (1985) illustrations. However, several species could not be unambiguously assigned to any group and remain as Micrathena incertae sedis (see below).
Micrathena cornuta group
Diagnosis: Females can be diagnosed by the presence of a pair of tubercles or spines in the carapace (Magalhães & Santos, 2011: figs 33, 34; except M. pungens), spiny fourth coxae (Fig. 18), reniform spermathecae (Levi, 1985: figs 805, 823, 855; Magalhães & Santos, 2011: fig. 38; except M. osa, in which it is rounded), epigynal lateral keels and epigynum without lobe, frequently conical and ventrally projected (Magalhães & Santos, 2011: figs 36, 37). Males can be diagnosed by the presence of spiny setal bases at the margins of the carapace (except M. pungens) and by the palpus lacking a terminal apophysis, with a long, distal tegular projection, a large conductor that projects beyond the margin of the tegulum (Magalhães & Santos, 2011: fig. 39), and a large and modified paracymbium with a dorsal lobe (Magalhães & Santos, 2011: fig. 40, DL).

Scanning electron microscopy images of species included in the current study. Fig. 18. Chaetacis aureola, female fourth leg, ventral. Fig. 19. Micrathena horrida, epigynum, and book lung covers, anterolateral. Circles indicate anterior apodemes. Fig. 20. Micrathena nigrichelis, epigynum, subventral. Fig. 21. Micrathena nigrichelis, male copulatory bulb, mesal. Abbreviations: BP, basal projection of the median apophysis; C, conductor; CL, conductor lobe; CO, copulatory openings; CS, coxal spines; E, embolus; EL, epigynum lobe; FSB, femoral setal bases; LP, epigynum lateral plates; MA, median apophysis rim; PM, paramedian apophysis; R, radix; SF, booklung stridulating files; TA, terminal apophysis; TB, epigynum transverse bar; TP, tegular projection.
Composition: This group corresponds to species previously allocated in Chaetacis, plus M. pungens. Eleven species are included: M. abrahami comb. nov., M. aureola comb. nov., M. bandeirante comb. nov., M. carimagua (Levi, 1985) comb. nov., M. cornuta comb. nov., M. cucharas (Levi, 1985) comb. nov., M. necopinata Chickering, 1960 comb. nov., M. osa comb. nov., M. picta comb. nov., M. pungens, M. woytkowsii (Levi, 1985) comb. nov.
Micrathena funebris group
Diagnosis: As for the species (see Levi, 1985: 588).
Composition: Monotypic.
Micrathena furcula group
Diagnosis: Females can be distinguished by the wide carapace with a straight anterior margin and a hairy thoracic region (Levi, 1985: fig. 170), and by the abdomen, which is wide, short, and has no spines except for one or two pairs of short posterior spines (Levi, 1985: fig. 170) (except M. digitata and M. furva, which have lateral spines). The epigynum has a transverse bar and looks like a bird's head laterally (Levi, 1985: figs 173, 180). Males can be distinguished from those of other groups whose members have a coxal hook by the following combination of characters: rounded thoracic region, abdomen short and rectangular and with a pattern of large, dorsal, paired guanine spots (Levi, 1985: fig. 174). The terminal apophysis always covers the embolus and has a rounded tip (Levi, 1985: figs 128, 163). There is no macroseta in the palpal patella (except in M. digitata and M. furva).
Composition: Ten species: Micrathena bimucronata, M. cubana, M. digitata, M. furcula, M. furva (Keyserling, 1892), M. mitrata, M. patruelis, M. rufopunctata, M. saccata, M. similis.
Micrathena gracilis group
Diagnosis: Females vary greatly in abdomen and carapace shape. Nevertheless, all can be distinguished by having a ventrally projected epigynal lobe that is laterally excavated (Fig. 19, EL). Most males are unknown; the ones described are minute in comparison to females and can be diagnosed by the palpus with a membranous digitiform projection in the basal projection of the median apophysis and a dorsally pointing conductor (Fig. 15, DP, CL).
Composition: Seven species: M. forcipata, M. gracilis, M. horrida, M. margeritaLevi, 1985, M. glyptogonoidesLevi, 1985, M. spinulataF.P.-Cambridge, 1904, M. striataF.P.-Cambridge, 1904.
Micrathena guerini group
Diagnosis: Females of this group often lack the anterior pair of spines (except M. crassispina, M. guanabara, and M. lindenbergi), having a pair of lateral and two large pairs of posterior spines. There is a transverse bar in the epigynum that is frequently, but not always, elongated, forming a scape-like structure (Fig. 20, TB). The epigynum lateral plates are swollen, forming distinct sclerotized cheeks (Fig. 20, LP). Males can be distinguished from other Micrathena males with a coxal hook by the elongated thoracic region and the abdomen dorsum with a median dark band with guanine spots by its sides (Magalhães & Santos, 2011: fig. 15). The terminal apophysis may be entirely fused to the embolus (e.g. M. nigrichelis, M. fissispina; Fig. 21, TA + E) or have a membranous projection (e.g. M. gaujoni, M. bifida; Fig. 17, TAP).
Composition: Eighteen species: Micrathena atuncelaLevi, 1985, M. bifida, M. crassispina (C.L. Koch, 1836), M. fissispina, M. gaujoni, M. guanabara, M. guerini, M. gurupiLevi, 1985, M. kochalkaiLevi, 1985, M. lindenbergi Mello-Leitão, 1940, M. milesSimon, 1895, M. nigrichelis, M. pilatonLevi, 1985, M. raimondi (Taczanowski, 1879), M. realiLevi, 1985, M. rubicundula (Keyserling, 1864), M. shealsi Chickering, 1960, M. teresopolisLevi, 1985.
Micrathena kirbyi group
Diagnosis: Females can be diagnosed by having ten pairs of abdominal spines, the fourth the longest (Levi, 1985: figs 262, 267). The epigynum has a transverse bar that is narrow laterally (Levi, 1985: figs 254, 268). Males have a coxal hook and a rounded thoracic region. The palpus has a sharp basal projection at the median apophysis that points towards the apex of the embolus and a basal membrane in the conductor (Levi, 1985: fig. 259).
Composition: Three species: Micrathena armigera (C.L. Koch, 1837), M. kirbyi, M. macfarlanei Chickering, 1961.
Micrathena lepidoptera group
Diagnosis: Females can be diagnosed by the unique trifid lateral spines in the abdomen (Levi, 1985: figs 526, 535) and by the pair of ventral, blunt spines beside the spinnerets (Levi, 1985: figs 525, 534). There is no stridulatory surface on the booklung covers. The genitalia of the males are similar to those of the M. triangularispinosa group: the basal projection of the median apophysis is large and covers the rim, and the paracymbium is laterally projected (Levi, 1985: figs 532, 541). The terminal apophysis, however, is large and wide, rather than thin, and covers the embolus in mesal view (Levi, 1985: figs 531, 540).
Composition: Two species: Micrathena decorata, M. lepidoptera.
Micrathena militaris group
Diagnosis: This corresponds to Levi's (1985) M. militaris and M. spinosa groups. Females have a sculptured sternum and at most four pairs of abdominal spines, none of which are anterior (Levi, 1985: figs 638, 712, 726). The median posterior eyes lack a tapetum altogether and the lateral eyes may not be juxtaposed. There is no stridulatory surface on the booklung covers. The epigynum lobe is laterally excavated, forming two pockets to which the male's embolus and terminal apophysis may remain attached after mating (e.g. M. militaris, M. spinosa, M. sexspinosa; Levi, 1985: figs 605, 729). The male genitalia are quite varied, but all have a simple or bifid digitiform projection in the palpal tibia that is diagnostic (but also present in M. swainsoni) (Levi, 1985: figs 681, 718). There is no palpal patella macrosetae (except M. lata) and the tibiae and femora lack strong spines and macrosetae, having only normal setae.
Composition: Eighteen species: Micrathena anchicayaLevi, 1985, M. banksiLevi, 1985, M. brevipes, M. cocaLevi, 1985, M. cyanospina, M. donaldi Chickering, 1961, M. furcata, M. guayasLevi, 1985, M. hamifera Simon, 1897, M. lata, M. militaris, M. petrunkevitchiLevi, 1985, M. pichincha, M. reimoseri Mello-Leitão, 1935, M. sagittata, M. sexspinosa (Hahn, 1822), M. soaresiLevi, 1985, M. spinosa.
Micrathena plana group
Diagnosis: Females have a hairy carapace and hairs on the abdomen dorsum, which is flattened. There are often several pairs of lateral spines (usually four; five in M. ruschii; Levi, 1985: fig. 328; Gonzaga & Santos, 2004: fig. 1). There is no stridulatory surface in the booklung covers. The genitalia are quite similar to that of species in the M. furcula group, although some species have spermathecae with two compartments (see char. 86). Males differ from other Micrathena males with a coxal hook by having a rounded thoracic region and by the abdomen, which is wider anteriorly and has two dark bands in the lateral margins (Magalhães & Santos, 2011: figs 17, 18). The basal projection of the median apophysis is faint and small, and frequently is not fused to the radix, and the rim is only slightly bent when compared to species in the M. furcula, M. guerini, and M. kirbyi groups (Fig. 17; Magalhães & Santos, 2011: fig. 19). The terminal apophysis may have a membranous projection (Fig. 17, TAP).
Composition: Seventeen species: Micrathena alvarengaiLevi, 1985, M. bananalLevi, 1985, M. brevispina (Keyserling, 1864), M. duodecimspinosa (O.P.-Cambridge, 1890), M. excavata, M. exlinaeLevi, 1985, M. huanucoLevi, 1985, M. lencaLevi, 1985, M. martaLevi, 1985, M. molesta Chickering, 1960, M. parallela (O.P.-Cambridge, 1890), M. plana, M. quadriserrataF.O.P.-Cambridge, 1904, M. ruschii, M. triangularis (C.L. Koch, 1836), M. triserrata, M. tziscaoLevi, 1985.
Micrathena schreibersi group
Diagnosis: Females can be diagnosed by the glabrous thoracic region of the carapace, by the constricted sternum (except M. clypeata, in which it is shield-shaped; see char. 37), lack of the small median ventral apodeme, epigynum with lateral keels (Levi, 1985: figs 569, 575), and spermathecae with two compartments. The epigynum may (Levi, 1985: fig. 577) or may not (Levi, 1985: fig. 547) have a transverse bar. Males may present a median constriction in the abdomen (not present in M. clypeata and M. balzapamba) (Levi, 1985: figs 548, 579) and have a distinct palpus structure, lacking a terminal apophysis, having a subsquarish tegular margin (Levi, 1985: fig. 571) and a large, flattened retrolateral lobe of the paracymbium (Levi, 1985: fig. 563).
Composition: Six species: M. clypeata, M. balzapamba, M. embiraLevi, 1985, M. schreibersi, M. spitzi, M. vigorsi.
Micrathena swainsoni group
Diagnosis: As for the species. See Levi (1985: 571). Additionally, females lack a tapetum in the median posterior eyes and have an epigynum without lobe. Males have a very large conductor, which holds the embolus and occupies most of the mesal face of the tegulum.
Composition: Monotypic.
Micrathena triangularispinosa group
Diagnosis: All species in this group are small-sized Micrathena, with a dome-shaped carapace (not so in M. schenkeli and M. ucayali) and at most four pairs of abdominal spines (Levi, 1985: fig. 490). The median posterior eyes lack a tapetum. The epigynum has a very rounded bulge and a small, frequently pointed lobe (Magalhães & Santos, 2011: fig. 11). Males have a carapace darker than that of females and a large, median guanine spot in the otherwise dark abdomen (Levi, 1985: fig. 487). The palpus structure is distinct, with a very sclerotized conductor apex that is notched, a drop-shaped, sclerotized conductor lobe, a thin and membranous terminal apophysis that is parallel to the embolus, and a large, sclerotized basal projection of the median apophysis that covers the rim (Fig. 16; Levi, 1985: fig. 467).
Composition: Eleven species: Micrathena acuta, M. annulata Reimoser, 1917, M. bicolor (Keyserling, 1864), M. evansi, M. flaveola (Perty, 1839), M. jundiai, M. peregrinatorum (Holmberg, 1883), M. schenkeli, M. triangularispinosa (De Geer, 1778), M. ucayaliLevi, 1985, M. yanomamiMagalhães & Santos, 2011.
Micrathena incertae sedis
The following species were assigned by Levi (1985) to the M. kirbyi and M. guerini groups. We have not examined specimens of them and thus we are not certain where they belong, although all of them should be within the distal part of the M. clypeata clade. These are M. agriliformis (Taczanowski, 1879), M. bogotaLevi, 1985, M. coroicoLevi, 1985, M. crassa (Keyserling, 1864), M. elongata (Keyserling, 1864), M. fidelis (Banks, 1909), M. lucasi (Keyserling, 1864), M. pupa Simon, 1897, M. stuebeli (Karsch, 1886), and M. zilchi Kraus, 1955. Micrathena agriliformis, M. bogota, M. elongata, and M. pupa possibly form a monophyletic group (characterized by a long and narrow abdomen with short spines) nested within the M. guerini group; M. crassa possibly belongs in the M. furcula group; and M. coroico and M. zilchi possibly belong in the M. guerini group. However, these are only tentative suggestions of placement and should be considered carefully only after examination of specimens.
ACKNOWLEDGEMENTS
We are grateful to all curators of institutions from which we borrowed specimens for this study and for the hospitality during our visits. In particular, we wish to thank Antonio D. Brescovit (IBSP), Anne-Elise Leguin (MNHN), Dana de Roche (SINMNH), Erica Portela (INPA), Laura Leibensperger (MCZ), Nancy L.M. Hung (MPEG), and Gunvi Lundberg and Torbjörn Kronestedt (NRM) for kindly helping us in our specimen survey in the collections. We would also like to thank A.A. Nogueira and A.D. Brescovit for providing a male specimen of M. cyanospina when males of this species were still undescribed. Kin Master Produtos Químicos provided a free sample of pancreatin and the staff of Centro de Microscopia da UFMG helped with the scanning electron microscopy images. A. R. Pepato kindly helped us with the Bayesian analysis. TNT is freely available because of the sponsorship of The Willi Hennig Society. Early versions of the manuscript were greatly improved by comments from A. R. Pepato and I. Agnarsson. This study was financially supported by a PROBIC/FAPEMIG and a CNPq scholarship to I. L. F. Magalhães and by grants from CNPq (Procs. 472976/2008-7 and 300498/2009-8) and Instituto Nacional de Ciência e Tecnologia dos Hymenoptera Parasitóides da Região Sudeste Brasileira (http://www.hympar.ufscar.br/) to A. J. Santos.
Appendices
APPENDIX 1
List of characters
The list below includes all characters used in the parsimony and Bayesian analyses. All measurements were taken preferentially on the left side of the specimens. Continuous characters recoded as discrete for the Bayesian analysis were recoded as absence−presence characters, when applicable, or using natural gaps observed. Continuous characters marked with an asterisk (*) were excluded from the Bayesian analyses. The retention and consistency indexes (CI and RI) are based on the maximum fit tree (Fig. 4).
Continuous characters [mean CI = 0.291, mean RI = 0.466]
- 1
Female, carapace , length (Fig. 1, CL). For the Bayesian analysis, this was combined with character 13 to express sexual dimorphism (Scharff & Coddington, 1997, char. 61) and coded as (0) monomorphic (carapace length of females less than two times that of males); (1) dimorphic (carapace length of females more than two times that of males). [CI = 0.182, RI = 0.428]
- 2
Female, caparace, thoracic region, width*(Fig. 1, CW). Measured in dorsal view in the line of the thoracic fovea. [CI = 0.274, RI = 0.528]
- 3
Female, carapace, lateral thoracic rim, width (Fig. 1, RW). Measured in dorsal view in the line of the thoracic fovea. For the Bayesian analysis, this was coded as thoracic rim (0) absent; (1) present. [CI = 0.211, RI = 0.626]
- 4
Female, carapace, transversal distance between anterior lateral eye and posterior median eye (Fig. 1, ES). For the Bayesian analysis, this was coded as (0) less than 30% of the length of the carapace; (1) more than 40% of the length of the carapace. [CI = 0.346, RI = 0.374]
- 5
Female, carapace, clypeus, height. Measured from the margin of the carapace to the anterior margin of the anterior median eyes. For the Bayesian analysis, this was coded as (0) less than 24% of carapace length; (1) more than 34% of carapace length. [CI = 0.382, RI = 0.513]
- 6
Female, chelicera, basal segment, length (female)*. [CI = 0.404, RI = 0.568]
- 7
Female, femur I, length* (Fig. 2, FL). [CI = 0.247, RI = 0.439]
- 8
Female, femur IV, length*. [CI = 0.212, RI = 0.446]
- 9
Female, abdomen, width (Fig. 1, AW). Measured in dorsal view in the line of the second pair of primary apodemes. For the Bayesian analysis, this was coded as (0) carapace length more than 20% of abdomen width; (1) carapace length less than 15% of abdomen width. [CI = 0.231, RI = 0.298]
- 10
Female, abdomen, first posterior spine, length (Fig. 2, FSL). For the Bayesian analysis, this was coded as first posterior spine (0) absent; (1) present (Fig. 2, PS1). [CI = 0.435, RI = 0.325]
- 11
Female, abdomen, second posterior spine, length (Fig. 2, SSL). For the Bayesian analysis, this was coded as second posterior spine (0) absent; (1) present (Fig. 2, PS2). [CI = 0.35, RI = 0.553]
- 12
Female, abdomen, spinneret cone, length (Fig. 2, SCL). In Micrathena and Chaetacis, the spinneret ring is on the tip of a ventral projection that forms a cone basal to the spinnerets. Its length was measured in lateral view from its base (usually coinciding with a median apodeme; see character 69) to its ventral border. For the Bayesian analysis, this was coded as spinneret cone (0) absent; (1) present. [CI = 0.326, RI = 0.756]
- 13
Male, carapace, length*. [CI = 0.229, RI = 0.476]
- 14
Male, chelicerae, basal segment, length*. [CI = 0.41, RI = 0.555]
- 15
Male, femur I, length*. [CI = 0.225, RI = 0.538]
- 16
Male, femur IV, length*. [CI = 0.21, RI = 0.376]
- 17
Male, palp, bulb, length* (Fig. 22, BL). [CI = 0.281, RI = 0.36]
- 18
Male, palp, bulb, width* (Fig. 22, BW). [CI = 0.29, RI = 0.225]

Scanning electron microscopy images of species included in the current study. Fig. 22. Wagneriana dimastophora, male copulatory bulb, mesal. Fig. 23. Micrathena nigrichelis, female eye region, dorsolateral. Arrow indicates the post-ocular macroseta. Fig. 24. Gasteracantha. cancriformis, female carapace, dorsolateral. Fig. 25. Micrathena plana, female carapace, dorsolateral. Abbreviations: ALE, anterior lateral eye; AME, anterior median eye; BL, bulb length; BW, bulb width; C, conductor; CH, cephalic hump; E, embolus; LSR, lateral setae rows; MA, median apophysis rim; P, pleura; PLE, posterior lateral eye; PM, paramedian apophysis; PME, posterior median eye; R, radix; TA, terminal apophysis; TF, thoracic fovea; TP, tegular projection.
Female somatic characters [mean CI = 0.45, mean RI = 0.678]
- 19
Carapace, coloration: (0) light brown [Pantone Matching System (PMS) 1215C, 1245C, 1395C]; (1) dark brown (PMS 1545C, 4975C, BlackC); (2) orange (PMS 159C). [CI = 0.222, RI = 0.682]
- 20
Carapace, longitudinal median pigmented stripe: This stripe (and the lateral pigmented bands, see next character) are darker in colour than the rest of the carapace (usually dark brown or black). (0) absent; (1) present (Fig. 26, MPS). [CI = 0.333, RI = 0.667]
- 21
Carapace, longitudinal lateral pigmented bands: (0) absent; (1) present (Fig. 26, LPB). [CI = 0.125, RI = 0.682]
- 22
Carapace, cephalic region, curved macrosetae: (0) present (Fig. 23, arrow); (1) absent (Magalhães & Santos, 2011: fig. 33). In most araneid spiders examined, there are one or more macrosetae immediately posterior to the lateral eyes that are curved and distinct in shape from the remaining carapace setae. These are absent in Chaetacis, Gasteracantha, Xylethrus, Aspidolasius, and Enacrosoma.[CI = 0.2, RI = 0.556]
- 23
Carapace, cephalic region, tubercles (Levi, 1985): (0) absent; (1) present (Magalhães & Santos, 2011: figs 33, 34, CT). [CI = 1, RI = 1]
- 24
Carapace, cephalic region, lateral eyes, position in relation to each other (Levi, 1985): (0) juxtaposed (Figs 24, 25; Magalhães & Santos, 2011: fig. 33); (1) apart by at least one diameter (Levi, 1985: fig. 704). [CI = 0.5, RI = 0.8]
- 25
Carapace, cephalic region, posterior median eyes, tapetum (Scharff & Coddington, 1997, char. 51): (0) present (Scharff & Coddington, 1997: fig. 38); (1) absent. [CI = 0.25, RI = 0.786]
- 26
Carapace, cephalic region, height (Scharff & Coddington, 1997, char. 47): (0) low; (1) high (Fig. 24, CH). In the current study, we considered a spider to have a ‘high’ cephalic region when there are two distinct bumps immediately posterior to the eyes (as in Aspidolasius and Xylethrus; Levi, 2002: figs 36, 50). This is a modification of the original character from Scharff & Coddington (1997), who required the cephalon to be rectangular and set off from the thorax to be considered as ‘high’. [CI = 1, RI = 1]
- 27
Carapace, thoracic region, height (Levi, 1985, 2002): (0) low (Fig. 25); (1) high (Fig. 24; Levi, 2002: figs 46, 82); (2) domed (Levi, 1985: fig. 471). [CI = 0.333, RI = 0.714]
- 28
Carapace, thoracic region, pilosity: (0) hirsute (Fig. 25); (1) with reduced setae (Magalhães & Santos, 2011: fig. 33); (2) glabrous (Fig. 24). The pilosity of the thoracic region refers to the median portion of the thorax and should not be confused with the setae in the lateral portions of the carapace (see next character). [CI = 0.25, RI = 0.76]
- 29
Carapace, thoracic region, lateral edges, setae: (0) absent (Magalhães & Santos, 2011: fig. 33); (1) present (Figs 24, 25, LSR). [CI = 0.333, RI = 0.818]
- 30
Carapace, thoracic region, fovea: (0) present (Fig. 25, TF; Magalhães & Santos, 2011: Fig. 33, Fo); (1) absent (Fig. 24). [CI = 1, RI = 1]
- 31
Carapace, thoracic region, fovea, shape: (0) slit-like (Scharff & Coddington, 1997: fig. 31); (1) circular (1–2, 22–25; Magalhães & Santos, 2011: figs 15, 18, 21, 33, Fo). [CI = 1, RI = 1]
- 32
Carapace, thoracic region, lateral dimples (Levi, 1985): (0) absent; (1) present (Magalhães & Santos, 2011: fig. 33, Di). [CI = 0.111, RI = 0.429]
- 33
Carapace, thoracic region, number of lateral dimples: (0) three pairs (Levi, 2002: fig. 46; Magalhães & Santos, 2011: fig. 33, Di); (1) two pairs; (2) one pair. [CI = 1, RI = 1]
- 34
Carapace, thoracic region, second and third lateral dimples, shape (Levi, 1985): (0) circular (Levi, 2002: fig. 46); (1) sulci-like (Magalhães & Santos, 2011: fig. 33). [CI = 1, RI = 1]
- 35
Cephalothorax, pleura, pilosity: (0) hirsute (Fig. 24); (1) glabrous. [CI = 0.2, RI = 0.5]
- 36
Sternum, coloration: (0) light brown; (1) dark brown; (2) white (PMS 607); (3) orange. [CI = 0.25, RI = 0.625]
- 37
Sternum, shape: (0) shield-shaped (Fig. 27); (1) with a slight constriction between the third and fourth pairs of coxae (Fig. 28). [CI = 0.5, RI = 0.75]
- 38
Sternum, ventral surface, shape (Levi, 1985): (0) flat (Figs 27, 28); (1) sculptured (Fig. 29); (2) domed (Levi, 1985: figs 508, 510). [CI = 0.667, RI = 0.857]
- 39
Sternum, posterior end, shape: (0) pointed (Figs 27, 28, 29); (1) notched (Levi, 2002: fig. 29). [CI = 0.333, RI = 0]
- 40
Chelicerae, prolateral teeth row, teeth number: (0) four; (1) five; (2) six. [CI = 0.5, RI = 0.667]
- 41
Chelicerae, retrolateral teeth row, teeth number: (0) two; (1) three; (2) four; (3) Five. [CI = 0.75, RI = 0]
- 42
Legs, femora, setal bases, shape: (0) flat; (1) projected, usually conical or slightly rounded (Fig. 18, FSB). [CI = 0.25, RI = 0.625]
- 43
Leg IV, coxae, texture (Levi, 1985): (0) smooth; (1) tuberculate or spiny (Fig. 18). [CI = 1, RI = 1]
- 44
Abdomen, sclerotization: (0) light; (1) heavy. Spiders in general have a very lightly sclerotized abdomen. Here, we considered an abdomen to be heavily sclerotized if it has at least two of the following characteristics: (1) a rigid cuticle, hard to deform with forceps, (2) lateral striae, or (3) dorsal secondary apodemes. [CI = 1, RI = 1]
- 45
Abdomen, dorsum, pilosity: (0) hirsute; (1) glabrous. [CI = 0.167, RI = 0.667]
- 46
Abdomen, median dorsal guanine spots: (0) absent; (1) present (Levi, 1985: figs 526, 668). [CI = 0.2, RI = 0.6]
- 47
Abdomen, dorsum, apodemes, shape (Gonzaga & Santos, 2004): (0) circular; (1) oval and with a median scar (Gonzaga & Santos, 2004: fig. 1). [CI = 0.5, RI = 0]
- 48
Abdomen, dorsum, secondary apodemes, arrangement (Scharff & Coddington, 1997: char. 57): (0) in a single row; (1) scattered. [CI = 0.333, RI = 0.667]
- 49
Abdomen, anterior pair of spines (Levi, 1985): (0) absent; (1) present (Figs 1, 2, AS). [CI = 0.167, RI = 0.722]
- 50
Abdomen, first pair of lateral spines (Levi, 1985): (0) absent; (1) present (Figs 1, 2, LS1). [CI = 0.143, RI = 0.25]
- 51
Abdomen, first pair of lateral spines, position: (0) in a straight line with apodemes (Fig. 1); (1) anteriorly positioned (Levi, 1985: figs 565, 647). The first pair of lateral spines may coincide exactly with the first pair of primary apodemes or be anterior to them. In the second condition, they may resemble anterior spines in being anteriorly directed. [CI = 0.2, RI = 0.75]
- 52
Abdomen, second pair of lateral spines (Levi, 1985): (0) absent; (1) present (Figs 1, 2, LS2). [CI = 0.2, RI = 0.5]
- 53
Abdomen, third pair of lateral spines (Levi, 1985): (0) absent; (1) present (Figs 1, 2, LS3). [CI = 0.111, RI = 0.5]
- 54
Abdomen, fourth pair of lateral spines (Levi, 1985): (0) absent; (1) present (Gonzaga & Santos, 2004: fig. 1). [CI = 0.5, RI = 0.75]
- 55
Abdomen, lateral spines, shape (Levi, 1985): (0) simple (Figs 1, 2); (1) trifid (Levi, 1985: figs 526, 535). [CI = 1, RI = 1]
- 56
Abdomen, first pair of posterior spines, colour: (0) black; (1) red (PMS Red 032C, 193C, 1805C); (2) brown; (3) white. [CI = 0.188, RI = 0.278]
- 57
Abdomen, first pair of posterior spines, red ring around base: (0) absent; (1) present (Fig. 9, Micrathena schreibersi). [CI = 0.111, RI = 0.2]
- 58
Abdomen, posterior spine lobe (Levi, 1985): (0) absent; (1) present (Fig. 30, PSL). [CI = 0.25, RI = 0.25]
- 59
Abdomen, third pair of posterior spines (Levi, 1985): (0) absent; (1) present (1–2, 26–32, PS3). [CI = 0.1, RI = 0.571]
- 60
Abdomen, posterior spines, arrangement: (0) dorsoventral (1–2, 26–32); (1) lateral (Levi, 1985: figs 377, 419, 526, 535; Gonzaga & Santos, 2004: fig. 1). In Micrathena species with a flattened abdomen, the posterior spines lie laterally in position to one another rather than being arranged in a dorsoventral fashion. [CI = 0.333, RI = 0.5]
- 61
Abdomen, median posterior spine (Levi, 2002): (0) absent; (1) present (Levi, 2002: figs 60, 63, 66). [CI = 0.5, RI = 0.5]
- 62
Abdomen, setal bases, shape (Scharff & Coddington, 1997: char. 67): (0) tight and circular; (1) wide and hooded (12–17, 18–21; Scharff & Coddington, 1997: fig. 47; Magalhães & Santos, 2011: fig. 35). [CI = 0.111, RI = 0.652]
- 63
Abdomen, venter, tubercle anterior to spinnerets: (0) absent; (1) present (12–17, 26–32, ST). In several Micrathena species, there is a rounded swelling anterior to the spinnerets. [CI = 0.333, RI = 0.9]
- 64
Abdomen, venter, blunt spines (Levi, 1985): (0) absent; (1) present (Fig. 31; Levi, 1985: figs 347, 525, 534). [CI = 0.333, RI = 0.333]
- 65
Abdomen, sclerotized ring around spinnerets (Levi, 1985; Scharff & Coddington, 1997: char. 65): (0) absent; (1) present (Scharff & Coddington, 1997: fig. 46). [CI = 0.5, RI = 0.857]
- 66
Abdomen, sclerotized ring, shape: (0) flat (Levi, 1985: figs 573, 662); (1) with a posterior lip (Levi, 1985: figs 347, 534, 608). [CI = 0.25, RI = 0.75]
- 67
Abdomen, ventral row of apodemes, small apodeme: (0) absent; (1) present (Fig. 31, SA). All Micrathena and Chaetacis females examined possess a median, ventral row of apodemes. The anterior-most one is the smallest, almost an indistinct dot near the epigynum. [CI = 0.167, RI = 0.737]
- 68
Abdomen, ventral row of apodemes, large apodeme: (0) absent; (1) present (12–17, 26–32, LMA). This is the largest ventral apodeme and occupies an intermediate position between the small and the spinnerets apodemes. [CI = 0.5, RI = 0.9]
- 69
Abdomen, ventral row of apodemes, spinnerets apodeme: (0) absent; (1) present (12–17, 26–32, SpA). The spinnerets apodeme is almost contiguous to the spinnerets ring. It is located in the swelling anterior to the spinnerets when this structure is present, or in the corresponding region when it is not. [CI = 1, RI = 1]
- 70
Abdomen, booklung cover surface, texture (Scharff & Coddington, 1997: char. 64): (0) wrinkled (Scharff & Coddington, 1997: fig. 44); (1) smooth. [CI = 0.125, RI = 0.708]
- 71
Abdomen, booklung cover wrinkles, shape (Levi, 1985; Scharff & Coddington, 1997: char. 63): (0) normal; (1) modified into stridulatory surface (Fig. 19, SF; Scharff & Coddington, 1997: fig. 43). [CI = 1, RI = 1]
- 72
Abdomen, booklung cover wrinkles, spacing between wrinkles (Hinton & Wilson, 1970): (0) wide (Fig. 19); (1) narrow (Magalhães & Santos, 2011: fig. 36). In M. horrida and M. gracilis, the stridulatory files are widely spaced and easily seen under the stereoscopic microscope. In other Micrathena and Chaetacis, the spacing between files is much narrower and frequently can only be seen under light microscopy or scanning electron microscopy. [CI = 1, RI = 1]
- 73
Abdomen, sclerotized plate anterior to epigynum: (0) absent; (1) present (26–32, 33–41). A distinct, rectangular plate fused to the anterior side of the epigynum is present between the booklungs of several Micrathena species and can be distinguished by being more sclerotized than the surrounding cuticle. [CI = 0.333, RI = 0.857]

Species included in this study. Fig. 26. Micrathena furcata, female carapace, dorsal. Fig. 27. Micrathena digitata, female sternum, ventral. Fig. 28. Micrathena spitzi, female sternum, ventral. Fig. 29. Micrathena spinosa, female sternum, ventral. Fig. 30. Micrathena digitata, abdomen end, lateral. Fig. 31. Micrathena lepidoptera, female abdomen, ventral. Fig. 32. Micrathena spinosa, epigynum and booklungs, ventral. Abbreviations: EP, epigynum sclerotized plate; LMA, large median apodeme; LPB, lateral pigmented bands; MPS, median pigmented stripe; PSL, posterior spine lobe; SA, small apodeme; SpA, spinnerets apodeme; SR, spinnerets sclerotized ring; ST, spinnerets tubercle; VS, ventral spines. Scale bars: 26, 29, 30, 31, 32 = 1 mm; 27, 28 = 0.5 mm.
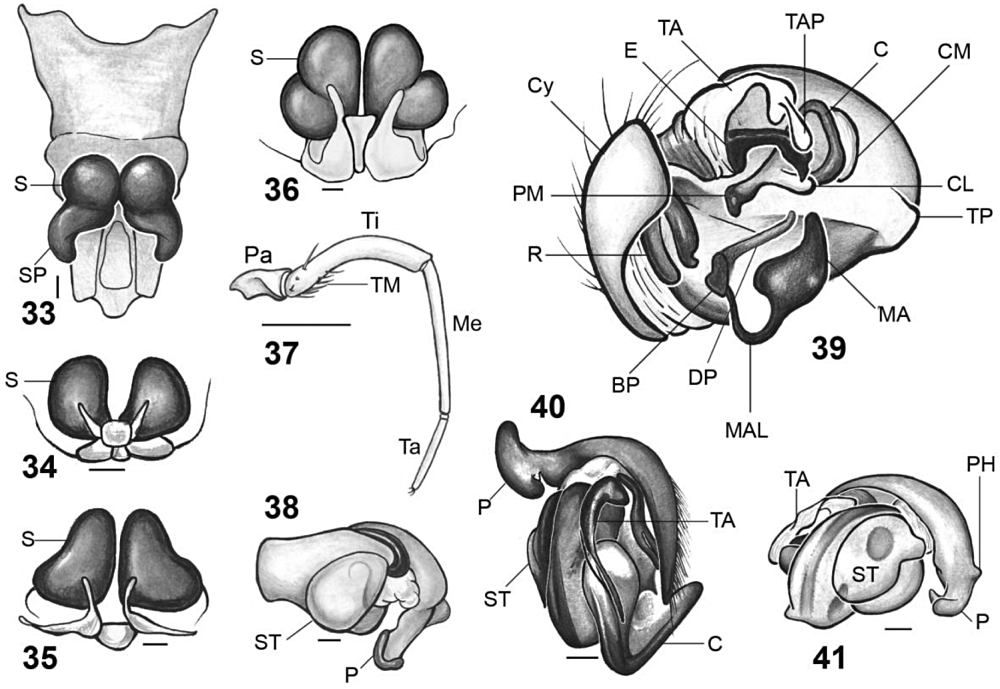
Species included in this study. Fig. 33. Micrathena spinosa, female internal genitalia, dorsal, cleared. Fig. 34. Micrathena horrida, female internal genitalia, dorsal, cleared. Fig. 35. Micrathena fissispina, female internal genitalia, dorsal, cleared. Fig. 36. Micrathena schreibersi, female internal genitalia, dorsal, cleared. Fig. 37. Chaetacis aureola, male first tibia, lateral. Fig. 38. Micrathena schreibersi, male palpus, retrolateral. Fig. 39. Micrathena bifida, male palpus, mesal. Fig. 40. Micrathena swainsoni, male palpus, apical. Fig. 41. Micrathena bifida, male palpus, retrolateral. Abbreviations: BP, basal projection of the median apophysis; C, conductor; CL, conductor lobe; CM, conductor basal membrane; Cy, cymbium; DP, digitiform projection of the median apophysis; E, embolus; MA, median apophysis rim; MAL, median apophysis lobe; Me, metatarsus; P, paracymbium; Pa, patella; PH, paracymbium hump; PM, paramedian apophysis; R, radix; S, spermathecae; SP, spermathecae projections; ST, subtegulum; Ta, tarsus; TA, terminal apophysis; TAP, terminal apophysis projection; Ti, tibia; TM, tibial macrosetae; TP, tegular projection. Scale bars = 0.1 mm except 40 = 0.5 mm.
Female genitalic characters [mean CI = 0.586, mean RI = 0.802]
- 74
Epigynum, bulge, shape (Levi, 1985): (0) domed (Magalhães & Santos, 2011: figs 11, 13); (1) conical and ventrally projected (Magalhães & Santos, 2011: figs 31, 37); (2) flat (as in Aspidolasius). [CI = 0.667, RI = 0.5]
- 75
Epigynum, anterior pair of apodemes: (0) absent; (1) present (Fig. 19, circles). A pair of apodemes is present in the anterior face of the bulge of the epigynum of several Micrathena species. [CI = 0.333, RI = 0.913]
- 76
Epigynum, lobe (Levi, 1985): (0) absent (Magalhães & Santos, 2011: figs 31, 37); (1) present (Figs 19, 20, EL). The lobe is a sclerotized, rounded outgrowth that projects from the tip of the posterior face of the epigynal bulge. We do not consider the Araneus scape as homologous to the lobe present in several araneid genera sampled in this study because is it a long, membranous structure attached to the anterior face of the epigynal bulge. [CI = 0.125, RI = 0.417]
- 77
Epigynum, lobe, orientation (Levi, 1985): (0) posterior (Fig. 20); (1) ventral (Fig. 19). [CI = 1, RI = 1]
- 78
Epigynum, lobe, shape (Levi, 1985): (0) dorsoventrally flattened (Fig. 20); (1) laterally flattened (Fig. 19). [CI = 0.333, RI = 0.778]
- 79
Epigynum, lobe, shape: (0) regular; (1) M. lepidoptera-like (Levi, 1985: figs 527, 538). In M. lepidoptera and M. decorata, the lobe is pointed at the tip, lightly sclerotized, and arises from a membranous base in the posterior face of the epigynum. [CI = 1, RI = 1]
- 80
Epigynum, transverse bar (Levi, 1985): (0) absent; (1) present (Fig. 20; Levi, 1985: figs 3, 5). [CI = 0.5, RI = 0.938]
- 81
Epigynum, lateral plates, shape: (0) flat; (1) swollen (Fig. 20; Levi, 1985: figs 146, 152). The posterior lateral plates of the epigynum may be dark and swollen, forming two sclerotized cheeks by the side of the posterior median plate. [CI = 1, RI = 1]
- 82
Epigynum, lateral plates, keels (Levi, 1985): (0) absent; (1) present (Levi, 1985: figs 569, 575; Magalhães & Santos, 2011: figs 36, 37). [CI = 0.5, RI = 0.889]
- 83
Epigynum, median plate, shape: (0) flat; (1) projected (Levi, 1985: figs 14, 36, 148). When projected, the median plate has a laterally visible bump that extends posteriorly to the lateral plates. [CI = 0.5, RI = 0.667]
- 84
Epigynum, copulatory openings, size: (0) large (Levi, 1985: figs 657, 686); (1) small and concealed beneath lateral plates (Fig. 20, CO). In most Micrathena and Chaetacis, the copulatory openings are rather inconspicuous and can only be seen under a fold between the median and lateral plates. In other araneid genera, the copulatory openings are large and can be seen in posterior view. [CI = 0.333, RI = 0.875]
- 85
Spermathecae, shape: (0) rounded (Figs 33, 34, 36, S); (1) elongated; (2) reniform (Fig. 35; Magalhães & Santos, 2011: fig. 38); (3) coiled. [CI = 0.75, RI = 0.8]
- 86
Spermathecae, compartments: (0) one (Figs 34, 35); (1) two, separated by a slight constriction (Fig. 36). [CI = 0.167, RI = 0.444]
- 87
Spermathecae, conical projections: (0) absent; (1) present (Fig. 33, SP). [CI = 1, RI = 1]
Male somatic characters [mean CI = 0.393, mean RI = 0.669]
- 88
Carapace, coloration: (0) light brown; (1) dark brown. [CI = 0.167, RI = 0.75]
- 89
Carapace, shape: (0) rounded (Magalhães & Santos, 2011: fig. 18); (1) elongated to oval (Magalhães & Santos, 2011: figs 15, 21). [CI = 0.333, RI = 0.889]
- 90
Palp, endite, tooth (Scharff & Coddington, 1997: char. 45): (0) absent; (1) present (Scharff & Coddington, 1997: fig. 36). [CI = 0.25, RI = 0.5]
- 91
Coxa I, hook and femur II, groove (Scharff & Coddington, 1997: chars 33, 34): (0) absent; (1) present (Scharff & Coddington, 1997: fig. 27). Scharff & Coddington (1997) coded these two structures in two different characters. However, no species that possesses one of these structures and lacks the other has been reported. Thus, we consider them to be functionally linked as part of the same mechanism (the coxal hook is thought to fit the femoral groove), and code them under a single character. [CI = 0.167, RI = 0.737]
- 92
Tibia I, shape (Levi, 1985): (0) straight; (1) curved (Fig. 37). [CI = 1, RI = 1]
- 93
Tibia I, dorsal and lateral macrosetae: (0) present (Fig. 37; Scharff & Coddington, 1997: fig. 3); (1) absent. The dorsum of the first and second tibia may or may not be armed with several strong, pointed macrosetae, which are thick and distinct from the remaining setae. [CI = 0.333, RI = 0.778]
- 94
Tibia II, dorsal and lateral macrosetae: (0) present (Scharff & Coddington, 1997: fig. 3); (1) absent. [CI = 0.2, RI = 0.75]
- 95
Tibia II, paired ventral macrosetae rows: (0) absent; (1) present. [CI = 0.2, RI = 0.692]
- 96
Femur I and II, dorsal and lateral macrosetae: (0) present (Magalhães & Santos, 2011: figs 15, 18); (1) absent (Magalhães & Santos, 2011: fig. 20). [CI = 0.2, RI = 0.636]
- 97
Femur I dorsal and lateral macrosetae, shape: (0) same as femur II macrosetae; (1) stronger than femur II macrosetae (Magalhães & Santos, 2011: figs 15, 18). [CI = 0.125, RI = 0.5]
- 98
Femur II, median ventral macrosetae row: (0) absent; (1) present (Magalhães & Santos, 2011: fig. 14). A distinct line of strong macrosetae, fitting between the paired ventral tibial macrosetae (see char. 95), may be present in the ventral face of the second femur. [CI = 0.25, RI = 0.4]
- 99
Trochanter IV, stout macrosetae (Scharff & Coddington, 1997: char. 32): (0) absent; (1) present (Scharff & Coddington, 1997: fig. 26). [CI = 0.5, RI = 0]
- 100
Abdomen, dorsal coloration pattern: (0) plain (Magalhães & Santos, 2011: fig. 21); (1) dark with a single, large guanine spot (Levi, 1985: fig. 487); (2) with a dark median stripe and light sides (Magalhães & Santos, 2011: fig. 15); (3) with two posterior paired guanine spots (Levi, 1985: fig. 717); (4) with many paired, large guanine spots (Levi, 1985: fig. 174); (5) plain, with two lateral dark bands (Magalhães & Santos, 2011: fig. 18). The dorsal coloration pattern in Micrathena males is very variable, but some recurrent states may be recognized. The most common state (plain) includes not only all those species that have no distinct pattern of coloration, but also some species that have unique patterns that could not be assigned to any of the other states. [CI = 0.625, RI = 0.842]
- 101
Abdomen, shape (Scharff & Coddington, 1997: char. 59): (0) rounded; (1) rectangular (Magalhães & Santos, 2011: figs 15, 18, 21); (2) inverted U-shaped (Scharff & Coddington, 1997: fig. 33). [CI = 0.667, RI = 0.875]
- 102
Abdomen, median constriction (Levi, 1985): (0) absent; (1) present (Levi, 1985: figs 548, 579). [CI = 0.5, RI = 0.5]
- 103
Abdomen, median nipples: (0) absent; (1) present (Levi, 1985: figs 548, 561). [CI = 0.5, RI = 0.75]
- 104
Abdomen, posterior tubercles: (0) absent (Magalhães & Santos, 2011: fig. 17); (1) present (Magalhães & Santos, 2011: figs 14, 21). A series of tiny tubercles, probably homologous to the females' spines, may be present in the posterior face of the abdomen. [CI = 0.111, RI = 0.619]
- 105
Abdomen, posterior tubercles, shape: (0) short and blunt (Magalhães & Santos, 2011: fig. 14); (1) long and pointed (Magalhães & Santos, 2011: fig. 21). [CI = 0.333, RI = 0.5]
- 106
Abdomen, posterior end, shape (Levi, 1985): (0) continuous; (1) pseudosegmented (Levi, 1985: figs 771, 781). [CI = 1, RI = 1]
Male genitalic characters [mean CI = 0.536, mean RI = 0.775]
- 107
Palpal bulb, tegulum, margin, shape: (0) rounded; (1) subsquarish (Fig. 38). [CI = 0.333, RI = 0.5]
- 108
Palpal bulb, tegulum, margin, projection: (0) absent; (1) present (12–17, 18–21, 33–41, TP). [CI = 0.063, RI = 0.375]
- 109
Palpal bulb, tegulum, margin, projection, length (Levi, 1985): (0) short (12–17, 18–21, 33–41, TP); (1) very long (Levi, 1985 figs 790, 851; Magalhães & Santos, 2011: fig. 39, Te). [CI = 1, RI = 1]
- 110
Palpal bulb, stipes (Scharff & Coddington, 1997: char. 19): (0) present (Scharff & Coddington, 1997: fig. 18); (1) absent. [CI = 1, RI = 1]
- 111
Palpal bulb, terminal apophysis (Scharff & Coddington, 1997: char. 22): (0) present (12–17, 18–21, 33–41, TA); (1) absent (Levi, 1985: fig. 9; Magalhães & Santos, 2011: fig. 39). [CI = 0.2, RI = 0.667]
- 112
Palpal bulb, terminal apophysis, position relative to embolus (Levi, 1985): (0) running alongside it (12–17, 33–41, TA; Levi, 1985: fig. 465); (1) covering it (12–17, 18–21, TA). In several Micrathena species, the terminal apophysis is large and wide and covers the embolus, which is therefore not entirely visible in mesal view. [CI = 0.5, RI = 0.944]
- 113
Palpal bulb, terminal apophysis, shape (Levi, 1985): (0) simple (12–17, 18–21, 33–41, TA; Levi, 1985: fig. 465) (1) large and sculptured (Fig. 14, TA; Levi, 1985: fig. 731). Micrathena species in the M. spinosa group present state 1, having a massive, embolus-supporting terminal apophysis with a large opening near its base. These two structures break off during mating, remaining attached to the female's epigynum. Females of these species with attached male parts are frequently found in museum collections. [CI = 1, RI = 1]
- 114
Palpal bulb, terminal apophysis, connectivity to embolus (Levi, 1978): (0) free (Figs 14, 16, 17); (1) fused (12–17, 18–21, 33–41). The embolus and terminal apophysis have a common base and are articulated to one another and to the radix or stipes (when present). In several species, however, the embolus and terminal apophysis are partially or totally fused rather than articulated. [CI = 0.143, RI = 0.571]
- 115
Palpal bulb, terminal apophysis and embolus, degree of connectivity to embolus: (0) partially fused (12–17, 33–41); (1) entirely fused (Fig. 21; Levi, 1985: fig. 6, A). When these two structures are fused, they can usually still be individualized as they are connected only until their middle. Some species, however, have them entirely fused, and the embolus can be seen emerging from the terminal apophysis as a tiny point with an apical opening. [CI = 0.5, RI = 0.5]
- 116
Palpal bulb, terminal apophysis, membranous projection: (0) absent; (1) present (18–21, 33–41, TAP; Magalhães & Santos, 2011: fig. 16, TAP). A drop-shaped or elongated, lightly sclerotized projection may hang from the distal portion of the terminal apophysis. [CI = 0.333, RI = 0.667]
- 117
Palpal bulb, conductor, attachment: (0) at the edge of the tegulum (Fig. 14, C); (1) at the middle of the tegulum (12–17-22–25, 33–41, C). This character refers to where the base of the conductor inserts, not to the size or shape of the structure. For instance, in Chaetacis, in which the conductor is large and extends beyond the tegulum, it is still considered attached at the middle of the tegulum. [CI = 0.333, RI = 0.6]
- 118
Palpal bulb, conductor, position: (0) not projected (12–17, 18–21, 33–41, C); (1) projected over the tegulum (Magalhães & Santos, 2011: fig. 39, C). [CI = 1, RI = 1]
- 119
Palpal bulb, conductor, apex, shape: (0) rounded (12–17, 18–21, 33–41, C); (1) pointed (Fig. 14, C). [CI = 0.143, RI = 0.7]
- 120
Palpal bulb, conductor, apex, notch: (0) absent; (1) present (Fig. 16, C). [CI = 0.2, RI = 0.5]
- 121
Palpal bulb, conductor, fold: (0) absent; (1) present (Fig. 17, C). The distal margin of the conductor may form a fold that serves as a bed to the embolus, into which it fits. [CI = 0.5, RI = 0.75]
- 122
Palpal bulb, conductor, gutter: (0) absent; (1) present (Fig. 22, C). In Wagneriana and Actinosoma, the conductor forms a gutter where the embolus fits. The position of the embolus in relation to the conductor is also characteristic of species with a gutter-shaped conductor and occurs in other araneid genera (e.g. Alpaida; I. L. F. Magalhães, pers. observ.). [CI = 1, RI = 1]
- 123
Palpal bulb, conductor, sclerotization: (0) not sclerotized (Levi, 2002: fig. 236); (1) with a sclerotized apex (Levi, 2002: fig. 223). [CI = 0.125, RI = 0.667]
- 124
Palpal bulb, conductor, retrolateral membrane: (0) absent; (1) present (Fig. 39, CM; Magalhães & Santos, 2011: fig. 39, CM). [CI = 0.333, RI = 0.667]
- 125
Palpal bulb, conductor, lobe (Levi, 1985): (0) absent; (1) present (12–17, 18–21, 33–41, CL). For a description of the difference between the terms ‘conductor lobe’ and ‘paramedian apophysis’, see char. 127. [CI = 0.143, RI = 0.455]
- 126
Palpal bulb, conductor, lobe, shape: (0) digitiform (18–21, 33–41, CL); (1) sclerotized and drop-shaped (Fig. 16, CL); (2) subsquarish and pointing dorsally (Fig. 15, CL); (3) large and covering the conductor apex (Fig. 14, CL). [CI = 1, RI = 1]
- 127
Palpal bulb, paramedian apophysis (Scharff & Coddington, 1997: char. 8): (0) absent; (1) present (18–21, 22–25, 33–41, PM; Magalhães & Santos, 2011: figs 16, 19, PM). Scharff & Coddington (1997) termed this character ‘conductor entire/with lobe’. Thus, what they called ‘lobe’ is not actually homologous to what we (and Levi, 1985) call ‘conductor lobe’, but rather to our ‘paramedian apophysis’. [CI = 0.2, RI = 0.833]
- 128
Palpal bulb, paramedian apophysis, shape: (0) digitiform (18–21, 33–41, PM); (1) large and oval (Levi, 1985: figs 549, 562); (2) bent (Fig. 22, PM). In M. spitzi and M. balzapamba, the paramedian apophysis is very large, oval, and displaced from its usual position to a more frontal location, near the embolus. In Wagneriana, Actinosoma, and Cyclosa it is strongly bent towards the median apophysis. [CI = 1, RI = 1]
- 129
Palpal bulb, median apophysis, rim (Levi, 1985): (0) present (12–17-22–25, 33–41, MA); (1) absent (Figs 14, 15). We here consider the rim to be the main part of the median apophysis, and the only part that is usually present in most araneid genera. [CI = 0.2, RI = 0.556]
- 130..
Palpal bulb, median apophysis, rim, sclerotization: (0) heavily sclerotized; (1) lightly sclerotized. A median apophysis is considered heavily sclerotized when it is dark and more sclerotized than the tegulum. [CI = 0.167, RI = 0.5]
- 131
Palpal bulb, median apophysis, rim, tooth (Levi, 1985): (0) absent; (1) present. [CI = 1, RI = 1]
- 132
Palpal bulb, median apophysis, rim, shape (Levi, 1985): (0) flat (Fig. 16, MA); (1) bent towards the embolus (12–17-22–25, MA). [CI = 0.333, RI = 0.882]
- 133
Palpal bulb, median apophysis, rim, shape: (0) simple (12–17, 18–21, MA); (1) bifid (Fig. 39, MA). [CI = 1, RI = 1]
- 134
Palpal bulb, median apophysis, base, shape: (0) short (12–17, 18–21, MA); (1) extended beneath radix (Fig. 22, MA). [CI = 1, RI = 1]
- 135
Palpal bulb, median apophysis, basal projection (Levi, 1985): (0) absent (Fig. 22); (1) present (12–17, 18–21, 33–41, BP; Magalhães & Santos, 2011: figs 16, 19, 22, 39, BP). In Micrathena and Chaetacis, the median apophysis has a projection that is partially fused to the frontal face of the radix. This projection is variable in shape and size; it may be large and very sclerotized or only an indistinct scar in the base of the median apophysis. [CI = 0.5, RI = 0.9]
- 136
Palpal bulb, median apophysis, basal projection, shape: (0) squarish (Fig. 21, BP; Levi, 1985: fig. 9); (1) curved and covering the rim (Fig. 16, BP); (2) semicircular and distally detached (Fig. 14, BP); (3) tooth-shaped (Magalhães & Santos, 2011: figs 22, 39). [CI = 0.5, RI = 0.727]
- 137
Palpal bulb, median apophysis, lobe (Levi, 1985): (0) absent; (1) present (12–17, 33–41, MAL; Levi, 1985: fig. 9, lobe). [CI = 0.5, RI = 0.941]
- 138
Palpal bulb, median apophysis, digitiform projection (Levi, 1985): (0) absent; (1) present (Figs 14, 15, DP; Levi, 1985: fig. 9). [CI = 0.167, RI = 0.375]
- 139
Cymbium, paracymbium, size (Levi, 1985): (0) small; (1) well developed (12–17, 33–41, P). In Micrathena and Chaetacis, the paracymbium is very large when compared to the same structure in other araneid spiders of similar size and often has extra lobes or a modified shape. [CI = 1, RI = 1]
- 140
Cymbium, paracymbium, position (Levi, 1985): (0) running alongside the cymbium (Fig. 41, P); (1) laterally projected (Fig. 40, P). [CI = 0.5, RI = 0.923]
- 141
Cymbium, paracymbium, retrolateral lobe, shape: (0) rounded (Figs 40, 41, P); (1) flattened (Levi, 1985: figs 9, 425, 563); (2) elongated (Levi, 1985: figs 719, 820; Magalhães & Santos, 2011: fig. 40, RL). [CI = 0.5, RI = 0.714]
- 142
Cymbium, paracymbium, hump (Levi, 1985): (0) absent; (1) present (Fig. 41, PH). [CI = 1, RI = 1]
- 143
Cymbium, paracymbium, dorsal lobe: (0) absent; (1) present (Magalhães & Santos, 2011: fig. 40, DL). [CI = 0.333, RI = 0.667]
- 144
Palpal tibia, digitiform projection (Levi, 1985): (0) absent; (1) present (Levi, 1985: figs 631, 681). [CI = 0.5, RI = 0.857]
- 145
Palpal tibia, digitiform projection, shape: (0) simple (Levi, 1985: fig. 631); (1) bifid (Levi, 1985: fig. 681). [CI = 1, RI = 1]
- 146
Palpal patella, large macroseta (Scharff & Coddington, 1997: char. 4): (0) present (Scharff & Coddington, 1997: fig. 5); (1) absent. [CI = 0.2, RI = 0.556]
AUTAPOMORPHIES
This section lists characters that are autapomorphic, most of them only in the context of this study, and that have been used in the Bayesian analyses. The taxa that present each autapomorphic state are indicated in brackets. Characters are organized in the order of appearance of taxa.
- 1
Female, carapace, posterior eye row, orientation (Scharff & Coddington, 1997: char. 54): (0) recurved; (1) procurved. [Argiope argentata]
- 2
Female, abdomen, sides, shape: (0) plain; (1) undulating. [Argiope argentata]
- 3
Female, carapace, silvery hairs: (0) absent; (1) present. [Argiope argentata]
- 4
Palpal bulb, embolus, hook: (0) absent; (1) present. [Argiope argentata]
- 5
Palpal bulb, conductor, shape: (0) lamellar; (1) twisted. [Argiope argentata]
- 6
Female, abdomen, dorsal ladder-like coloration pattern: (0) absent; (1) present. [Araneus venatrix]
- 7
Epigynum, annulated scape: (0) absent; (1) present. [Araneus venatrix]
- 8
Palpal bulb, subterminal apophysis (Scharff & Coddington, 1997: char. 20): (0) absent; (1) present. [Araneus venatrix]
- 9
Palpal bulb, median apophysis, bifid prong (Scharff & Coddington, 1997: char. 11): (0) absent; (1) present. [Araneus venatrix]
- 10
Palpal bulb, subtegulum, size: (0) small, occupying half of the palpal bulb in retrolateral view; (1) very large, occupying most of the palpal bulb in retrolateral view. [Aspidolasius branicki]
- 11
Palpal bulb, embolus, size and shape: (0) straight to slightly curved; (1) very long and coiled [Aspidolasius branicki]; (2) wide and scythe-like [Micrathena lata].
- 12
Epigynum, dorsal depressions flanking the lobe (Levi, 1999): (0) absent; (1) present (Levi, 1999: fig. 31). [Cyclosa fililineata]
- 13
Palpal bulb, conductor, frontal lobe (Levi, 1999): (0) absent; (1) present (Levi, 1999: fig. 41). [Cyclosa fililineata]
- 14
Female, abdomen, frontal condyles (Scharff & Coddington, 1997: char. 58): (0) absent; (1) present. [Gasteracantha cancriformis]
- 15
Female, abdomen, genital tubercle (Scharff & Coddington, 1997: char. 27): (0) absent; (1) present. [Gasteracantha cancriformis]
- 16
Female, abdomen, anterior projection that fits on sternum notch (Levi, 1996): (0) absent; (1) present. [Hypognatha belem]
- 17
Female, abdomen, dorsal sclerites (Levi, 1996): (0) absent; (1) present. [Hypognatha belem]
- 18
Male, carapace, median clypeal projection (Levi, 1996): (0) absent; (1) present. [Hypognatha belem]
- 19
Male, carapace, lateral eyes, position (Levi, 1996): (0) sitting on the carapace; (1) on the tips of lateral projections. [Hypognatha belem]
- 20
Palpal bulb, embolus base, shape: (0) tubular; (1) flat, wide and enlarged (Levi, 1996: fig. 126). [Hypognatha belem]
- 21
Palpal bulb, embolus, orientation (Scharff & Coddington, 1997: char. 24): (0) clockwise; (1) anticlockwise. [Hypognatha belem]
- 22
Palpal bulb, extra tegular projection: (0) absent; (1) present (Fig. 22). [Wagneriana dimastophora]
- 23
Palpal bulb, paramedian apophysis, size (Levi, 1996): (0) short; (1) very long (Levi, 1996: figs 276, 281). [Xylethrus superbus]
- 24
Epigynum, transverse bar, shape: (0) entirely attached to the epigynal bulge; (1) with a constriction near the base (Levi, 1985: fig. 57). [Micrathena nigrichelis]
- 25
Female, abdomen, spinnerets ring, anterior protuberance: (0) absent; (1) present. [Micrathena clypeata]
- 26
Female, abdomen, fourth pair of lateral spines, coloration (Levi, 1985): (0) dark red to black; (1) yellow. [Micrathena excavata]
- 27
Female, carapace, setae (Levi, 1985): (0) short and sparse; (1) long and very numerous (Levi, 1985: figs 169, 170) [Micrathena furcula]
- 28
Palpal bulb, median apophysis, basal projection, orientation: (0) attached to the mesal face of the tegulum; (1) detached and pointing towards the embolus. [Micrathena kirbyi].
- 29
Epigynum, transverse bar, anterior margin, shape (Levi, 1985): (0) concave to plain; (1) convex. [Micrathena plana]
- 30
Female, abdomen, M. ruschii first pair of lateral spines: (0) absent; (1) present. [Micrathena ruschii]
- 31
Palpal bulb, conductor apex, shape (Magalhães & Santos, 2011): (0) simple; (1) divided into two parts (Magalhães & Santos, 2011: fig. 19). [Micrathena ruschii]
- 32
Palpal bulb, terminal apophysis, size: (0) at least as long as embolus; (1) shorter than half the length of the embolus. [Micrathena triserrata]
- 33
Female, abdomen, median dark longitudinal band: (0) absent; (1) present. [Micrathena decorata].
- 34
Epigynum, bulge, anterior face, shape: (0) entire; (1) with two lateral excavations (Levi, 1985: fig. 545). [Micrathena spitzi]
- 35
Palpal bulb, embolus, apex, shape: (0) simple; (1) bifid. [Micrathena vigorsi]
- 36
Palpal bulb, tegulum, distal margin, size: (0) normal; (1) very enlarged (Levi, 1985: fig. 504). [Micrathena acuta]
- 37
Cymbium, paracymbium, sclerotization: (0) as sclerotized as the rest of the cymbium; (1) much more sclerotized than the rest of the cymbium. [Micrathena acuta]
- 38
Cymbium, dorsal projection: (0) absent; (1) present (Levi, 1985: figs 488, 489). [Micrathena schenkeli]
- 39
Palpal bulb, median apophysis, scythe-like projection: (0) absent; (1) present. [Micrathena funebris]
APPENDIX 2
List of material examined
The following material was examined both for discrete character survey and for continuous measurements. Species and collecting localities are organized in alphabetical order. Coordinates in parentheses were taken from specimens labels, whereas those in brackets were retrieved using Google Earth or the geoLoc tool of the speciesLink website (http://splink.cria.org.br/geoloc?&setlang=en).
Actinosoma pentacanthum. BRAZIL. Amazonas. Campinas, Rio Autaz [7°7′S, 72°5′W], A. Roman coll ., 27.viii.2010, NRM (1♀); Cururuzinho, A. Roman coll., 28.x.2010, NRM (1♂). Bahia. Prado, Parque Nacional do Descobrimento [17°4′S, 39°18′W], A. Nemésio coll., 7.x.2009, UFMG 3367 (1♀). Mato Grosso. Poconé, Fazenda Boa Vista [16°15′S, 56°37′W], 29.viii.1987, MNRJ 14596 (1♂). Rio de Janeiro. Macaé, Lagoa das Garças [22°22′S, 41°46′W], D. Almeida coll., xi.2001, MNRJ 3454 (2♀ 1♂). Unspecified location. A. Leitão coll., MNRJ 321 (5♀ 1♂). Araneus venatrix. BRAZIL. Minas Gerais. Belo Horizonte, Estação Ecológica da UFMG (19°58′S, 43°58′W), E.S.S. Álvares & E.O. Machado coll., 10–23.i.2001, UFMG 14 (1♂); Caratinga, Estação Biológica de Caratinga [19°47′S, 42°8′W], E.S.S. Álvares coll., 13.v.2000, UFMG 662 (1♀); Dionísio, [19°49′S, 42°45′W], R. Loyola coll., 30.iv.2001, UFMG 925 (1♀); Santa Bárbara, Estação de Preservação e Desenvolvimento Ambiental de Peti [19°58′S, 42°9′W], E.S.S. Álvares & T. Rodrigues coll., 9.iv.2004, UFMG 1647 (1♀); Santana do Riacho, Parque Nacional Serra do Cipó (19°20′S, 43°31′W), E.S.S. Álvares & E.O. Machado coll., 14.ii.2001, UFMG 475 (1♀). São Paulo. Barueri, [23°30′S, 46°52′W], K. Lenko coll., 20.i.1966, MZSP 5208 (2♀ 1♂). Argiope argentata. BRAZIL. Minas Gerais. Belo Horizonte, Alto Santa Lúcia [19°55′S, 43°56′W], C.S. Azevedo coll., 15.x.1998, UFMG 144 (1♀); Belo Horizonte, Estação Ecológica da UFMG (19°58′S, 43°58′W), E.S.S. Álvares & E.O. Machado coll., 25.xi.1999, UFMG 15 (1♂); ditto, 10–23.i.2001, UFMG 19 (1♂); Belo Horizonte, [19°55′S, 43°56′W], A.J. Santos et al. coll., vi−viii.1993, UFMG 161 (11♀ 2♂); Marliéria, Parque Estadual do Rio Doce [19°43′S, 42°45′W], T. Rodrigues coll., 14.xi.2003, UFMG 1680 (1♀); Prudente de Morais, Fazenda Sapé[19°29′S, 44°10′W], E.S.S. Álvares coll., 3–4.ii.2001, UFMG 299 (1♀ 2♂); ditto, 2.i.2000, UFMG 1847 (1♂); Santana do Riacho, Cardeal Mota [19°10′S, 43°42′W], E.S.S. Álvares coll., 15.vii.2001, UFMG 1121 (1♂); Santana do Riacho, Parque Nacional Serra do Cipó[19°10′S, 43°42′W], E.S.S. Álvares & E.O. Machado coll., 20–23.xii.2000, UFMG 482 (1♀); ditto, 21–24.vii.2000, UFMG 484 (2♀). Aspidolasius branicki. BRAZIL. Acre. Senador Guiomard, Reserva Extrativista Catuaba [10°10′S, 67°50′W], E.F. Morato coll., 2002, IBSP 158659 (1♂); ditto, IBSP 158667 (1♂); ditto, IBSP 158691 (1♀); ditto, IBSP 158752 (1♂). Pará. Novo Progresso, Serra do Cachimbo (9°16′18.6″S, 54°56′22.9″W), D.R. Santos-Souza coll., 9.ix.2003, MPEG 6102 (1♂). ECUADOR. Napo. Estación Biológica Jatun-Sacha, (1°3′57″S, 77°37′W), A.J. Santos coll., 1–5.xii.2009, UFMG 3366 (2♀). Chaetacis aureola. BRAZIL. Amazonas. Barcelos, Lago Javalaca, Rio Demeni (0°16′15″S, 62°44′50″W), A.A. Nogueira et al. coll., 17.viii.2008, IBSP 120528 (1♂). Bahia. Una, Reserva Biológica de Una [15°18′S, 39°3′W], A.D. Brescovit et al. coll., 15–28.xi.2000, IBSP 46460 (1♂). Espírito Santo. São Mateus, Reserva Florestal Vale do Rio Doce [18°44′S, 39°50′W], A.D. Brescovit et al. coll., 5–12.i.1998, IBSP 16950 (2♀ 2♂). Pará. Arapari, Margem Esquerda do Rio Tocantins [8°58′S, 57°11′W], B.M. Mascarenhas et al. coll., 27.iii.1977, MPEG 2877 (1♀); Belém, Parque Ambiental do Utinga (1°26′0″S, 48°25‴W), G.H.F. Azevedo et al. coll., 12.ii.2010, UFMG 3770 (5♀); Novo Progresso, Serra do Cachimbo (9°21′89″S, 55°2′1″W), D.R. Santos-Souza coll., 16.ix.2003, MPEG 4152 (1♂); Novo Progresso, (7°9′7″S, 55°18′20″W), J.O. Dias coll., 20.xi.2005, MPEG 4487 (1♀). C. bandeirante. BRAZIL. Mato Grosso. Indiavaí, Sítio Dona Júlia, Rio Sepotuba [15°29′S, 58°34′W], J. Raizer coll., 1.iii.2002, IBSP 48980 (2♀). Mato Grosso do Sul. Corumbá, Passo do Lontra [19°0′S, 57°39′W], J. Raizer et al. coll., iv.1998, IBSP 70783 (3♂). São Paulo. Presidente Epitácio, Usina Hidrelétrica Engenheiro Sérgio Motta [21°45′S, 52°5′W], Equipe IBSP coll., 16.i−13.ii.1999, IBSP 160897 (1♀); ditto, IBSP 160898 (3♀ 2♂); ditto, IBSP 23255 (1♂); ditto, IBSP 23245 (4♂); Rosana, Distrito de Primavera, Usina Hidrelétrica Engenheiro Sérgio Motta [22°34′S, 53°3′W], Equipe IBSP coll., i−ii.2000, IBSP 29905 (10♀). C. cornuta. BRAZIL. Acre. Rio Branco, Reserva Extrativista Humaitá[9°58′S, 67°48′W], R.S. Vieira coll., 18.viii.1995, IBSP 15617 (2♀); Sena Madureira, Seringal Santo Antônio [9°4′S, 69°39′W], B. Patterson coll., 15–18.ix.1973, MCZ 91711 (1♀). Pará. Melgaço, Estação Científica Ferreira Penna [1°48′S, 50°43′W], D.F. Candiani coll., 8.vi.2004, MPEG 2826 (1♀); ditto, B. M. Mascarenhas coll., 5.ix.1999, MPEG 2827 (1♀); ditto, J.A.P. Barreiros coll., 16.iv.2002, MPEG 2828 (1♀). COLOMBIA. Putumayo. Puerto Asis, [0°30′N, 76°30′W], MCZ 91700 (1♀ 1♂); ditto, MCZ 91725 (1♀). C. picta. BRAZIL. Goiás. Itajaí, Fazenda Lindos Campos (19°49′31″S, 51°32′24″W), Equipe Jauru coll., iv.2004, IBSP 78161 (1♀). Mato Grosso. Barra dos Bugres, [15°4′S, 57°11′W], A. Cerruti coll., xi.1983, MNRJ 2540 (1♀); Indiavaí, Sítio Dona Júlia, Rio Sepotuba [15°29′S, 58°34′W], J. Raizer coll., 1.iii.2002, IBSP 160899 (1♂); Xavantina, [14°40′S, 52°20′W], H. Sick coll., 17.ii.1947, MZSP 1243 (2♀). Mato Grosso do Sul. Inocência, Fazenda Lagoinha (19°17′3″S, 51°3′6″W), Equipe Jauru coll., iv.2004, IBSP 78114 (1♂); Paraíso, Fazenda Pedra Branca (19°11′18″S, 52°53′46″W), Equipe Jauru coll., iv.2004, IBSP 78139 (1♀). Minas Gerais. Brasilândia de Minas, Fazenda Brejão [17°0′S, 46°0′W], R.A.F. Redondo & F.A. Perini coll., 4.iv.2004, UFMG 1535 (1♀). Pará. Santarém, [2°25′S, 54°42′W], MNHN 16119 (1♂). Pernambuco. Recife, Reserva Florestal Dois Irmãos [8°3′S, 34°54′W], R.L.C. Baptista coll., ii.1988, MNRJ 14620 (1♀). Sergipe. Itabaiana, Povoado da Ribeira [10°47′S, 37°26′W], N.A.C. Zyngier coll., 25.ix.1999, IBSP 24143 (3♀). Cyclosa fililineata. BRAZIL. Minas Gerais. Nova Lima, RPPN Mata Samuel de Paula (20°00′S, 43°52′W), J.P.P. Pena-Barbosa et al. coll., 15.x.2006, UFMG 2451 (1♂); ditto, UFMG 2452 (1♀); ditto, UFMG 2784 (4 ♀ 1♂); ditto, UFMG 2785 (1♀ 4♂); ditto, 29–30.iv.2007, UFMG 2786 (1♂). Enacrosoma anomalum. BRAZIL. Acre. Rio Branco, Reserva Extrativista Humaitá[9°58′S, 67°48′W], Equipe IBSP.SMNK coll., 12.iv.1996, IBSP 15798 (1♂). Amazonas. Tamaniqua, Paranã Teiú, Juruá[2°37′S, 65°43′W], F.N.A.A. Rego & C.A. Rheims coll., 19.ix.2003, IBSP 81632 (1♀). Bahia. Ilhéus, CEPLAC [14°48′S, 39°1′W], A.D. Brescovit et al. coll., 11.iv.1998, IBSP 19165 (1♀); ditto, IBSP 18897 (2♂). Pará. Altamira, Castelo dos Sonhos (8°17′15″S, 54°59′55″W), D.F. Candiani coll., 12.xi.2005, MPEG 4469 (1♀). Rio de Janeiro. Volta Redonda, Floresta da Cicuta [22°31′S, 44°7′W], Equipe Biota coll., 11–18.vi.2001, IBSP 96263 (1♂). Gasteracantha cancriformis. BRAZIL. Bahia. Itamaraju, [17°2′S, 39°31′W], UFMG 683 (2♀). Espírito Santo. Colatina, (19°20′S, 40°33′W), T. Souza coll., IBSP 87978 (6♀). Minas Gerais. Lagoa Santa, [19°37′S, 43°52′W], I.L.F. Magalhães coll., 9–12.iv.2009, UFMG 1144 (1♀); Prudente de Morais, Fazenda Sapé (19°30′S, 44°7′W), E.S.S. Álvares coll., UFMG 276 (1♀ 1♂). Rio de Janeiro. Rio de Janeiro, Pão de Açúcar [22°56′23″S, 43°9′12″W], E.H. Wienskoski coll., i.2007, MNRJ 5007 (1♂). Rio Grande do Sul. MNRJ 41684 (1♀ 2♂). São Paulo. Biritiba-Mirim, Margem do Rio Biritiba [23°34′S, 46°2′W], Equipe IBSP coll., 10.x.2002, IBSP 117549 (1♂); São Paulo, Reserva CUASO [21°59′S, 49°59′W], F.S.Cunha coll., 9.vi.1999, IBSP 32901 (1♂); São Sebastião, [23°47′S, 45°25′W], E.S.S. Álvares coll., xi.2003, UFMG 1783 (1♀). Hypognatha belem. BRAZIL. Alagoas. Murici, Estação Biológica do Murici (9°15′S, 35°51′W), Equipe Biota coll., 13–22.ix.2003, IBSP 84203 (1♂); ditto, IBSP 84206 (1♂). Bahia. Una, Reserva Biológica de Una [15°18′S, 39°3′W], A.D. Brescovit et al. coll., 13.iv.1998, IBSP 18037 (1♀); ditto, A.D. Brescovit et al. coll., 15–28.xi.2000, IBSP 45758 (1♀). Micrathena acuta. BRAZIL. Acre. Rio Branco, Reserva Experimental Catuaba (10°4′24.7″S, 67°37′26.6″W), G.H.F. Azevedo & A.J. Santos coll., 30.xi.2010, UFMG 1282 (1♀ 2♂); Rio Branco, Reserva Extrativista Humaitá[9°58′S, 67°48′W], Equipe IBSP.SMNK coll., 12.iv.1996, IBSP 15767 (1♀). Amazonas. Umarituba, Rio Negro [0°20′S, 66°33′W], A. Roman coll., 20.iv.1924, NRM (1♀); Rio Itacoaí, J.C. Melo-Carvalho & A. Viegas coll., v.1950, MNRJ 14589 (1♂). Bahia. Una, Reserva Biológica de Una [15°18′S, 39°3′W], A.D. Brescovit et al. coll., 15–28.xi.2000, IBSP 45930 (1♀); ditto, IBSP 46445 (1♂); ditto, IBSP 46948 (1♀); ditto, IBSP 47024 (1♂). Roraima. Ilha de Maracá, [3°22′N, 61°26′W], S. Harris coll., 26.xi.1997, MNRJ 2558 (3♀). ECUADOR. Napo. Estacíon Biológica Jatun-Sacha, (1°3′57″S, 77°37′0″W), A.J. Santos coll., 1–5.xii.2009, UFMG 3365 (2♀); Napo-Galeras, (0°44′0″S, 77°35′28″W), A.J. Santos coll., 27.xi.2009, UFMG 3358 (1♂). M. balzapamba. ECUADOR. Bolívar. Balzapamba, [1°46′S, 79°11′W], Wm. Clarke-Macintyre coll., 26.v.1938, AMNH (1♀); ditto, AMNH (1♂); ditto, MCZ (1♀); ditto, vi.1938, AMNH (1♀). Los Ríos. Montalvo, [1°47′S, 79°17′W], Wm. Clarke-Macintyre coll., 20.iv.1938, AMNH (1♀). M. bifida. PERU. Junín. Uticyacu, [6°37′S, 78°47′W], F. Woytkowski coll., iii.1948, MCZ 91721 (1♀ 1♂). M. brevipes. COSTA RICA. Heredia. Puerto Viejo, Estacíon Biologica La Selva [10°25′N, 84°00′W], W. Eberhard coll., 1986, MCZ 91648 (1♀); ditto, vi.1982, MCZ 91662 (1♀); ditto, ii.1986, MCZ 91701 (4♂). M. clypeata. BOLIVIA. Unspecified location. MNHN 15704 (12♀). BRAZIL. Acre. Rodrigues Alves, Parque Nacional da Serra do Divisor [7°44′S, 72°38′W], L. Resende & R. Vieira coll., 10.iii.1997, IBSP 12483 (4♀); ditto, R.S. Vieira coll., 21.xi.1996, IBSP 9472 (2♂). Amazonas. Borba, Balneário do Lira [4°23′S, 59°35′W], Equipe IBSP coll., 22.iv.1996, IBSP 15490 (1♂); Manaus, Cabo Frio, Reserva do Projeto Dinâmica Biológica de Fragmentos Florestais (2°30′S, 60°0′W), F.N.A.A. Rego coll., 14.iii.2002, IBSP 78198 (1♂). ECUADOR. Napo. Estación Biológica Jatun-Sacha, (1°3′57″S, 77°37‴W), A.J. Santos coll., 1–5.xii.2009, UFMG 3360 (2♂ 1♀). NO DATA. MNHN 4999 (11♂). M. cyanospina. BRAZIL. Amazonas. Manaus, Reserva KM 41 [3°6′S, 60°1′W], C.M.P. Leite coll., 30.vii−2.ix.2008, IBSP 119795 (1♀); Presidente Figueiredo, Usina Hidrelétrica de Balbina [2°2′S, 60°2′W], Equipe IBSP coll., 1987.1988, IBSP 10818 (1♀); São Gabriel da Cachoeira, Pico da Neblina, Bebedouro Novo (0°44′53.88″S, 65°58′31.8″W), A. Nogueira coll., 30.ix.2007, INPA-AR 6279 (1♂). Pará. Cachimbo, [8°56′S, 54°54′W], Werner coll., xi.1955, MZSP 8262 (3♀). M. decorata. COLOMBIA. Magdalena. Cerro Lagile, Sierra Nevada de Santa Marta [10°56′N, 73°33′W], J.A. Kochalka coll., 30.iv.1975, MCZ 91645 (1♀). Socorpa Mission. Sierra de Perijá, [9°21′N, 72°59′W], B. Malkin coll., 21.viii.1968, MCZ 91666 (1♀). M. digitata. BRAZIL. Minas Gerais. Santana do Riacho, Parque Nacional da Serra do Cipó, Portaria Palácio (19°15′S, 43°31′W), E.S.S. Álvares & E.O. Machado coll., 10.xii.2001, UFMG 1350 (2♀). Pará. Belém, [1°27′S, 48°29′W], T. McGrath coll., vii.1971, MCZ 91670 (1♀). Rio de Janeiro. Rio de Janeiro, Parque Nacional da Tijuca [22°58′S, 43°15′W], E.S.S. Álvares coll., ii.2004, UFMG 1778 (1♂); Rio de Janeiro, Sumaré[22°57′S, 43°15′W], H. Sick coll., i.1946, AMNH (1♂); Rio de Janeiro, [22°57′6″S, 43°12′42″W], H. Levi & L. Levi coll., 30.iii.1983, MCZ 91664 (2♀); A. Roman coll., NRM (1♀). São Paulo. Cubatão, Mata de Encosta da Copebrás (23°50′6″S, 46°23′52″W), A. Nogueira et al. coll., vi−ix−xii.2008, IBSP 135651 (1♀ 2♂); Jundiaí, Reserva Biológica Municipal da Serra do Japi (23°13′S, 45°56′W), A.D. Brescovit et al. coll., 2004, IBSP 68689 (1♂). Unspecified location. USNM (1♂). NO DATA. S. Wagner coll., 1910, MNHN (1♀). M. evansi. BRAZIL. Amazonas. Rio Autaz, Caapiranga [3°17′S, 61°13′W], A. Roman coll., 30.viii.1914, NRM (1♀); Rio Autaz, Santa Amélia [3°24′S, 58°57′W), A. Roman coll., 11.ix.1914, NRM (1♂). Bahia. Itamaraju, [17°2′S, 39°31′W], MNRJ 14585 (1♀); Jussari, Fazenda São Francisco [15°11′S, 39°31′W], CEPLAC coll., 27.xi.1969, MNRJ 14584 (1♀); Mascote, Fazenda Palestina [15°33′S, 39°16′W], CEPLAC coll., 09.vi.1968, MNRJ 14581 (1♀); Prado, Fazenda Furado [17°21′S, 39°13′W], CEPLAC coll., 13.ix.1970, MNRJ 14588 (1♀). Minas Gerais. Santa Bárbara, Estação de Preservação e Desenvolvimento Ambiental de Peti [19°58′S, 42°9′W], C.S. Azevedo coll., 12.xi.1999, UFMG 685 (1♀). Pará. Belém, [1°27′S, 48°29′W], M.E. Galiano coll., viii.1971, MNRJ 2526 (2♀ 1♂); Melgaço, Estação Científica Ferreira Penna (1°44′18.02″S, 51°27′48.01″W), 18.xi.2005, MPEG 16753 (1♂); Novo Progresso, Serra do Cachimbo (9°22′02.9″S, 55°01′11.9″W), A.B. Bonaldo coll., 18.ix.2003, MPEG 6076 (1♂). NO DATA. MNHN 16025 (1♀). M. excavata. BOLIVIA. Unspecified location. MNHN 15701 (3♀). BRAZIL. Minas Gerais. Belo Horizonte, Estação Ecológica da UFMG (19°58′S, 43°58′W), B.T. Faleiro coll., 11.ix.2009, UFMG 3349 (1♀); ditto, I.L.F. Magalhães coll., 24.iv.2009, UFMG 990 (1♀); Cataguases, Estação Ecológica Água Limpa (21°22′22″S, 42°42′53″W), V.B. Rodrigues & D.C. Cavalari coll., iv.2010, UFMG 4131 (1♀); ditto, v.2010, UFMG 4132 (1♀); Marliéria, Parque Estadual do Rio Doce (19°48′S, 42°38′W), Equipe Biota coll., 01–10.ix.2003, IBSP 94952 (2♂). Pará. Melgaço, Estação Científica Ferreira Penna [1°48′S, 50°43′W], A.B. Bonaldo coll., 25.iii.2002, MPEG 2774 (1♀ 1♂); Novo Progresso, Serra do Cachimbo (9°16′49″S, 54°56′32″W), D.D. Guimarães coll., 15.ix.2003, MPEG 4151 (1♂). Santa Catarina. Paulo Lopes, Parque Estadual da Serra do Tabuleiro (27°77′S, 48°63′W), Equipe Biota coll., 10–20.ii.2002, IBSP 96551 (1♂). M. fissispina. BRAZIL. Alagoas. Murici, Estação Biológica do Murici [9°18′S, 35°56′W], Equipe Biota coll., 13–22.ix.2003, IBSP 84155 (1♂). Bahia. Iguassú, A. Roman coll., NRM (1♂); Itamaraju, [17°2′S, 39°31′W], UFMG 667 (1♂); Jussari, Fazenda São Francisco [15°11′S, 39°31′W], CEPLAC coll., 27.xi.1969, MNRJ 14582 (1♂); Una, Reserva Biológica de Una [15°18′S, 39°3′W], A.D. Brescovit et al. coll., 15–28.xi.2000, IBSP 46417 (2♀). Espírito Santo. Linhares, [19°25′S, 40°3′W], G.Q. Romero coll., 25–30.viii.2002, IBSP 36743 (2♀); Presidente Kennedy, Ferrous Mineração (20°1′S, 40°5′W), I.S. Oliveira coll., 17–21.i.2010, UFMG 3494 (2♀). Paraíba. Areia, Reserva da Mata do Pau de Ferro [6°57′S, 35°41′W], A.D. Brescovit et al. coll., 23–29.ix.1999, IBSP 84233 (1♂). ECUADOR. Unspecified location. MNHN 10510 (1♂). NO DATA. MNHN 19236 (3♀); UFMG (1♀). M. funebris. MEXICO. Chiapas. Escuintla, [15°19′N, 92°40′W], N. Banks coll., MCZ 91715 (4♀). Jalisco. Chamela, [19°31′N, 105°4′W], W. Eberhard coll., 1.x.1989, MCZ (1♀ 3♂). M. furcata. ARGENTINA. Buenos Aires. Paraná de Las Palmas y Canal 6, [34°16′S, 58°33′W], M.E. Galiano coll., v.1963, MCZ 91723 (2♀). BRAZIL. Espírito Santo. Castelo, [20°36′S, 41°12′W], M. Alvarenga coll., xi.1976, AMNH (1♂). Minas Gerais. Catas Altas, Parque Natural do Caraça [20°4′S, 43°24′W], E.S.S. Álvares coll., 29.x.2005, UFMG 1243 (1♀). Paraná. Araucária, [25°34′S, 49°25′W], O. Mielke coll., i.v.1967, MCZ 91718 (1♀); Volta Grande, [25°27′S, 50°19′W], B. Hentel coll., MNRJ 3859 (1♀). São Paulo. Águas da Prata, [21°55′S, 46°43′W], A.T. Costa coll., 27.xi.1969, MNRJ 3874 (2♀); Boracéia, [22°10′S, 48°45′W], L.T. Filho coll., 28.ii.1949, MZSP 11184 (2♂); Itapeva, [23°58′S, 48°52′W], D. McGrath & S.M. Camazine coll., xii.1970, MCZ 91714 (2♀). M. furcula. BRAZIL. Minas Gerais. Marliéria, Parque Estadual do Rio Doce (19°39′30.1″S, 42°34′25.2″W), B.T. Faleiro coll., 21.viii.2010, UFMG 4453 (1♀). COSTA RICA. Puntarenas. Parque Nacional Corcovado, [8°32′N, 83°34′W], R.W. Matthews coll., 31.viii.1982, MCZ 91179 (1♀). PANAMA. Barro Colorado Island. Canal Zone, [9°9′N, 79°50′W], A.M. Chickering coll., viii.1936, IBSP 5015 (1♀); ditto, P. Raw coll., ix, MCZ 91713 (2♀); ditto, A.M. Chickering coll., vi−vii.1934, MCZ 91736 (1♂); ditto, P. Raw coll., MZSP 11226 (1♀). M. gaujoni. ECUADOR. Napo. Cosanga, Estacíon Biológica Yanayacu (0°35′57″S, 77°53′26″W), A.J. Santos coll., 24–30.xi.2009, UFMG 3355 (8♀ 1♂). M. gracilis. COSTA RICA. Guanacaste. Palo Verde, [10°18′N, 84°49′W], J.A. Coddington coll., 18.vii.1980, MCZ 91676 (1♀). San Jose. San Antonio de Escazu, [9°54′N, 84°07′W], x.1981, MCZ 91675 (1♂). MEXICO. Veracruz. Catemaco, [18°25′N, 95°6′W], F. Coyle coll., 23.vi.1982, MCZ 91673 (1♀); Orizaba, [18°50′N, 97°6′W], N. Banks coll., MCZ 91674 (1♂). Yucatan. Ruínas de Chichen Itza, (20°40′N, 88°34′W), W. Maddison & R.S. Anderson coll., 19–20.vii.1983, MCZ 91672 (1♀ 1♂). USA. Massachusetts. Belchertown, [42°16′N, 72°24′W], J.Lisk & P.Terrasi coll., vii.2005, MCZ 66591 (1♀). Virginia. Surry County, Pipsico Scout Res. [37°7′N, 76°52′W], E. Sabath coll., 14–20.vii.1968, MCZ 91677 (12♀ 1♂). M. guanabara. BRAZIL. Espírito Santo. Santa Teresa, [19°56′S, 40°36′W], A. Ruschi coll., MNRJ 2545 (2♀). Minas Gerais. Cataguases, Estação Ecológica Água Limpa (21°22′22″S, 42°42′53″W), V.B. Rodrigues & D.C. Cavalari coll., iv.2010, UFMG 4133 (1♀); ditto, v.2010, UFMG 4134 (1♂); ditto, UFMG 4135 (1♀). Paraná. Antonina, Reserva do Rio Cachoeira [25°27′S, 48°42′W], Hubert coll., 13–19.iv.2004, IBSP 116189 (1♂). Rio de Janeiro. Cachoeiras de Macacu, Reserva Ecológica de Guapiassú (22°25′S, 42°44′W), Equipe Biota coll., 08–12.xi.2001, IBSP 95026 (1♂); Itatiaia, Vale da Grama, Rio do Marimbondo [22°29′S, 44°33′W], E.H. Wienskoski coll., 01–03.i.2008, MNRJ 5009 (1♀); Rio de Janeiro, Corcovado [22°58′S, 43°15′W], S. Harris coll., 20.vi.1987, MNRJ 3862 (2♀); Rio de Janeiro, Jardim Botânico [22°58′1″S, 43°13′24″W], A.B. Kury coll., 21.xi.1993, MNRJ 503 (1♂); Rio de Janeiro, Parque Nacional da Tijuca [22°57′S, 43°15′W], E.S.S. Álvares coll., ii.2004, UFMG 1753 (1♀). São Paulo. Cotia, Reserva Florestal Morro Grande [23°37′S, 46°56′W], A.A. Nogueira et al. coll., 09.iii.2003, IBSP 83457 (1♂). Unspecified location. Praia da Armação, S. Harris & R.L.C. Baptista coll., 31.x.1986, MNRJ 3877 (2♀). M. guerini. COLOMBIA. Antioquia. San Vicente, [6°16′N, 75°19′W], M.A. Serna coll., 29.vii.1986, MCZ 91663 (1♀). Valle. Saladito, [10°63′N, 74°67′W], IV.1979, MCZ 91653 (1♀); ditto, W. Eberhard coll., iv.1977, MCZ 91719 (1♂). M. horrida. BRAZIL. Minas Gerais. Marliéria, Parque Estadual do Rio Doce (19°38′S, 42°34′W), Equipe Biota coll., 1–10.ix.2003, IBSP 94926 (1♀); Santa Bárbara, Estação de Preservação e Desenvolvimento Ambiental de Peti [19°58′S, 42°9′W], A.J. Santos et al. coll., 5–6.vi.2010, UFMG 4137 (1♀). Pernambuco. Recife, Horto Dois Irmãos [8°3′S, 34°54′W], A.D. Brescovit coll., 10.ii.1998, IBSP 15289 (1♂). São Paulo. São Paulo, Campus do Instituto Butantan [23°31′S, 46°37′W], 14.x.2008, IBSP 120095 (1♀ 1♂). COSTA RICA. Heredia. Puerto Viejo, Estacíon Biologica La Selva [10°25′N, 84°00′W], W. Eberhard coll., i.1978, MCZ 91646 (2♂). CUBA. Santiago de Cuba. Cuchillo de Guajimero, P.J. Darlington coll., 22.vii.1936, MCZ 91659 (3♀). ECUADOR. Napo. Estación Biológica Jatun-Sacha, (1°3′57″S, 77°37‴W), A.J. Santos coll., 1–5.xii.2009, UFMG 3362 (1♀). PANAMA. Barro Colorado Island. Canal Zone, [9°9′N, 79°50′W], A.M. Chickering coll., viii.1950, IBSP 3921 (3♀ 2♂); ditto, N. Banks coll., 13.vii, MCZ 91712 (2♂). M. jundiai. BRAZIL. Minas Gerais. Nova Lima, RPPN Mata Samuel de Paula (20°0′S, 43°52′W), J.P.P. Pena-Barbosa et al. coll., 30.iv.2007, UFMG 2474 (1♂); ditto, 13–15.x.2006, UFMG 2475 (1♀); ditto, 29–30.iv.2007, UFMG 3187 (1♂); Santa Bárbara, Estação de Preservação e Desenvolvimento Ambiental de Peti [19°58′S, 42°9′W], A.J. Santos et al. coll., 5–6.vi.2010, UFMG 4136 (1♀); Santana do Riacho, Parque Nacional Serra do Cipó (19°22′S, 43°36′W), B.T. Faleiro coll., 21.iv.2010, UFMG 4017 (1♀). Rio de Janeiro. Cachoeiras de Macacu, Reserva Ecológica de Guapiassú[22°27′S, 42°39′W], Equipe Biota coll., 8–12.xi.2001, IBSP 95042 (1♀); ditto, IBSP 95093 (1♂); Volta Redonda, Floresta da Cicuta [22°31′S, 44°7′W], Equipe Biota coll., 11–18.vi.2001, IBSP 96148 (1♀); ditto, IBSP 96222 (1♂). M. kirbyi. BRAZIL. Acre. Rodrigues Alves, Parque Nacional da Serra do Divisor [7°44′S, 72°38′W], L. Resende & R. Vieira coll., 10.iii.1997, IBSP 12472 (3♀). Amazonas. Manaus, Mata da Universidade Federal do Amazonas [3°6′S, 60°1′W], R.L.C. Baptista et al. coll., 13.v.2006, MNRJ 14578 (2♂); Manaus, Reserva Adolpho Ducke [2°57′S, 59°55′W], L. Aquino coll., 4.viii.1987, INPA-AR 412 (1♀ 1♂). Mato Grosso. Aripuanã, Reserva Humboldt (10°11′S, 59°48′W), B.C. Ratcliffe coll., 16–22.iii.1977, INPA-AR 414 (1♀). Pará. Melgaço, Estação Científica Ferreira Penna [1°48′S, 50°43′W], B.J.F. da Silva coll., 11–20.vii.1998, MPEG 2797 (1♂); ditto, F.B. Aires coll., 16.xi.2001, MPEG 2804 (1♂). ECUADOR. Napo. Estacíon Biológica Jatun-Sacha, (1°3′57″S, 77°37′0″W), A.J. Santos coll., 1–5.xii.2009, UFMG 4015 (9♀); ditto, UFMG 4016 (3♀). M. lata. BRAZIL. Amazonas. Manaus, [3°6′S, 60°1′W], K. Lenko coll., 1.ix.1962, MZSP 7687 (1♂). Bahia. Camacan, Fazenda Santa Úrsula [15°25′S, 39°29′W], CEPLAC coll., 19.xii.1968, MNRJ 14574 (1♀); ditto, 8.i.1969, MNRJ 14575 (1♀); Camacan, [15°25′S, 39°29′W], CEPLAC coll., MNRJ 2509 (2♀); Coaraci, Fazenda Boa Esperança [14°38′S, 39°33′W], CEPLAC coll., 22.vii.1970, MNRJ 14577 (1♀); Gandu, Fazenda Pedra Branca [13°44′S, 39°29′W], CEPLAC coll., 5.ii.1970, MNRJ 14573 (1♀); Gandu, Fazenda São Rafael [13°44′S, 39°29′W], CEPLAC coll., 8.v.1969, MNRJ 14583 (1♀); Mascote, Fazenda Palestina [15°33′S, 39°16′W], CEPLAC coll., 9.vi.1968, MNRJ 14576 (1♀). Espírito Santo. Santa Teresa, [19°55′S, 40°36′W], B.A. Soares coll., 29.ix.1942, MZSP 7982 (1♂). Rio Grande do Sul. Caxias do Sul, Vila Oliva [29°10′S, 51°10′W], P. Pio Buckleg coll., MNRJ 2504 (1♀). ECUADOR. Napo. Napo-Galeras, (0°44′0″S, 77°35′28″W), A.J. Santos coll., 27.xi.2009, UFMG 3356 (1♂). M. lepidoptera. COLOMBIA. Boyacá. Rio Dpan, xi−xii.1945, AMNH (2♀). Magdalena. Cerro Lagile, Sierra Nevada de Santa Marta [10°56′N, 73°33′W], J.A. Kochalka coll., 24.ii.1974, MCZ 91669 (1♂). M. nigrichelis. BRAZIL. Minas Gerais. Belo Horizonte, Estação Ecológica da UFMG [19°55′S, 43°56′W], I.L.F. Magalhães coll., 24.iv.2009, UFMG 988 (1♀); Conceição do Mato Dentro, Tabuleiro [19°2′S, 43°25′W], F.S. Leite & E.O. Machado coll., 24.iv.2003, UFMG 1508 (2♀); ditto, UFMG 1525 (1♀); Ouro Preto, Parque Estadual do Itacolomi (20°22′S, 32°32′W), K.P. Santos et al. coll., 11–13.iv.2008, UFMG 2148 (1♂); ditto, UFMG 2149 (1♀); ditto, UFMG 2369 (1♀ 1♂); Santa Bárbara, Estação de Preservação e Desenvolvimento Ambiental de Peti [19°58′S, 42°9′W], E.S.S. Álvares & T. Rodrigues coll., 7.iv.2004, UFMG 1254 (1♀); ditto, UFMG 1258 (1♀); ditto, UFMG 1283 (1♂); ditto, UFMG 1640 (1♀); ditto, UFMG 1645 (1♀); Santana do Riacho, Parque Nacional Serra do Cipó (21°15′S, 43°31′W), E.O. Machado coll., 6.iii.2002, UFMG 1693 (1♀). Rio de Janeiro. MNHN 8508 (1♂). São Paulo. Mogi das Cruzes, [23°30′S, 46°11′W], E.K. Kashimata & R. Martins coll., v.2001, IBSP 56354 (3♀); Teodoro Sampaio, Parque Estadual Morro do Diabo (22°31′S, 52°18′W), Equipe Biota coll., 24–31.iii.2003, IBSP 118371 (1♂); ditto, IBSP 118372 (1♂). NO DATA. MNHN 8404 (5♂). M. patruelis. BRAZIL. Amazonas. São José, Itacoatiara, Ilha do Soreano [9°38′S, 67°5′W], F.N.A.A. Rego & C.A. Rheims coll., 6.xi.2003, IBSP 81837 (1♂). Bahia. Iguassú, A. Roman coll., NRM (2♀); ditto, A. Roman coll., 7.viii.1924, NRM (2♂); ditto, A. Roman coll., 6.vii.1924, NRM (4♀). Mato Grosso. Porto Estrela, Cáceres [15°19′S, 57°14′W], H.F. Japyassúcoll., 27.ii.2002, IBSP 43638 (1♀). Minas Gerais. Ouro Preto, Parque Estadual do Itacolomi (20°22′S, 32°32′W), K.P. Santos et al. coll., 11–13.iv.2008, UFMG 2150 (1♂). Pernambuco. Jaqueira, RPPN Frei Caneca [8°43′S, 35°47′W], A.S.M. Silva coll., 24.ix.2006, IBSP 78776 (1♀). M. pichincha. ECUADOR. Pichincha. Alluriquín, [0°19′S, 78°59′W], S. & J. Peck coll., 27.vi.1975, MCZ 24042 (1♀); Tandapi, [0°26′S, 78°46′W], L. Pena coll., 15–20.vi.1965, NRM (1♀); Tandayapa, [0°0′N, 78°40′W], L. Burnham coll., 20.ii.1979, MCZ 24047 (1♂). M. plana. BRAZIL. Minas Gerais. Belo Horizonte, Estação Ecológica da UFMG [19°55′S, 43°56′W], I.L.F. Magalhães coll., 25.iv.2009, UFMG 982 (1♂); ditto, 24.iv.2009, UFMG 992 (5♀ 1♂); Lagoa Santa, [19°37′S, 43°52′W], I.L.F. Magalhães coll., 10.iv.2009, UFMG 956 (1♀ 1♂); ditto, UFMG 958 (8♀ 3♂); Nova Lima, RPPN Mata Samuel de Paula (20°00′S, 43°52′W), J.P.P. Pena-Barbosa et al. coll., 29–30.iv.2007, UFMG 2484 (1♂); ditto, UFMG 2485 (1♀); Prudente de Morais, Fazenda Sapé (19°30′S, 44°7′W), E.S.S. Álvares coll., xii.1998, UFMG 277 (2♀); Santa Bárbara, Estação de Preservação e Desenvolvimento Ambiental de Peti [19°58′S, 42°9′W], E.S.S. Álvares & T. Rodrigues coll., 9.iv.2004, UFMG 1268 (1♀); Sete Lagoas, Serra Santa Helena [19°27′S, 44°14′W], E.S.S. Álvares coll., 22.iii.2005, UFMG 1565 (1♀). Pará. Santarém, [2°25′S, 54°42′W], MNHN 16123 (11♂). M. pungens. BRAZIL. Amazonas. Manaus, Fazenda Dimona (2°20′28″S, 60°5′51″W), A.J. Santos coll., 4–10.viii.2009, UFMG 3354 (1♀); São Gabriel da Cachoeira, [0°19′S, 67°18′W], 13.x.1990, INPA-AR 0384 (3♀ 3♂). Pará. Belém, Parque Ambiental do Utinga (1°26′0″S, 48°25‴W), G.H.F. Azevedo et al. coll., 12.ii.2010, UFMG 3769 (1♀ 1♂); Tucuruí, [3°58′S, 49°37′W], Equipe IBSP coll., viii.1984, IBSP 5486 (2♂). ECUADOR. Napo. Estación Biológica Jatun-Sacha, (1°3′57″S, 77°37‴W), A.J. Santos coll., 1–5.xii.2009, UFMG 3363 (1♀). VENEZUELA. Bolívar. Parque Nacional Canaima, [5°20′N, 61°30′W], S. Angel coll., 7.xi.2001, IBSP 37072 (3♀ 1♂). M. ruschii. BRAZIL. Espírito Santo. Santa Teresa, [19°56′S, 40°36′W], A. Ruschi coll., MNRJ 2566 (1♀); ditto, [19°56′S, 40°36′W], A. Ruschi coll., MNRJ 4255 (1♀). Rio de Janeiro. Itatiaia, Parque Nacional do Itatiaia (22°27′17″S, 44°36′29.8″W), G.H.F. Azevedo et al. coll., 15–22.ii.2011, UFMG (3♀ 1♂). NO DATA. MNHN 20445 (1♀). M. sagittata. USA. Florida. Jackson County, [30°42′N, 85°11′W], W.A. Shear coll., 18.vii.1973, MCZ 91732 (1♀). Texas. Saratoga, [30°17′N, 94°31′W], J. Bequaert coll., x.1960, MCZ 91733 (1♂). Virginia. Surry County, Pipsico Scout Res. [37°7′N, 76°52′W], E. Sabath coll., 14–20.vii.1968, MCZ 91724 (2♂). West Virginia. Aurora, [39°19′N, 79°33′W], N. Banks coll., 7–14.viii, MCZ 91730 (3♀ 2♂); Oakvale, [37°20′N, 80°57′W], W.A. Shear coll., MCZ 91731 (1♂). M. schenkeli. BRAZIL. Goiás. Chapadão do Céu, Parque Nacional das Emas (18°5′S, 52°55′W), 10–15.x.2009, UFMG 3351 (1♀). Mato Grosso. Chapada dos Guimarães, Parque Nacional da Chapada dos Guimarães [15°26′S, 55°45′W], E.S.S. Álvares coll., 14.ii.2000, UFMG 676 (2♀). Mato Grosso do Sul. Corumbá, Passo do Lontra [19°0′S, 57°39′W], A.D. Brescovit coll., vii.1999, IBSP 23913 (1♀); ditto, J. Raizer coll., 2001–2002, IBSP 94290 (1♀ 1♂). São Paulo. Onda Verde, Fazenda São João [20°37′S, 49°17′W], F. Rane coll., i.1946, MZSP 7341 (1♂); Pindamonhangaba, [22°55′S, 45°28′W], R. Martins & I. Knysak coll., 8–10.iv.1998, IBSP 20045 (1♂); Primavera, Usina Hidrelétrica Engenheiro Sérgio Motta [21°59′S, 49°59′W], Equipe IBSP coll., i−ii.2000, IBSP 29925 (1♀). M. schreibersi. BRAZIL. Alagoas. Murici, Estação Biológica do Murici (9°12′S, 35°54′W), A. Nemésio coll., 8.ix.2009, UFMG 3345 (1♀). Amazonas. Manaus, Fazenda Dimona (2°20′28″S, 60°5′51″W), A.J. Santos coll., 4–10.viii.2009, UFMG 3353 (1♂); Manaus, Reserva do Projeto Dinâmica Biológica de Fragmentos Florestais [3°6′S, 60°1′W], E.M. Venticinque coll., ii−ix.2001, IBSP 55559 (1♂). Bahia. Porto Seguro, Estação Ecológica Pau-Brasil [16°25′S, 39°4′W], A.D. Brescovit et al. coll., 20.iv.1998, IBSP 17836 (1♂); Porto Seguro, Parque Nacional do Monte Pascoal [16°25′S, 39°4′W], A.D. Brescovit et al. coll., 22.iv.1998, IBSP 18480 (1♂); Una, Reserva Biológica de Una [15°18′S, 39°3′W], A.D. Brescovit et al. coll., 15–28.xi.2000, IBSP 46939 (2♀); ditto, IBSP 47009 (2♀). Minas Gerais. Caratinga, Estação Biológica de Caratinga [19°47′S, 42°8′W], E.O. Machado coll., 8.vii.2001, UFMG 1118 (1♂); Piedade de Caratinga, (19°45′S, 42°5′W), I.S. Oliveira coll., 21–22.vii.2009, UFMG 3348 (1♀). ECUADOR. Napo. Estación Biológica Jatun-Sacha, (1°3′57″S, 77°37‴W), A.J. Santos coll., 1–5.xii.2009, UFMG 3359 (2♀). NO DATA. MNHN 10564 (1♀). M. spinosa. BRAZIL. Pará. Belém, Benevides, Ambiente Antrópico CMNP [1°27′S, 48°29′W], D.R. Santos-Souza coll., 30.iv.2001, MPEG 4755 (1♂); Belém, Museu Paraense Emílio Goeldi, Campus de Pesquisa (1°27′3″S, 48°26′40″W), F. Burnett coll., 10.vi.2001, MPEG 4664 (1♂); Marabá, Serra Norte, Caldeirão (6°0′23″S, 50°17′50″W), M. Zanuto coll., 16.i.1984, MPEG 4240 (1♀). ECUADOR. Napo. Estación Biológica Jatun-Sacha, (1°3′57″S, 77°37‴W), A.J. Santos coll., 1–5.xii.2009, UFMG 3364 (1♀). NO DATA. AMNH (2♀ 1♂). M. spitzi. BRAZIL. Espírito Santo. Santa Teresa, [19°55′S, 40°36′W], 1–2.iv.1969, MZSP 13075 (1♂). Minas Gerais. Belo Horizonte, Estação Ecológica da UFMG (19°58′S, 43°58′W), E.S.S. Álvares coll., xii.2000, UFMG 3350 (1♂); Belo Horizonte, Museu de História e Jardim Botânico da UFMG [19°55′S, 43°56′W], U. Oliveira coll., 14.v.2009, UFMG 889 (1♀). Rio de Janeiro. Petrópolis, [22°30′S, 43°11′W], MNRJ 42490 (2♀). São Paulo. Água Funda, [23°32′S, 46°37′W], H.M. Canter coll., 16.iii.1961, MZSP 7384 (1♂); Caucaia do Alto, [23°41′S, 47°1′W], A. Nogueira & F.S. Cunha coll., iii.2003, IBSP 94606 (1♂); Cotia, [23°36′S, 46°55′W], 12.iii.2003, MZSP 23880 (1♂); Itapevi, (23°33′S, 46°55′W), V. Castilho & C. Bertim coll., 22–25.iii.1999, IBSP 38047 (2♀); São Paulo, Parque Estadual Serra da Cantareira [23°23′S, 46°35′W], R. Pinto-da-Rocha et al. coll., 26.iv.2001, MZSP 24614 (3♀). NO DATA. UFMG (1♀). M. swainsoni. BRAZIL. Mato Grosso do Sul. Anaurilândia, [22°11′S, 52°43′W], F.S. Cunha & J.P.L. Guadanucci coll., 05–11.iii.2001, IBSP 39359 (9♀ 6♂). Minas Gerais. Curvelo, [18°45′S, 44°25′W], A.A. Azevedo coll., 3.v.1999, UFMG 1119 (2♀ 1♂); Lagoa Santa, [19°37′S, 43°52′W], I.L.F. Magalhães coll., 10.iv.2009, UFMG 955 (1♀). M. triserrata. MEXICO. Campeche. Xpujil, Chicanna Ruins (18°32′N, 89°31′W), W. Maddison coll., 12–14.vii.1983, MCZ 91668 (2♀ 3♂). Quintana Roo. Francisco Villa, Kohunlich Ruins (18°26′N, 88°48′W), W.Maddison & R.S.Anderson coll., 14–17.vii.1983, MCZ 91661 (1♀). M. vigorsi. BRAZIL. Amazonas. São Gabriel da Cachoeira, Pico da Neblina [0°19′S, 67°18′W], A. Nogueira coll., 23.ix.2007, INPA-AR 6288 (1♂); ditto, 13.x.2007, INPA-AR 6289 (1♀). Roraima. Ilha de Maracá, [3°22′N, 61°26′W], S. Harris coll., 26.xi.1997, MNRJ 2554 (1♀). ECUADOR. Napo. Estacíon Biológica Jatun-Sacha, (1°3′57″S, 77°37′0″W), A.J. Santos coll., 1–5.xii.2009, UFMG 3346 (3♀). PERU. Loreto. Ampiyacu, [3°51′S, 70°41′W], J. Becker coll., i.1980, MNRJ 14592 (1♀). Wagneriana dimastophora. BRAZIL. Minas Gerais. Cataguases, Estação Ecológica Água Limpa (21°22′22″S, 42°42′53″W), V.B. Rodrigues & D.C. Cavalari coll., iv.2010, UFMG 4116 (1♀); Nova Lima, RPPN Mata Samuel de Paula (20°0′S, 43°52′W), J.P.P. Pena-Barbosa et al. coll., 14.x.2006, UFMG 2454 (1♀ 1♂); ditto, UFMG 2788 (5♀). Santa Catarina. Paulo Lopes, Parque Estadual da Serra do Tabuleiro (27°77′S, 48°63′W), Equipe Biota coll., 10–20.ii.2002, IBSP 96531 (1♂). São Paulo. Peruíbe, Estação Ecológica Juréia.Itatins [24°18′S, 47°0′W], A.D. Brescovit et al. coll., 17–21.iii.1997, IBSP 9714 (1♀ 1♂). Xylethrus superbus. BRAZIL. Amazonas. Coari, Porto Urucu (4°48′23″S, 65°2′5″W), L. Miglio coll., 9.vii.2006, MPEG 15396 (1♀); Coari, Porto Urucu (4°51′27″S, 65°4′46″W), C.A.C. Santos Jr. coll., ix.2006, MPEG 15397 (1♀). Goiás. Araguari, R. Spitz coll., iii.1930, MZSP 7718 (2♀). Pará. Novo Progresso, Serra do Cachimbo (9°16′18.6″S, 54°56′22.9″W), D.R. Santos-Souza coll., 9.ix.2003, MPEG 6074 (1♂).




