Embryo developmental events and the egg case of the Aleutian skate Bathyraja aleutica (Gilbert) and the Alaska skate Bathyraja parmifera (Bean)
Abstract
Embryo development events were correlated with egg-case changes for the Aleutian skate Bathyraja aleutica and the Alaska skate Bathyraja parmifera. Yolk absorption underwent two phases: that of steady absorption during early development and that of rapid yolk absorption during the final development stages. Total length (LT) for 50% of the pre-hatching embryos egg-case jelly disappearance was 92·04 mm (range 81–102 mm) and 99·36 mm (range 81–100 mm) for B. aleutica and B. parmifera, respectively, allowing the inner chamber to open to seawater flow. The tail filament underwent three phases of growth: rapid elongation during early development (<100 mm embryo LT), stasis of tail filament length during the remainder of embryo development and rapid absorption soon after hatching. Complete tail filament development coincided with the disappearance of egg-case jelly. Clasper buds first developed at embryos >70 mm LT for both species and the sex ratio was 1:1 well before hatching. Egg cases that were devoid of an ova or developing embryo were c. 5·0 and 6·5% of the egg cases examined for B. aleutica and B. parmifera, respectively. Measurements showed that egg cases containing only egg jelly were smaller in both width and length than those possessing an ova. Embryo stages were punctuated with distinct events that correlated with egg case changes controlling the internal environment of the developing embryo.
Introduction
Skate life history can be characterized as being more similar to large mammals than to most fish species (Frisk et al., 2001; Charnov, 2002). Skates depend on a relatively stable environment and high offspring survival, producing young that are relatively large and precocious. Juveniles emerge resembling miniature adults and do not undergo developmental stages in the open environment (Winemiller & Rose, 1992; Luer et al., 2007), foregoing the high mortality rates that many fishes experience (Wourms et al., 1988; Pratt & Casey, 1990). A highly developed egg case that encompasses the embryo during the long development periods (Berestovskii, 1994; Hamlett et al., 2005; Hoff, 2007) provides protection and serves as a modulating mechanism, regulating the embryos contact with the outside environment. Physical changes to the egg case and internal chamber are triggered by the embryo, all orchestrated to provide the critical physical requirements necessary for successful growth and development (Koob & Cox, 1990; Berestovskii, 1994; Hamlett & Koob, 1999). This study focused on embryo stage and physical changes in the egg case, and the synchrony between the two in an attempt to modulate the embryonic environment during development. Through an understanding of elasmobranch requirements for the early life-history stages, critical habitat necessary for successful reproduction may be identified.
The Aleutian skate Bathyraja aleutica (Gilbert) and the Alaska skate Bathyraja parmifera (Bean) are two of the most abundant species in the eastern Bering Sea, comprising >95% of the estimated skate biomass (Hoff & Britt, 2005; Lauth & Acuna, 2007). The skate fauna of the eastern Bering Sea consists of over 13 species, however, populations are dominated by B. parmifera on the continental shelf (0–200 m) and B. aleutica on the upper continental slope (200–1200 m). Both species possess nursery sites along the shelf-slope interface (Hoff, 2007) and evidence suggests they depend on the stable environment provided by this habitat for successful reproduction. Reproductive studies have shown both species to have moderate longevity, slow growth and late maturity at large sizes (>1000 mm, total length, LT) (Ebert, 2005; Matta & Gunderson, 2007). These species are most probable targets of directed fisheries due to their large size, high abundance and accessibility. Due to growing concern for elasmobranchs increased mortality incurred by directed fisheries, management plans for Alaskan skates are currently being considered (Matta & Gunderson, 2007). This study focuses on the first step in providing early life-history information that may aid in determining essential fish habitat requirements for these species.
Materials and methods
Egg cases and embryos of the two species were collected from nursery sites in the south-eastern Bering Sea (Hoff, 2007) using bottom trawl gear (83-112 net) similar to that used by the Alaska Fisheries Science Center (AFSC) standard eastern Bering Sea bottom trawl survey (Lauth & Acuna, 2007). The sites were sampled seasonally, approximately once every 60 days over a 14 month period, to obtain a series of developmental stages for each species. A random sub-sample of egg cases was collected from each sampling period and preserved in 10% buffered formalin while at sea. Specimens remained in formalin for ≤2 years before data collection.
Juvenile (post-hatching) Alaska skates were collected during the standard AFSC eastern Bering Sea groundfish survey during July 2006 (Lauth & Acuna, 2007). Juvenile B. aleutica were collected during the AFSC biennial eastern Bering Sea upper continental slope survey conducted in 2004 (Hoff & Britt, 2005). Dead specimens were preserved in 10% formalin at sea and stored in 70% ethanol before measurements.
Egg-case condition and measurements
Egg cases were examined and scored based on external physical condition. Egg-case condition codes were defined as: (1) egg case newly deposited; colour light tan, brown or dark yellow with pliable case and keels; posterior horn inside margins mostly intact; surface clean and free of all fouling invertebrate growth; byssal threads thick, adherent and abundant; (2) egg case previously deposited, colour dark brown with leathery texture and stiff keels; posterior horn inside margin frayed and thinned, surface moderately clean with evidence of little fouling invertebrate growth on the surface; byssal threads present either thick or adherent to mostly lost and (3) egg case old, colour deep brown to charcoal black with papery texture; posterior horns thinned and mostly frayed; surface sparsely to completely covered with fouling invertebrate growth including barnacles, anemones, snail eggs and bryozoans; byssal threads reduced, dark and mostly absent.
Egg-case width and length were measured to the nearest 0·5 mm after preservation following Ishiyama & Ishihara (1977). Egg-case width was measured at mid-case including the keels, and length was measured from the centres of the posterior and anterior margins and did not include the horns.
Embryo stages and measurements
After measurement, egg cases were cut open by making a slice down the right side (lateral cut running posterior to anterior) to expose the contents and avoid cutting the yolk or embryo. Yolk diameters were measured to the nearest 0·1 mm with electronic digital calipers. A piecewise linear model (Neter et al., 1996) was applied to yolk size at embryo LT data sets for both species. The piecewise model performed an iterative process to determine the least sum of squares for the data set and identified an inflection point, estimating the two best fitting models to the data set.
A determination of developmental stage of each embryo was made using six stage criteria. Embryo developmental stages were modified from those of Hitz (1964) and Berestovskii (1994) and were as follows: (1) egg case includes a large yolk mass surrounded by transparent egg jelly that fills the inner chamber including the horns; blastodisc may be present but no visible embryo development is apparent or embryos are microscopic, transparent and not visible with the naked eye; (2) embryo development has begun and embryo is visible; incomplete disc formation and disc does not form the most anterior portion of the body; eyes and branchial filaments have formed; embryo is visible and unpigmented, egg jelly has begun to disappear and may be limited to the horns and posterior and anterior ends of the chamber in late stage; (3) embryo disc has completely formed, connecting anteriorly; disc and tail begin pigmentation and late stages show heavy disc pigmentation; disc thorn development begins; disc diameter is smaller than yolk diameter; egg jelly has disappeared from horns and chamber; (4) embryo is fully pigmented; tail filament is fully formed and distinguishable from the rest of the tail; disc thorns and tail thorns are distinguishable; yolk sac diameter ≥10 mm; (5) embryo is fully developed; yolk sac diameter <10 mm; often yolk sac is completely absorbed and only small button remains on embryo abdomen; disc is completely formed and disc thorns are emergent and (6) ‘gel’, embryo, yolk and ovum are absent; chamber is filled with egg jelly only.
Embryo LT, tail filament length and disc width at the widest diameter were measured using either digital calipers or a ruler to the nearest 0·5 mm. To determine the size at clasper development embryos were scored as to the presence or absence of claspers protruding from the base of the pelvic fins.
Post-hatching juveniles measurements included LT, disc width and tail filament length measured to the nearest 0·5 mm. A qualitative analysis of gut contents was conducted on B. parmifera juveniles to determine if exogenous feeding had occurred.
Egg cases were assessed for the presence of horn egg jelly for all stages and the full size range of embryos encountered. The presence of egg jelly in the egg case chamber and horns was determined by breaking off the left posterior horn and determining if the horn was hollow (egg jelly absent) or full (egg jelly present). The state of egg jelly in the horn was confirmed by noting the egg jelly state in the egg-case chamber surrounding the yolk and embryo. A simple logistic regression model was used to estimate the coefficients of the logistic response function curve for absence of horn egg jelly (Conrath & Musick, 2002). The logistic model used was: Pr= [1 +e−(a +bx)]−1, where Pr= the proportion of individuals at each mm absent of horn egg jelly, x = embryo LT in mm and a and b are coefficients.
Results
Nine hundred egg cases of B. aleutica and 910 egg cases of B. parmifera were examined for contents and egg case and embryo measurements. Egg cases in stage 1, with no visible embryos, comprised 24·6% of B. aleutica and 36% of B. parmifera eggs examined, with the remaining 639 (71%) B. aleutica and 522 (57%) B. parmifera eggs containing visible embryos available for measurements (Table I).
| B. aleutica | B. parmifera | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Embryo stage | Embryo stage | |||||||||||||
| Gel | 1 | 2 | 3 | 4 | 5 | Total | Gel | 1 | 2 | 3 | 4 | 5 | Total | |
| Total examined | 45 | 216 | 397 | 125 | 102 | 15 | 900 | 60 | 328 | 284 | 148 | 82 | 8 | 910 |
| Disc width | 88 | 107 | 93 | 14 | 302 | 166 | 142 | 73 | 8 | 381 | ||||
| Sex determination | 166 | 122 | 102 | 15 | 405 | 130 | 146 | 82 | 8 | 358 | ||||
| L T | 394 | 125 | 102 | 15 | 636 | 279 | 148 | 82 | 8 | 509 | ||||
| Filament length | 94 | 101 | 95 | 15 | 305 | 107 | 129 | 71 | 8 | 307 | ||||
| Horn egg jelly state | 7 | 177 | 386 | 122 | 101 | 15 | 808 | 26 | 144 | 129 | 100 | 41 | 5 | 440 |
| Egg (yolk) diameter | 1 | 40 | 46 | 91 | 15 | 193 | 2 | 57 | 78 | 64 | 8 | 201 | ||
| Egg case length and width | 45 | 216 | 397 | 125 | 102 | 15 | 900 | 60 | 328 | 284 | 148 | 82 | 8 | 910 |
- L T, total length.
Bathyraja aleutica egg cases were predominantly in conditions 1 and 2 and rarely were found in a highly weathered state with epiphytic growth indicative of egg condition 3 (Fig. 1). Egg cases of B. parmifera occurred in conditions from 1 to 3 (Fig. 1), with all three case conditions common in those examined. Egg case conditions increased with increased embryo stages (Fig. 2) and showed a progression of distinct egg-case condition during embryo development.

Egg cases of (a) Bathyraja aleutica in two conditions (1 and 2) and (b) Bathyraja parmifera in three conditions (1, 2 and 3).
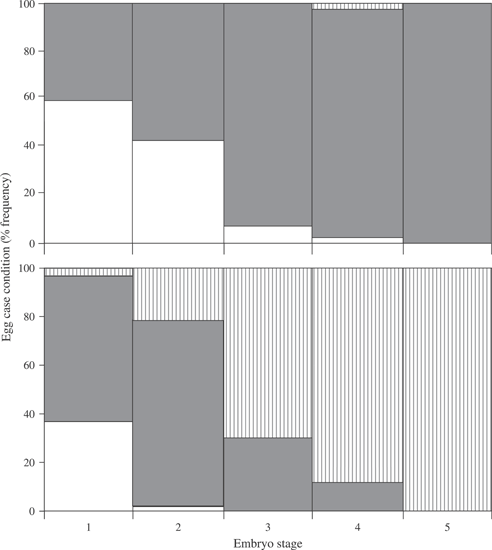
Frequency histograms of egg-case conditions [1 ( ), 2 (
), 2 (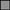 ) and 3 (
) and 3 ( )] at each embryo stage of development in (a) Bathyraja aleutica and (b) Bathyraja parmifera.
)] at each embryo stage of development in (a) Bathyraja aleutica and (b) Bathyraja parmifera.
Yolk-sac absorption proceeded at a relatively constant rate during early development and subsequently underwent a rapid change in relative absorption rate during late developmental stages. Piecewise models indicated that at embryo LT of 200 mm for B. aleutica and 176 mm for B. parmifera yolk absorption changed trajectories and the slopes of the two models were quite different (Fig. 3). At hatching embryos in stage 5 had completely absorbed all external yolk and examination of newly post-hatched juvenile B. parmifera still possessing tail filaments showed 100% had full stomachs from exogenous feeding with no signs of yolk ‘remnants’ in the gut cavity.
Yolk diameter at embryo total length (LT) for (a) Bathyraja aleutica and (b) Bathyraja parmifera. Inflection points ( ) indicate a change in the relative rate of yolk absorption during the last stage (5) of embryo development.
) indicate a change in the relative rate of yolk absorption during the last stage (5) of embryo development.
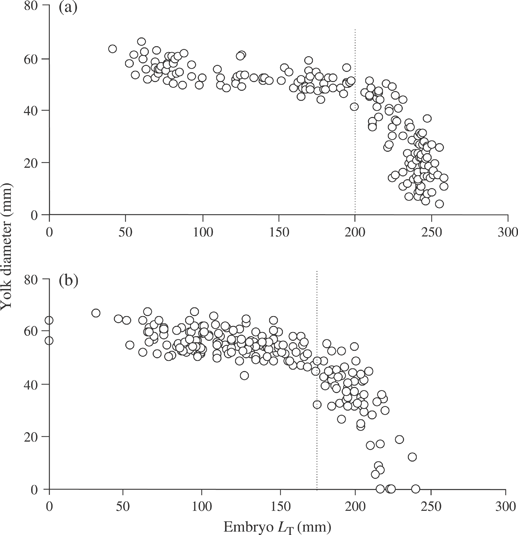
Embryos were elongated initially with a long tail and undeveloped disc through stage 2. Complete disc formation occurred during stage 3 and grew relatively rapid through hatching (Fig. 4). Growth patterns differed between pre-hatching and post-hatching fishes for both B. aleutica and B. parmifera. Post-hatching fishes followed a linear model for LT and disc growth, while pre-developing embryos followed a curvilinear exponential growth model (Fig. 5).
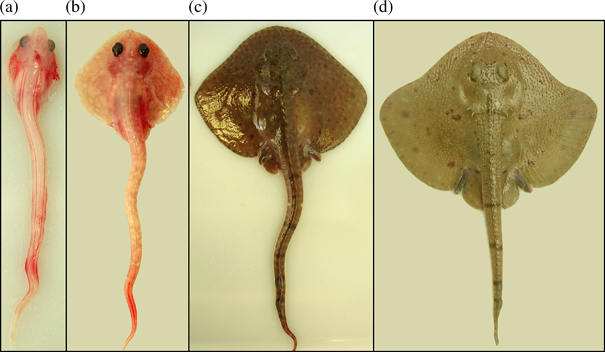
Embryo developmental stages of Bathyraja parmifera: (a) 2 (80 mm total length, LT), (b) 3 (110 mm LT), (c) 4 (184 mm LT) and (d) 5 (227 mm LT).
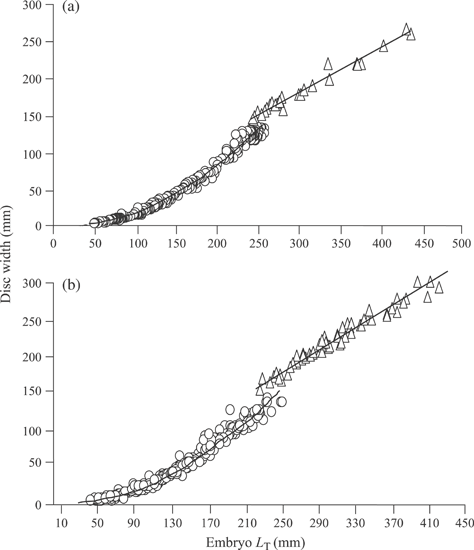
Growth in disc width and total length (LT) for pre-hatching (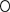 ) and post-hatching (
) and post-hatching ( ) (a) Bathyraja aleutica and (b) Bathyraja parmifera. The curves were fitted by: (a) pre-hatching y = 0·0007x2·1894 (r2= 0·99, n = 302) and post-hatching y = 0·602x + 1·3426 (r2= 0·98, n = 24) and (b) pre-hatching y = 0·0015x2·1031 (r2= 0·98, n = 391) and post-hatching y = 0·7187x − 1·0831 (r2= 0·96, n = 59).
) (a) Bathyraja aleutica and (b) Bathyraja parmifera. The curves were fitted by: (a) pre-hatching y = 0·0007x2·1894 (r2= 0·99, n = 302) and post-hatching y = 0·602x + 1·3426 (r2= 0·98, n = 24) and (b) pre-hatching y = 0·0015x2·1031 (r2= 0·98, n = 391) and post-hatching y = 0·7187x − 1·0831 (r2= 0·96, n = 59).
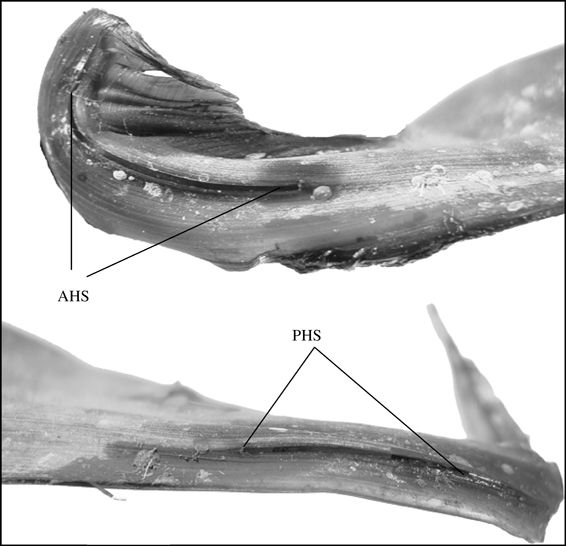
Egg-case jelly disappeared beginning inside the egg case chamber nearest the developing embryo and proceeded outwards to the anterior and posterior edges, with the horn egg jelly disappearing last, opening the posterior and anterior egg case horn slits (Fig. 6). The largest embryo that still maintained egg jelly in the posterior horn of the egg case was 102 mm LT for both species, and the smallest embryo that did not contain horn egg jelly was 81 mm LT for B. aleutica and 100 mm LT for B. parmifera (Fig. 7). Applying coefficients estimated from the logistic model determined, the LT at 50% (LT50) disappearance of horn egg jelly to be 92·04 mm LT for B. aleutica and 99·36 mm LT for B. parmifera (Fig. 7).
Detail of the anterior horn slit (AHS) and posterior horn slit (PHS) on the egg case of Bathyraja parmifera after egg jelly has disappeared and slits have opened to the flow of sea water.
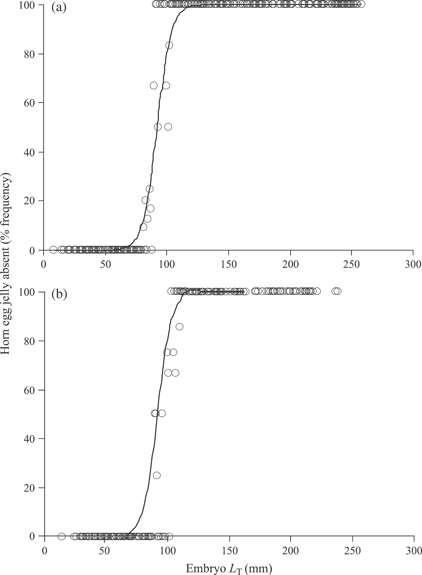
The logistic response curve applied to posterior horn egg jelly disappearance at embryo total length (LT) for (a) Bathyraja aleutica and (b) Bathyraja parmifera. For both species, egg jelly disappeared in embryos by 100 mm LT. The curves were fitted by: (a) y = 100 [1 + e−(−16·2218 + 0·176x)]−1 (n = 636, P < 0·001) and (b) y = 100 [1+ e−1(−22·752 + 0·229x)]−1 (n = 418, P = 0·001).
The tail filament developed as an extension on the posterior caudal region and was positioned in the posterior egg case horn during late development stages (Fig. 8). The tail filament grew rapidly during early development and was subsequently followed by a period of stasis or little filament growth during the late embryo stages. A piecewise regression model indicated that tail filament growth changed trajectories near 100 mm LT for both species (Fig. 9). There was little filament length change after fishes reached 100 mm LT with a mean ± length of 29·05 ± 2·57 mm (n = 232) for B. aleutica and 23·50 ± 2·34 mm (n = 217) for B. parmifera. In both species, the tail filament was quickly reabsorbed after hatching and by 300 mm LT was nearly absent.
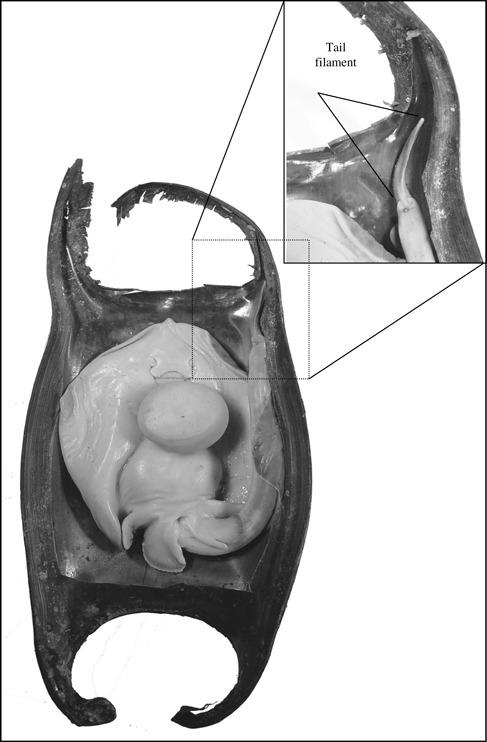
Bathyraja parmifera late-stage 4 embryo in its egg case. Inset is the detail of the tail filament and its placement in the hollow posterior horn.
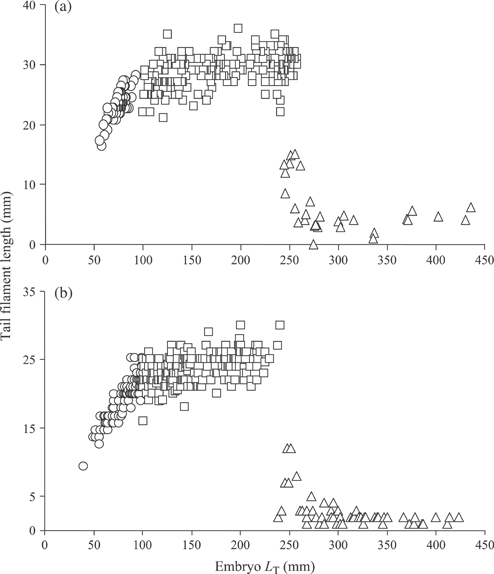
Growth of the tail filament for (a) Bathyraja aleutica and (b) Bathyraja parmifera. The tail filament underwent three phases of growth: rapid growth (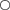 ), growth stasis (
), growth stasis ( ) and resorption soon after hatching (
) and resorption soon after hatching ( ).
).
Embryo clasper development began in early stage 2 and appeared as small, bud-like structures attached at the inside base of the pelvic fins. The claspers developed into elongated structures by hatching that were similar to those seen in post-hatching individuals. Clasper development for B. aleutica began c. 70–79 mm LT (smallest 70 mm LT), however, the sex ratio was not 1:1 until >90–99 mm LT suggesting a slightly larger size at clasper bud development (Fig. 10). Clasper development was first seen at 70–79 mm LT for B. parmifera and the sex ratio for >70 mm LT averaged 1:1.
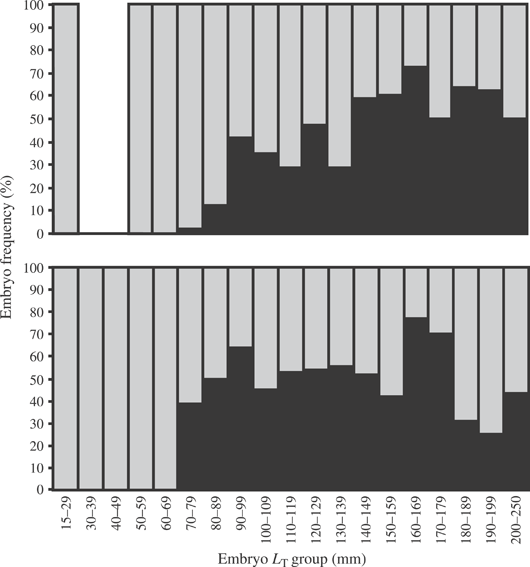
Proportion of embryos with claspers present ( ) and claspers absent (
) and claspers absent ( ) for (a) Bathyraja aleutica and (b) Bathyraja parmifera.
) for (a) Bathyraja aleutica and (b) Bathyraja parmifera.
Abnormal egg cases containing only egg jelly and devoid of any ovum or developing embryo comprised 5% of B. aleutica and 6·5% of B. parmifera egg cases examined. Abnormal eggs of both species were smaller in width and length when compared to normal egg cases containing an ovum and developing embryo (Fig. 11).
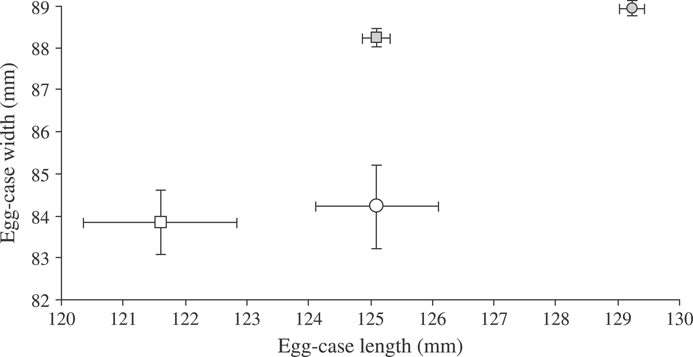
Mean ±s.e. size of egg cases of Bathyraja aleutica ( ,
,  ) and Bathyraja parmifera (
) and Bathyraja parmifera ( ,
,  ) containing egg jelly, and an ova or a developing embryo (
) containing egg jelly, and an ova or a developing embryo ( ,
,  ) and those containing only egg jelly (
) and those containing only egg jelly ( ,
,  ).
).
Tables II and III summarize the study findings on the developmental stages, embryo and egg case morphological progression, egg case condition and timing of key events in the development progress of the two skate species.
| Embryo stage | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Range | Range (mean) | Range (mean) | Range (mean) | Range (mean) | |
| Embryo size (mm) | 0 | 8–125 (67) | 108–201 (145) | 176–258 (232) | 235–258 (244) |
| Disc width (mm) | 0 | 5–29 (13) | 22–83 (43) | 65–135 (113) | 115–134 (127) |
| Tail filament (mm) | 0 | 15–35 (24) | 21–36 (28) | 22–35 (30) | 24–33 (30) |
| Yolk sac (mm) | 65–70 | 49–65 (56) | 41–61 (51) | 10–51 (29) | 4–11 (8) |
| Egg-case jelly | Chamber and horns | Disappears from chamber | Disappears from horns | Absent | Absent |
| Embryo development | Egg deposition, large yolk mass and no visible development | Embryo development, filament rapid growth and clasper development begins | Disc full formed, pigmentation on disc, and claspers developed, tail curls into horn and disc thorns begin forming | Embryo fully formed rapid yolk absorption | Complete yolk internalization and near emergence from egg |
| Egg-case shell condition | 1–2 | 1–2 | 2 | 2 | 2 |
| Egg-case progression | Colour light tan, case soft and thick, byssal threads abundant, chamber filled with egg jelly and horns slits closed | Colour changes to darker brown, case walls thin and become brittle, byssal thread thinning and adherent, egg jelly in chamber and horn disappears and horn slits open allowing influx of sea water | |||
| Embryo stage | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Range | Range (mean) | Range (mean) | Range (mean) | Range (mean) | |
| Embryo size (mm) | 0 | 15–110 (66) | 91–170 (128) | 158–239 (193) | 214–241 (222) |
| Disc width (mm) | 0 | 5–28 (14) | 21–72 (42) | 68–132 (98) | 114–136 (125) |
| Tail filament (mm) | 0 | 10–25 (20) | 18–27 (23) | 20–30 (25) | 22–30 (25) |
| Yolk sac (mm) | 50–57 (54) | 45–60 (51) | 39–59 (49) | 11–52 (35) | 0–8 (2) |
| Egg-case jelly | Chamber and horns | Disappears from chamber | Disappears from horns | Absent | Absent |
| Embryo development | Egg deposition, large yolk mass and no visible development | Embryo development, filament rapid growth and clasper development begins | Disc fully formed, pigmentation on disc, claspers developed, tail curls into horn and disc thorns begin forming | Embryo fully formed and rapid yolk absorption | Complete yolk internalization and emergence from egg |
| Egg-case shell condition | 1–2 | 2–3 | 2–3 | 3 | 3 |
| Egg-case progression | Colour light tan, case soft and thick, byssal threads numerous, chamber filled with egg jelly and horns slits closed | Colour darkens to dark brown, case hardening and walls thinning, byssal thread thinning and adherent, egg jelly in chamber and horn disappears and horn slits open allowing influx of sea water | Colour darkens to black, case brittle and thin-walled, byssal thread sparse and short, egg jelly absent and egg case open to sea water | ||
Discussion
Skate egg cases have been shown to possess anti-microbial and antifouling properties that may help protect the embryo during the early stages of development (Thomason et al., 1994, 1996). For B. parmifera, the antifouling mechanisms appeared to deteriorate with age as shown by the colour change and invertebrate growth seen on cases of late-stage embryos. Fouling, however, did not seem to affect the developing embryo as there was no evidence of fouling organism growth found in the inside chamber in any egg cases examined. The egg case of B. aleutica seemed less susceptible to fouling, possibly indicating antifouling properties remain intact longer than B. parmifera. For both species, these mechanisms may lessen naturally as the embryo develops beyond vulnerable periods with antifouling mechanisms no longer needed in later development stages. Egg cases of the big skate Raja binoculata Girard showed a similar correlation between the colour change and the embryo stage in which egg cases containing embryos near hatching had changed to dark black and possessed fouling barnacle growth (Hitz, 1964). The egg case of the spotted dogfish Scyliorhinus canicula (L.) was found to have antifouling, antibacterial and anti-settlement properties that help protect the embryo once exposed to sea water (Thomason et al., 1994, 1996).
Bathyraja parmifera and B. aleutica embryos absorbed yolk in two distinct phases: that of slow use during early development and a much more rapid use at later development stages. This pattern appears common for elasmobranchs and showed similar patterns in the slender smoothhound shark Gollum attenuatus (Garrick) (Yano, 1993), S. canicula (Lechenault et al., 1993) and the clearnose skate Raja eglanteria Bosc (Luer et al., 2007). Emergence from the egg case with a reserve of food supports the notion stated by Hoff (2007) that after hatching juvenile skates quickly move out of the nursery sites and may undergo long distance migrations. The food reserve may help them make this transition until they reach optimum habitat and began exogenous feeding.
The function of oviparous egg jelly is not clearly understood (Koob & Straus, 1998), but its presence may be important for successful early embryo development (Leonard et al., 1999). It has been suggested that egg jelly is used as a food source or as a physical protection of the egg yolk and embryo (Koob & Straus, 1998). This study showed that egg jelly disappeared early in embryo development (stage 2); therefore its use as a food source appears limited and the latter notion of protection is more plausible. Experimental studies on osmoregulation abilities of the spiny dogfish Squalus acanthias L. and R. binoculata suggested that egg jelly may be key in protecting early embryo stages from sea water until they develop the ability to osmoregulate (Evans, 1981; Read, 1968). Yolks of newly deposited eggs were extremely fragile and surrounding ectoderm and endoderm membranes were very thin and often broken upon examination. Later stage embryos possessed yolks with thickened membranes that were rarely broken with handling. Egg jelly may serve to maintain the fragile egg contents intact until yolk membranes have fully developed.
The disappearance of egg jelly and tail filament maximum growth occurred, at approximately one-third the total embryo development time for B. aleutica and B. parmifera (Hoff, 2007), similar to other oviparous species (Libby & Gilbert, 1960; Hitz, 1964; Kormanic, 1993; Koob & Straus, 1998). The synchronization of full tail filament development and the opening of the egg case to sea water suggests a co-ordinated system, ensuring a means for oxygen exchange and metabolic waste removal to meet the growing demands of the embryo. Experimental studies conducted on the little skate Leucoraja erinacea (Mitchill) suggested that the tail filament is important for setting up current flow-through the egg case and that oxygen demands of the growing embryo are the primary reason for the constant water exchange (Leonard et al., 1999).
The pattern of tail filament growth and absorption after hatching suggested it is primarily a pre-hatching phenomenon. Ishiyama (1958) found similar patterns for the raspback skate Bathyraja isotrachys (Günther) and others from Japanese waters to those demonstrated with species in the present study. For these Japanese species, tail filaments had been completely reabsorbed by 300 mm LT, shortly after juvenile emergence from the egg case. Ford (1971) found that for R. binoculata embryo tail filaments grew rapidly during early development and slowed during later stages of development, indicating the quick development and use of the tail filament during embryo development.
The occurrence of egg cases without an ovum or embryo containing only egg jelly has been reported for Amblyraja radiata (Donovan) (Templeman, 1982), as well as many other oviparous elasmobranchs (Templeman, 1982; Hamlett & Koob, 1999). Templeman (1982) reported that these egg cases took on unusual shapes and were smaller than normal eggs for R. radiata, which was also found for B. aleutica and B. parmifera. Causes for egg-case production containing only egg jelly may include mistakes in the synchrony of the ovary and the shell gland in newly mature individuals and females at the beginning or ending of a reproductive cycle in which the shell gland is producing egg cases and there are no developed ova. Egg cases and jelly are produced by the shell gland, independent of the ovum (Hamlett & Koob, 1999; Hamlett et al., 2005) and therefore egg-case production can be completed without a fertilized egg. Because egg cases are significantly smaller when they contain only egg jelly, causes may stem from the shell gland itself where the egg case is produced. Whatever the reason, non-viable eggs appear common in rajid species and may constitute >6% of all egg cases produced. This should be considered when estimating fecundity based on egg case production from aquaria observations (Libby, 1959; Libby & Gilbert, 1960; Holden et al., 1971; Holden, 1975; Mellinger, 1983; Castro et al., 1988; Berestovskii, 1994; Ellis & Shackley, 1995).
Skate oviparity appears highly specialized to ensure optimal survival of offspring (Dulvy & Reynolds, 1997). The egg case is a dynamic biological system supplying the developing embryo with all requirements until hatching. Embryo development processes of B. aleutica and B. parmifera suggest a synchronized design between the egg case and its contents, the embryo and the environment. Development of the skate embryo is punctuated with distinct events that were identified in this study. Skate reproductive behaviour and habitat selection undoubtedly play key roles in species survival. Elasmobranchs reflect a life-history strategy that relies on low production, low mortality and an unchanging environment for reproductive success (Winemiller & Rose, 1992). Ironically, all these same aspects make them highly vulnerable to population declines due to habitat disturbances, increased mortality due to fishing, global climate change and ecosystem changes. The understanding of these biological processes and their effect on oviparous elasmobranch species will help address conservation issues as they emerge. Conservation and management strategies that are based on a clear understanding of all fish life stages will be inherently more likely to succeed.
Acknowledgments
I thank the skippers and crew of the F.Vs Ocean Explorer, Sea Storm, Nordic Fury, Arcturus, Aldebaran and Great Pacific. Thanks to the scientists at the Alaska Fisheries Science Center that helped with this study, their dedication is greatly appreciated. I especially thank D. Stevenson, S. Kotwicki, S. Gaichas, R. Reuter, E. Iwamoto, E. Acuna, E. Jorgenson and C. Gredzens. Thanks to B. Voss for his tremendous help with the literature searches and obtaining research materials. Thanks to B. Lauth, R. Nelson, G. Stauffer and G. Walters for their support. Thanks to T. Essington, D. Gunderson, D. Kimura, T. Pietsch and C. Rooper, for their review and helpful suggestions to improve this manuscript. This project was supported by North Pacific Research Board (NPRB grant #415), Essential Fish Habitat funding in 2004 and 2006 and generous financial support from the Alaska Fisheries Science Center.




