Testing subspecies hypothesis with molecular markers and morphometrics in the Pacific white tern complex
Abstract
To test the validity of subspecies designations of the white tern, genetic and morphological data were used to assess differences among four putative Pacific subspecies Gygis alba candida, Gygis alba rothschildi, Gygis alba pacifica, and Gygis alba microrhyncha. The origin(s) of a recent colonization of Oahu was also examined using molecular data. Samples were collected from 209 birds, representing island groups of the North and South Pacific. Culmen length and depth, longest and shortest rectrix lengths, and wing chord measurements from an earlier dataset were compared. Mitochondrial DNA variation suggests that there are no phylogenetically distinct species within the Pacific Ocean. The genetic and morphological similarity of G. a. candida and G. a. rothschildi warrants merging them into one subspecies (G. a. candida). Gygis alba microrhyncha and G. a. pacifica are distinctly smaller and larger than the other two subspecies, respectively, but are not completely diagnosable across the morphological characters examined. Although the Pacific subspecies do not exhibit reciprocal monophyly, there is significant genetic differentiation among the two South Pacific groups, G. a. microrhyncha, G. a. pacifica, and all other Pacific subspecies. This differentiation warrants treating these two South Pacific groups as separate management units, but not species or subspecies. Finally, the recently established population of white terns on Oahu shared haplotypes with all subspecies, suggesting multiple origins from populations across the Pacific and confirming contemporary gene flow. © 2009 The Linnean Society of London, Biological Journal of the Linnean Society, 2009, 98, 586–595.
INTRODUCTION
Determining taxonomic status and systematic relationships among species is crucial for accurate biodiversity assessments and conservation management (Avise, 2004; Willerslev & Cooper, 2005). For example, the endangered Kemp's ridley sea turtle (Lepidochelys kempii) was previously thought to be related to a non-endangered species (olive ridley; Lepidochelys olivacea) but is now known to be a genetically distinct species (Bowen, Meylan & Avise, 1991). Similarly, the dusky seaside sparrow (Ammodramus maritimus nigrescens) was previously considered to be distinct subspecies based on morphological differences and was managed as a separate subspecies but was later shown to lack phylogenetic distinctiveness from other groups (Avise & Nelson, 1989). Designation of distinct, named taxonomic units can have a significant influence on how they are managed (Haig et al., 2006).
Many avian species are subdivided into multiple subspecies (American Ornithologists' Union, 1998) and the debate over its use and validity within avian taxonomy has provided opportunities for the discussion of subspecies designations. Several researchers have argued that intraspecific groups are evolutionarily important and deserve taxonomic recognition, whereas others question the validity of subspecies designations as they have been employed by the ornithological community (see forums on subspecies moderated by: Wiens, 1982; Phillimore & Owens, 2006). Those who support the importance of the role of subspecies in systematics and evolutionary biology suggest that, when made, subspecies designations should be supported by the existence of clearly recognizable characteristics, such as molecular markers, morphology, behavior, or other phenotypic parameters (Wilson & Brown, 1953; Moritz, 1994; Avise, 2000). Avise (2000) defined a subspecies as a group exhibiting reciprocal monophyly of multiple characteristics between populations. Patten & Unitt (2002) formalized Amadon's 75% rule (1949) suggesting that ‘to be a valid subspecies 75% of a population must lie outside 99% of the range of other populations for a given defining character or set of characters’. These definitions are two currently applied methods in defining subspecies.
The white tern is a small seabird found in subtropical and tropical oceans throughout the world. The American Ornithologists' Union (AOU, 1998) recognizes one species of white tern (Gygis alba, Sparrman 1786), and three subspecies: Gygis alba candida (common white tern; Pacific and Indian Ocean, except the Marquesas Islands), Gygis alba microrhyncha (little white tern; Marquesas Islands only), and Gygis alba alba (Atlantic white tern; Atlantic Ocean). Other taxonomists divide the complex into two distinct species, G. alba, which includes G. a. candida and G. a. alba, and G. microrhyncha of the Marquesas Islands (Pratt, Bruner & Berrett, 1987). The recognition of the two tropical Pacific subspecies (G. a. candida and G. a. microrhyncha) as distinct species is based on geographical and morphological differences. There are no subspecific distinctions in behavior or vocalizations.
Gygis alba candida is distributed throughout the Indian and Pacific Ocean, but absent in the Marquesas, whereas G. a. microrhyncha breeds in the Marquesas and Kiribati (Phoenix and Line Islands). Compared to G. a. candida, G. a. microrhyncha has a shorter length and depth of culmen; shorter length of retrices and wing chord; wider black eye-ring; and an overall smaller body size (Niethammer & Patrick, 1998; Olson, 2005). Pratt et al. (1987) treated G. a. microrhyncha as a distinct species to ‘encourage birders to look for both forms, and thus to aid our knowledge of their distribution and interactions’, at the same time acknowledging that the two may prove to be conspecific. Additionally, G. a. candida currently includes groups that were previously recognized as distinct subspecies: Gygis alba rothschildi (Northwestern Hawaiian Islands and Krusenstern Island), Gygis alba pacifica (Central and South Pacific), Gygis alba royana (Kermadec and Norfolk Islands), Gygis alba leucopes (Henderson and Ducie Islands), and Gygis alba monte, found throughout the Indian Ocean (AOU, 1998; Niethammer & Patrick, 1998).
In the Hawaiian Archipelago, white terns breed throughout the Northwestern Hawaiian Islands (NWHI), but breed only on Oahu within the main Hawaiian Islands (Niethammer & Patrick, 1998). A single pair of white terns was first observed breeding on Oahu in 1961 (Ord, 1961). However, a photograph dating from 1953 in the Bishop Museum (Honolulu, Hawaii) of Grenville Hatch showing a young, hand-raised white tern found on Oahu may suggest an earlier breeding date. The population has steadily increased and was comprised of at least 694 adults and 221 nests in 2003 (VanderWerf, 2003). The origin(s) of colonizers remains uncertain. It has been suggested that sailors brought several chicks from the Marquesas Islands in 1960 (R. Pyle, pers. comm.), whereas it has also been claimed that the birds may have colonized Oahu naturally from nearby colonies in the NWHI (E. N. Flint, pers. comm.). Additionally, Olson (2005) published morphological measurements (wing chord, longest and shortest retrix) of a 1924 museum specimen that was found on the northern point of Oahu. Based on these measurements, this likely storm-driven vagrant was the first record of G. a. microrhyncha in the Hawaiian Archipelago.
In the present study, we used criteria proposed by Avise (2000); reciprocal monophyly), and Patten et al. (2002; 75% rule), with both genetic and morphological data, to evaluate the support for G. a. microrhyncha as a separate species and current subspecies designations in the Pacific Ocean. Additionally, we used the genetic data to identify the source population(s) of the recently established population of white terns on the island of Oahu and to examine the partitioning of genetic variance among the four subspecific populations and the Oahu group, treating all individuals as if they were sampled from a single species in this analysis.
MATERIAL AND METHODS
Sample collection for genetic analysis
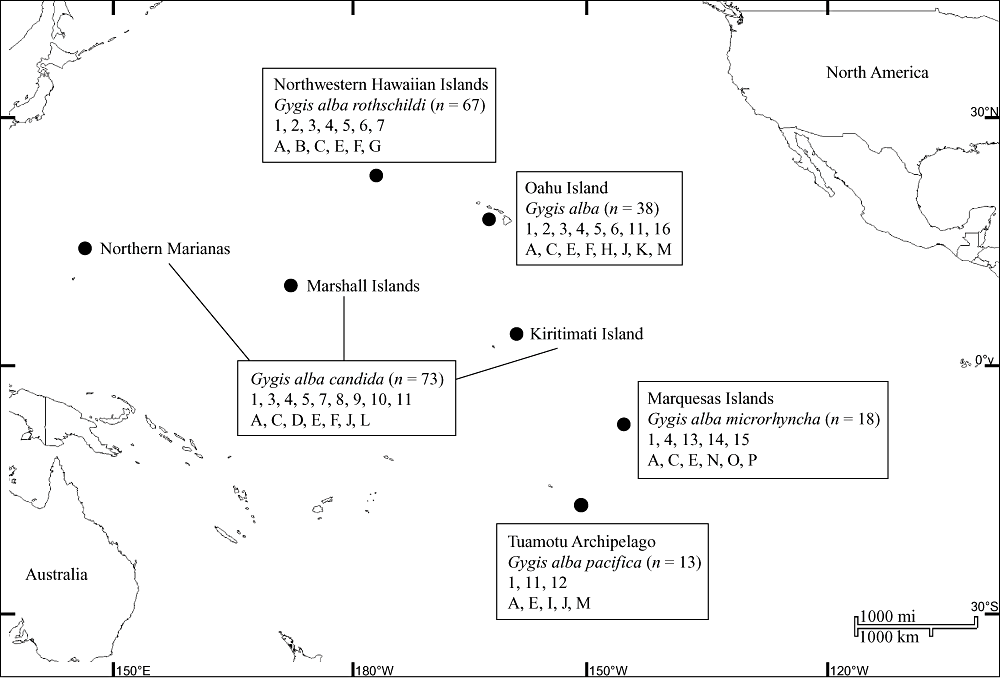
Tissue samples were collected from 209 white terns, representing island groups of the North and South Pacific (museum voucher numbers, locality and sample size are provided in the Appendix; Fig. 1). Because of the recent colonization of Oahu by unknown individuals, this population was treated as distinct. Toes and feathers were collected from museum specimens with forceps cleaned with 10% bleach between sampling. Samples from the field included molted primary feathers and body tissue from dead specimens and were collected and placed in pre-labelled bags. All samples were stored at −80 °C. Museum specimens were initially analysed separately from contemporary specimens, although no significant differences were detected and, as a result, museum and contemporary specimens were combined for all genetic analyses. Furthermore, the bird specimens used for genetic analysis were not used in the morphological analysis.
DNA isolation and sequencing
Genomic DNA (gDNA) was isolated from molted feathers or body tissue from freshly dead and museum specimens with the Qiagen Dneasy Tissue Kit (Qiagen, Inc.) in accordance with the manufacturer's instructions. Primers from Bridge, Jones & Baker (2005) were used to amplify a 502 bp portion of cytochrome b (Cyt b) and 516 bp of NADH dehydrogenase 2 gene (ND2). Because of the fragmentary nature of gDNA from museum samples, additional primers were designed (Table 1) from sequences downloaded from the National Center for Biotechnology Information (NCBI) database (accession numbers AY631290 and AY631362; Bridge et al., 2005).
| Primer sequences (5′- to 3′) | |
|---|---|
| Cytochrome b: Ta = 58 °C | CGG ACG AGG ATT CTA CTA TGGCGG GTT TGA TAT GTG GAG GT |
| CAC TTC CTC CTC CCC TTCGTA GGG GGC TTA GGA ACA GG | |
| NADH2: Ta = 50 °C | ATC GAG GCC GCA ATT AAA TAGGG GTT TTT GTT CAT GAG GTT |
| CAG CTG CAA TTG GCA TAA AAACG CGA GAC GAA GGT AGA AG |
DNA amplifications were performed via the polymerase chain reaction in 25-µL volumes that included 1–2 µL of gDNA, 10 × MangoTaq reaction buffer (Bioline, MA; 16 mm NH4, 67 mm Tris-HCl), 2 mm MgCl2, 0.4 mm of each dNTP, 3 µg of bovine serum albumin, 5 U of MangoTaq DNA polymerase (Bioline) and 0.3 mm of each primer. All reactions sets included negative controls containing sterile water in place of gDNA. Cycling parameters consisted of denaturation at 94 °C for 10 min followed by 35 cycles of 94 °C for 30 s, 50 °C (ND2) and 58 °C (Cyt b) for 40 s and 72 °C for 1 min, with a final extension of 10 min at 72 °C. Single-band products were verified with agarose gel electrophoresis and amplified products were purified using the Exosapit enzyme kit (USB Corp.) in accordance with the manufacturer's instructions. Cycle sequencing was carried out in both directions using the ABI BigDye terminator kit (Perkin-Elmer Applied Biosystems, Inc.) followed by electrophoresis on an ABI 3730XL at the University of Hawaii's Center for Advanced Studies in Genomics, Proteomics and Bioinformatics. All individuals were sequenced at least twice and locus identity was verified via BLAST searches (Altschul et al., 1990) against the NCBI Genbank sequence database. Sequences were edited and unambiguously aligned using the program SEQUENCHER, version 4.6 (Gene Codes Corporation) and amino acid sequences as a reference. All sequences have been deposited in the NCBI GenBank (Accession numbers EU516389 to EU516525 for Cyt b and EU516526 to EU516662 for ND2).
Sequence analysis
Phylogenetic patterns in the sequence data were examined in PAUP*, version 4.0b10 (Swofford, 2003) using a maximum parsimony (MP) and maximum likelihood (ML) analysis. For MP, all characters were unordered and of equal weight. A heuristic search was performed with the tree-bisection–reconnection (TBR) branch-swapping algorithm with 100 random sequence addition replicates and 1000 bootstrap replicates. The ML model and its parameters were estimated using MODELTEST, version 3.04 (Posada & Crandall, 1998) and a heuristic search option was performed with TBR branch-swapping with 1000 bootstrap replicates. DNASP, version 4.10.9 (Rozas et al., 2003) was used for calculations of diversity measures: haplotype diversity (h), nucleotide diversity (average number of nucleotide differences per site within groups; π), and sequence divergence (the average number of nucleotide differences per site between groups; dxy; Nei (1987)
The presence of extant ancestral haplotypes within populations violates the assumptions of bifurcating trees (Posada and Crandall 2001); therefore, we constructed statistical parsimony networks with a 95% confidence limit using TCS, version 1.21 (Clement, Posada & Crandall, 2000) to assess the genealogical relationships among haplotypes. Analysis of molecular variance (AMOVA) (Excoffier, Smouse & Quattro, 1992) was used to investigate the proportion of total genetic variation within and among populations (ΦST) using the software ARLEQUIN (Schneider, Roessli & Excoffier, 2000). ARLEQUIN was also used to estimate pairwise genetic distances between all population pairs and statistical significance was ascertained by conducting 10,000 permutations.
Morphometrics
Morphological data, including the mean, range, and sample sizes of culmen length and depth, longest and shortest retrix, and wing chord were obtained from Holyoak & Thibault (1976; Table 2). No morphological data were obtained from specimens used in the genetic analysis. Populations were divided into the same four groupings (excluding Oahu) as in the molecular analysis, G. a. candida (Marianas, Wake, Phoenix, Clipperton, Cocos, Christmas, Fanning, and Washington); G. a. rothschildi (NWHI); G. a. pacifica (Caroline, Palau, Bismarck, Solomon, Samoa, Fiji, Niue, Tonga, Danger, Austral, Society, Tuamotu, Rapa, Easter, Henderson, and Ducie); and G. a. microrhyncha (Marquesas).
| Gygis alba candida (N = 72) | Gygis alba rothschildi (N = 47) | Gygis alba pacifica (N = 394) | Gygis alba microrhyncha (N = 99) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | SE | Mean | Range | SE | Mean | Range | SE | Mean | Range | SE | |
| Culmen* | 38.1 | 36–42 | 0.2 | 37.0 | 33–40 | 0.2 | 41.8 | 37–46 | 0.1 | 36.0 | 31–39 | 0.2 |
| Culmen depth† | 8.7 | 7.6–9.8 | 0.1 | 8.5 | 8.0–9.2 | 0.04 | 9.2 | 7.9–10.9 | 0.03 | 6.7 | 5.5–8.0 | 0.05 |
| Longest retrix† | 109.2 | 97–120 | 0.6 | 109.0 | 101–118 | 0.6 | 114.9 | 103–145 | 0.4 | 79.0 | 73–95 | 0.4 |
| Shortest rectrix† | 68.9 | 61–77 | 0.4 | 68.0 | 64–74 | 0.3 | 72.5 | 62–83 | 0.2 | 64.0 | 60–75 | 0.3 |
| Wing chord† | 237.2 | 227–246 | 0.5 | 235.0 | 219–246 | 0.9 | 246.7 | 236–258 | 0.2 | 218.0 | 212–235 | 0.5 |
- * Indicates significant difference among all groups except between G. a. microrhyncha and G. a. rothschildi, G. a. candida, and G. a. rothschildi, P < 0.05.
- † Indicates significant difference among all groups except between G. a. candida and G. a. rothschildi, P < 0.05.
- Linear measurements are in millimetres. Combined geographic data are shown sensuHolyoak & Thibault (1976).
- G. a. candida = Marianas, Wake, Phoenix, Clipperton, Cocos, Christmas, Fanning, and Washington; G. a. rothschildi = Hawaii; G. a. pacifica = Caroline, Palau, Bismarck, Solomon, Samoa, Fiji, Niue, Tonga, Danger, Austral, Society, Tuamotu, Rapa, Easter, Henderson, and Ducie; G. a. microrhyncha = Marquesas. Each character compared among subspecies with a one-way analysis of variance followed by Tukey's test.
Standard errors of culmen length and depth, longest and shortest retrix, and wing chord were calculated (Sokal & Rohlf, 1981) using the mean, range, and sample sizes sensuHolyoak & Thibault (1976). Each character was then compared among subspecies with a one-way analysis of variance (ANOVA), followed by post-hoc tests (Tukey's test for unequal sample sizes) and the diagnosability index (Dij) (Patten et al., 2002).
RESULTS
Interspecific sequence diversity
The maximum likelihood model selected using the Akaike information criterion in MODELTEST, version 3.07 was the Hasegawa–Kishino–Yano model (HKY + I) with gamma distributed rate variation (alpha = equal) and invariant sites (pinvar = 0.9054) for Cyt b, and the transversional model (TVM) with gamma distributed rate variation (alpha = equal) and invariant sites (pinvar = 0) for ND2. Topologies obtained from MP and ML analysis of both genes were identical and resulted in an unresolved polytomy (data not shown). For Cyt b, the mean estimated sequence divergence was 0.34% with the highest divergence between G. a. rothschildi and G. a. pacifica (0.87%) and lowest the between G. a. rothschildi and G. a. candida (0.23%). For ND2, the mean estimated sequence divergence was 0.25%, with the highest between G. a. microrhyncha and G. a. pacifica (0.51%) and the lowest between G. a. rothschildi and G. a. candida (0.18%).
Intraspecific sequence diversity
Sequences from 209 birds resulted in 16 Cyt b haplotypes containing 18 polymorphic sites, 12 of which were parsimoniously informative. Sixteen ND2 haplotypes resulted in 17 polymorphic sites, 11 of which were parsimoniously informative (Table 3). The haplotype networks (Fig. 2) revealed multiple shared haplotypes among subspecies and a primary haplotype shared by all subspecies. The most common Cyt b haplotype (haplotype 1) was shared by 130 of 209 (62%) individuals and 15 haplotypes were identified from the remaining 81 individuals. For ND2, two haplotypes were shared among all putative subspecies with the most common haplotype, haplotype A, present in 128 of 209 (61% of all samples) individuals, and in haplotype E, 28 of 209 (13% of all samples) individuals; 14 haplotypes were identified from the remaining 53 individuals.
| Cytochrome b | n | π | h | Haplotype | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| Gygis alba candida | 73 | 0.0021 | 0.448 ± 0.07 | 54 | 0 | 4 | 3 | 2 | 0 | 4 | 1 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 0 |
| Gygis alba rothschildi | 67 | 0.0020 | 0.654 ± 0.06 | 37 | 12 | 5 | 6 | 4 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gygis alba pacifica | 13 | 0.0072 | 0.692 ± 0.08 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 4 | 0 | 0 | 0 | 0 |
| Gygis alba microrhyncha | 18 | 0.0043 | 0.549 ± 0.13 | 12 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 1 | 0 |
| Oahu Island | 38 | 0.0030 | 0.669 ± 0.08 | 21 | 4 | 2 | 6 | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| ND2 | n | π | h | Haplotype | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | ||||
| Gygis alba candida | 73 | 0.0017 | 0.443 ± 0.07 | 54 | 0 | 7 | 1 | 4 | 2 | 0 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 0 | 0 |
| Gygis alba rothschildi | 67 | 0.0016 | 0.744 ± 0.08 | 37 | 10 | 3 | 0 | 14 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gygis alba pacifica | 13 | 0.0037 | 0.549 ± 0.13 | 5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gygis alba microrhyncha | 18 | 0.0043 | 0.758 ± 0.08 | 8 | 0 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 1 |
| Oahu Island | 38 | 0.0025 | 0.599 ± 0.04 | 24 | 0 | 1 | 0 | 8 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
- The sample size (N), nucleotide diversity (π), and haplotype diversity (h) are shown.
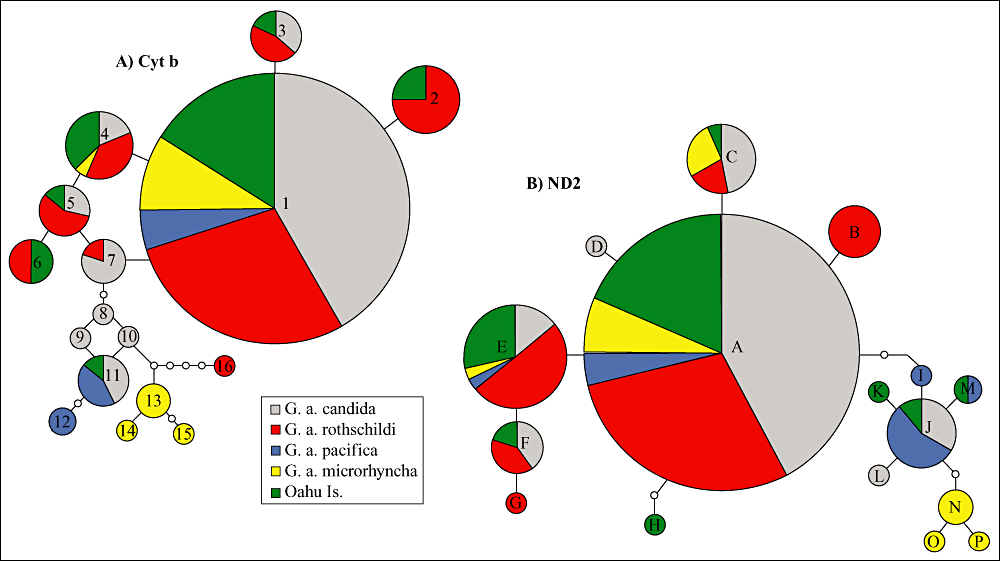
Statistical parsimony networks (95% parsimony limit) constructed using cytochrome b (A) and ND2 sequences (B) from the four subspecies of Gygis alba in the Pacific and an undetermined G. alba subspecies from Oahu. Haplotype designations correspond to those shown in Table 3 and Fig. 1. The diameter of the circles is proportional to the number of individuals represented by that haplotype. Each branch presents a single base pair mutation and white circles are hypothetical/unsampled haplotypes.
Gygis alba candida (N = 73) sequences provided nine haplotypes (three private) for Cyt b and seven haplotypes (two private) for ND2. Gygis alba rothschildi (N = 67) sequences provided eight haplotypes (one private) for Cyt b and six haplotypes (two private) for ND2. Gygis alba pacifica (N = 13) sequences provided three haplotypes (one private) for Cyt b and five haplotypes (one private) for ND2. Gygis alba micrhorhyncha (N = 18) sequences yielded five haplotypes (three private) for Cyt b and six haplotypes (three private) for ND2. Oahu (N = 38) sequences yielded seven haplotypes (none unique) for Cyt b and eight haplotypes (two private) for ND2.
Results from the AMOVA indicated significant genetic structure at the subspecies level for both Cyt b and ND2 (ΦST, Cytb = 0.167, P < 0.001; ΦST, ND2 = 0.146, P < 0.001; Table 4). Most pairwise comparisons between subspecies (seven out of nine for Cyt b and eight out of nine for ND2) were significantly different at both loci (P < 0.001; Table 5). For both genes, the greatest difference was between G. a. rothschildi and G. a. pacifica (ΦST, Cytb = 0.526, P < 0.01; ΦST, ND2 = 0.448, P < 0.01). The smallest difference for Cyt b is between G. a. rothschildi and Oahu (ΦST = –0.003, P > 0.05) and, for ND2, G. a. candida and Oahu (ΦST = 0.014, P > 0.05). Oahu individuals showed significant differentiation from G. a. pacifica and G. a. microrhyncha and little to no significant differentiation from G. a. candida and G. a. rothschildi. Among the groups, the Oahu population shared the most haplotypes with all other populations, (seven Cyt b and six ND2 haplotypes; Fig. 2, Table 2), which is also reflected in the only nonsignificant ΦST obtained in all pairwise calculations (Table 5).
| Source of variation | d.f. | Variance components | Percentage of variation | |
|---|---|---|---|---|
| Cytochrome b gene | Among populations | 4 | 0.135 Va | 16.67 |
| ΦST = 0.167 (P < 0.001) | Within populations | 204 | 0.675 Vb | 83.33 |
| ND2 gene | Among populations | 4 | 0.096 Va | 14.59 |
| ΦST = 0.146 (P < 0.001) | Within populations | 204 | 0.563 Vb | 85.41 |
- Each analysis of molecular variance uses the five populations shown in Fig. 1.
- d.f., degrees of freedom; Va & Vb are associate covariance components.
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1 Gygis alba candida | – | 0.051** | 0.452** | 0.112** | 0.0219 |
| 2 Gygis alba rothschildi | 0.069** | – | 0.526** | 0.187** | −0.003 |
| 3 Gygis alba pacifica | 0.361** | 0.448** | – | 0.242** | 0.393** |
| 4 Gygis alba microrhyncha | 0.179* | 0.265** | 0.083* | – | 0.099** |
| 5 Oahu Island – unknown | 0.014 | 0.031* | 0.285* | 0.146* | – |
- * P < 0.05,
- ** P < 0.01.
Morphometrics
One-way ANOVA indicated that all morphological characters showed significant variation among subspecies (Fculmen, 3,608 = 495.27; Fculmen depth, 3,608 = 658.99; Flongest retrix, 3,608 = 811.54; Fshortest retrix, 3,608 = 174.76; Fwing chord, 3,608 = 1214.95; all P < 0.000; Table 2). Tukey's test for unequal sample sizes showed that culmen depth, longest and shortest retrix, and wing chord were not significantly different between G. a. candida and G. a. rothschildi. Therefore, these two putative subspecies were merged for Amadon's ‘75% rule’ analysis. For bill length, only G. a. microrhyncha and G. a. pacifica were diagnosable (Dmp = 0.98 and Dpm = 1.07) from each other. Only G. a. microrhyncha was significantly distinguished from all other putative subspecies using bill depth and the longest retrix. Additionally, G. a. microrhyncha was distinguishable from every putative subspecies except G. a. rothschilid using the wing chord. Regardless of significant mean values among subspecies when evaluating the shortest retrix, the index values were all ≤ 0, and therefore not distinguishable under the ‘75% rule’.
DISCUSSION
In the present study, we have provided data on genetic and morphological differences among putative white tern subspecies. Analysis of mitochondrial (mt)DNA variation does not support G. a. microrhyncha as a phylogenetically distinct group from populations occurring in the Pacific at the species level. For each gene, all four subspecies share at least two haplotypes. The most common Cyt b haplotype was shared by more than 46% of individuals from each species and 38% for ND2. Despite the significant population structure recovered from the AMOVA analysis, the corresponding evolutionary partitions at the mtDNA level do not warrant the recognition of distinct subspecies within the Pacific. Analysis of the examined morphological characters examined failed to distinguish any of the putative subspecies with two exceptions. Bill length and longest retrix of G. a. microrhyncha, which were distinct from all other groups and vice versa.
Gygis alba candida is not significantly different genetically or morphologically from G. a. rothschildi to warrant specific or subspecific distinction. This result supports the merging of these two into one subspecies (G. a. candida). The neighbouring South Pacific subspecies (G. a. pacifica and G. a. microrhyncha) do not exhibit reciprocal monophyly at the mtDNA level but exhibit significant morphological variation. The morphological differences among G. a. pacifica, G. a. microrhyncha, and the North Pacific subspecies may be a reflection of adaptive differences, particularly between the neighbouring South Pacific groups, G. a. pacifica and G. a. microrhyncha. The difference in bill size between G. a. pacifica and G. a. microrhyncha could be an adaptation that reduces potential competition for food in the Marquesan waters. On the small islands with limited nest sites, the larger birds are perhaps better suited for the defense of nesting sites (Holyoak & Thibault, 1976). Gygis alba pacifica and G. a. microrhyncha should be considered as separate management units from each other and from North Pacific groups, but not as distinct species or subspecies. The lack of mtDNA structuring and significant morphological geographic variation is similar to that of the sooty tern, Onychoprion fuscata (Peck & Congdon, 2004), and subspecific morphological differences are most likely the result of processes such as phenotypic plasticity, selection, and natal philopatry rather than geographic barriers to gene flow. However, the observed mtDNA partitioning that we found in the white tern suggests insufficient female natal philopatry to generate morphological variation.
The Oahu population represents a genetic mixture of individuals from currently recognized subspecies throughout the Pacific Ocean. The individuals on Oahu share more haplotypes with other putative subspecies than any other group (i.e. seven Cyt b and six ND2 haplotypes). This supports the conclusion that the Oahu population is composed of individuals from multiple source populations. Because of its high genetic diversity, the Oahu population should be the focus of management efforts to maintain the most genetic variability within this species. Measurements of morphological characters should be obtained from the Oahu population to determine whether the high genetic diversity is also reflected in the morphology. Measurements of morphological characters of individuals from the Oahu population are needed for comparisons with other groups to help clarify patterns of morphological differentiation among populations.
There is growing concern over globally declining seabird populations (Gilman, Brothers & Kobayashi, 2005; Wolf et al., 2006; Baker et al., 2007; Garthe & Flore, 2007, Millus, Stapp & Martin, 2007). Developing management strategies to reduce the extinction risk for any species benefits from assessments of population structure, genetic diversity, breeding range, taxonomic status, and other parameters prior to population declines. The white tern is listed as a species of ‘least concern’ by the International Union for Conservation of Nature and Natural Resources (IUCN). However, global population trends in this species have yet to be quantified (IUCN, 2007) and white tern populations are not immune to the impacts of climate change, over-fishing, habitat destruction, disease, and invasive species, especially small mammalian predators. It is important to understand the population connectivity of the white tern to provide potential benchmarks for management needs.
Ongoing work with microsatellites and single copy nuclear DNA will provide insights into the fine scale population structure and taxonomic status of this species (e.g. subspecies, evolutionary significant and management units; Crandall et al., 2000; Fraser & Bernatchez, 2001; Moritz, 2002). Furthermore, including samples from subspecies in the Indian and Atlantic Ocean will provide a more comprehensive analysis of genetic variation within the entire white tern complex. The addition of nuclear markers and increased sampling will inform the conservation efforts to preserve the genetic diversity and evolutionary potential of this seabird. Further examination of this tropical tern could lead to the identification of historical and evolutionary processes that mediate genetic and morphological variation in seabirds.
ACKNOWLEDGEMENTS
This work was supported by NSF (Ecology, Evolution, and Conservation Biology program of the University of Hawaii; NSF grant #05385500 and DGE02-32016 to K. Y. Kaneshiro), and the Hawaii Audubon Society. We are very grateful to Eric VanderWerf, Phil Bruner, Corinne Chun Fujimoto, the US Fish and Wildlife Services and Hawaii Department of Land and Natural Resources for the collection of samples. We also thank the following individuals for their input, support, and assistance: BC-PD, Pat Aldrich, Erin Baumgartner, Brian Bowen, Matt Craig, Dave Duffy, Mike Dunford, Anu Gupta, Ken Hayes, Matt Iaachei, Matt Knope, Wing Hin Leung, Catherine Lippe, Marty Meyer, Cliff Morden, Sheldon Plentovich, Heather Spalding, Laurie Strommer, Andy Taylor, Alice Tran, Kim Tice, Eric VanderWerf, Les Watling, and Lindsay Young. This work was conducted under all necessary institutional, state, and federal wildlife permits.
Appendix
Voucher numbers for specimens used in the present study
| Subspecies | Museum | Catalogue numbers | Locality |
|---|---|---|---|
| Gygis alba candida | Brigham-Young University, Hawaii | 4088 | Saipan, CNMI |
| Gygis alba candida | Brigham-Young University, Hawaii | 4099 | Saipan, CNMI |
| Oahu Island – unknown | Brigham-Young University, Hawaii | 2575 | Sand Island, Oahu Island |
| Oahu Island – unknown | Brigham-Young University, Hawaii | 2611 | Sand Island, Oahu Island |
| Oahu Island – unknown | Brigham-Young University, Hawaii | 2613 | Sand Island, Oahu Island |
| Gygis alba candida | The Field Museum | 346132 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346133 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346134 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346135 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346136 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346137 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346138 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346139 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346140 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346141 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346142 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346143 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346144 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346145 | Enewetak, Marshall Islands |
| Gygis alba candida | The Field Museum | 346146 | Enewetak, Marshall Islands |
| Gygis alba microrhyncha | The Field Museum | 71024 | Hivaoa Is., Marquesas |
| Gygis alba microrhyncha | The Field Museum | 71025 | Hivaoa Is., Marquesas |
| Gygis alba microrhyncha | The Field Museum | 71026 | Hivaoa Is., Marquesas. |
| Gygis alba microrhyncha | The Field Museum | 71027 | Hivaoa Is., Marquesas |
| Gygis alba microrhyncha | The Field Museum | 71028 | Hivaoa Is., Marquesas |
| Gygis alba microrhyncha | The Field Museum | 71029 | Hivaoa Is., Marquesas |
| Gygis alba rothschildi | The Field Museum | 188923 | Laysan Is., NWHI |
| Gygis alba rothschildi | The Field Museum | 188924 | Laysan Is., NWHI |




