Effective long-term control of cardiac events with β-blockers in a family with a common LQT1 mutation
Abstract
The congenital long QT syndrome (LQTS) is characterized by a prolonged QT interval on the surface electrocardiogram and an increased risk of recurrent syncope and sudden cardiac death. Mutations in seven genes have been identified as the molecular basis of LQTS. β-blockers are the treatment of choice to reduce cardiac symptoms. However, long-term follow-up of genotyped families with LQTS has been rarely reported. We have clinically followed a four-generation family with LQTS being treated with β-blocker therapy over a period of 23 years. Seven family members were carriers of two amino acid alterations in cis (V254M-V417M) in the cardiac potassium channel gene KCNQ1. Voltage-clamp recordings of mutant KCNQ1 protein in Xenopus oocytes showed that only the V254M mutation reduced the IKs current and that the effect of the V417M variant was negligible. The family exhibited the complete clinical spectrum of the disease, from asymptomatic patients to victims of sudden death before β-blocker therapy. There was no significant reduction in QTc (556 ± 40 ms½ before therapy, 494 ± 20 ms½ during 17 years of treatment; n = 5 individuals). Of nine family members, one female died suddenly before treatment, three females of the second generation were asymptomatic, and four individuals of the third and fourth generation were symptomatic. All mutation carriers were treated with β-blockers and remained asymptomatic for a follow-up up to 23 years. Long-term follow-up of a LQT1 family with a common mutation (V254M) being on β-blocker therapy was effective and safe. This study underscores the importance of long-term follow-up in families with specific LQT mutations to provide valuable information for clinicians for an appropriate antiarrhythmic treatment.
Congenital long QT syndrome (LQTS) is an autosomal dominant disorder of abnormal cardiac excitability, which is characterized by a prolongation of the QT interval on the surface electrocardiogram (ECG) and by syncopal episodes and death due to ventricular tachyarrhythmias, typically of the torsades de pointes type (1). The disorder is caused by mutations in seven genes so far, of which six are coding for ion channel genes (2–7) and one, the Ankyrin B gene, for an membrane anchor protein that interacts with several membrane proteins including cardiac ion channels (8). LQTS has allelic heterogeneity with more than 200 different mutations identified in these genes. The clinical manifestation in mutation carriers may be largely heterogeneous within the same family or between patients with the same mutation and the electrophysiological in vitro phenotype of a specific mutation does not conclusively predict the clinical phenotype (9–11). Thus, additional factors such as gender, heart disease, or underlying arrhythmia play a modifying role in the phenotypic expression. Large-scale observations in genotyped individuals may be of importance to address age- and sex-dependent effects of disease manifestations and therapeutic response to β-receptor blocking agents, the current treatment of choice in LQTS.
Subjects and methods
Family population
A family of nine members with LQTS (Fig. 1) has been followed because of sudden cardiac death of individual III-3. Two of them [I-2 (QTc: 530ms1/2) and III-3 (QTc: 590 ms1/2)] died before any therapy (age 95 years and age 21 years, respectively). Five (II-2, III-1, III-2, III-5, and IV-1) of the remaining seven were followed up to 23 years; the mean follow-up of all was 17.9 ± 8.8 years. All patients underwent routine physical examination, resting 12-lead ECG including determination of baseline QTc according to Bazett's equation (12), heart rate, inspection of the T-wave morphology, exercise testing with QTc measurements, and Holter monitoring (not shown). A detailed history of LQTS-related events (syncope, seizures, palpitations, and cardiac arrest) was obtained. Previously published diagnostic criteria were applied to diagnose LQTS (13). For DNA diagnosis, an informed consent in accordance with the Institutional Review Board (IRB) guidelines of the University of Münster was obtained from all family members before participation in this study.
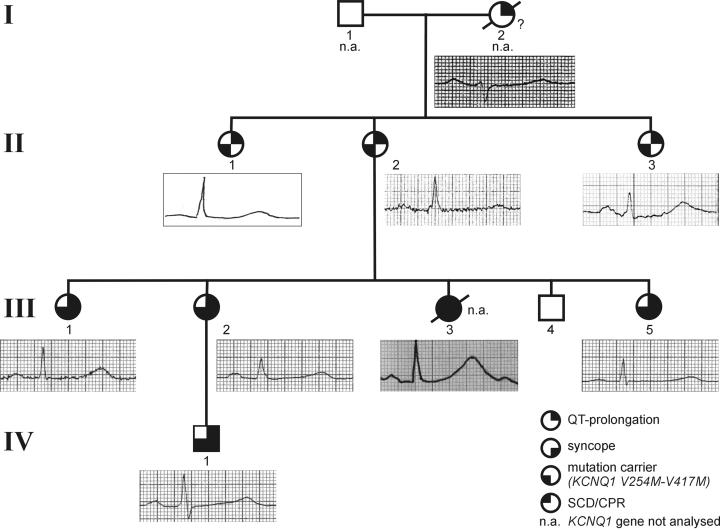
Pedigree of the family with the V254M-V417M mutations. Solid and partially solid circles (females) and squares (males) indicate symptomatic and asymptomatic carriers, respectively. Empty symbols represent non-carriers. Electrocardiogram recordings of the patients are taken from lead II.
Genetic analysis
DNA was isolated from venous ethylenediaminetetraacetic acid blood (14) of all available family members. Standard direct sequencing was used as previously described (15). Primers reported by Wang et al. (2), Splawski et al. (16), and Abbott et al. (6) were used to amplify the entire coding region of five LQTS genes (LQT1–3, LQT5, and LQT6); LQT4 and LQT7 have not been screened in this study. Mutagenically separated polymerase chain reaction (MS-PCR) was used as an independent method for mutation detection (17).
Generation of expression vectors and heterologous expression of wild-type and mutant KCNQ1
A fragment encoding the complete open reading frames of KvLQT1 or KCNE1 was amplified by PCR from a human kidney cDNA library or human genomic DNA, respectively, and subcloned into pGEM-HE vector. KCNQ1 mutants were introduced into KvLQT1 vector (QuickChange mutagenesis kit) (Stratagene, La Jolla, CA). In vitro transcription and heterologous expression in Xenopus oocytes was done as described previously (18). Stage IV and V oocytes were injected with 50 nl of cRNA mixtures (KCNQ1 and KCNE1 cRNA in a 1 : 1 molecular ratio). Concentration of wild-type and mutant-injected KCNQ1 was 0.1 µg/µl (2.5 ng/oocyte) and 0.03 µg/µl (0.75 ng/oocyte) for KCNE1. The total amount of cRNA was kept constant in coexpression experiments.
Electrophysiological recordings and data analysis in Xenopus oocytes
Current measurements were performed as described previously (18). Macroscopic currents were measured after applying a 4-s voltage pulse to +40 mV and normalized with respect to the wildtype control. The holding potential was held at−80 mV. Isochronal steady-state activation curves were derived from tail currents at −50 mV after stepping to test potentials ranging from−80 to +80 mV. Half-maximal activation potential (V1/2) and slope (k) were determined by fitting the data with a Boltzmann function G/Gmax = maximum amplitude/{1 + exp[(V – V1/2)/k]}. Data were normalized with respect to the saturation of the fit. For calculations, fitting and presentation of data, MS-EXCEL (Microsoft, Seattle, WA) and Igor Pro (WaveMetrics Inc., Lake Oswego, OR) were used. Statistical analysis was done by one-way ANOVA and a following Bonferroni t-test (SigmaStat) (SPSS Inc., Chicago, IL). Data are displayed as mean ± SEM.
Results
Clinical evaluation of the family
The family first came to medical attention in 1980 because of sudden death of a 21-year-old woman (III-3, Fig. 1). She had suffered from recurrent episodes of loss of consciousness with loss of motor control, pallor, and cyanosis since the age of six years. Initially, she was treated for a myoclonic epilepsy with hexamidine di-isethionate (Mylepsin®; 15 mg/kg) and carbamazepine (15 mg/kg). However, syncope frequently reoccurred. Because of concomitant depression, therapy with a tricyclic antidepressant (amitriptyline) was started four days before death. On the day of an appointment with her neurologist, she collapsed and was resuscitated because of ventricular fibrillation. After external electrical defibrillation, ECGs showed a marked QT interval prolongation of over 600 ms1/2 with giant T-waves (Fig. 1). The patient died 12 days later due to severe hypoxic cerebral damage.
Subsequently, eight of her family members of four generations were examined for LQTS. In seven (six female and one male), a QT interval prolongation was present (QTc 553 ± 42 ms½, n = 7). Four of the seven LQT patients (III-1, III-2, III-5, and IV-1) were also symptomatic with recurrent syncope. The three sisters of III-3 had been treated for ‘epilepsy’ since early childhood (age of onset: 6.7 ± 0.6 years) before LQTS (age at diagnose: 20.3 ± 6.4 years) was diagnosed (Table 1). All symptomatic family members belong to the third and fourth generations (Fig. 1), whereas members of the second generation (median age: 68.3 ± 2.5 years) and I-2 (died at age 95) were asymptomatic. All episodes of syncope occurred in early childhood and were triggered by physical activity.
| Patient, gender | Current age in 2003 (years) | QTcBazett (ms1/2) | Age (years) of 1st sycope | Symptoms before β-blocker tx | Trigger for symptoms | Years of β-blocker tx | Events during therapy |
|---|---|---|---|---|---|---|---|
| I-2, female | aged 95 years | 530 | – | – | – | – | – |
| II-1, female | 71 | 520 | – | – | – | 23 | Nonec |
| II-2, female | 68 | 560 | – | Dizziness | Excitement, fear | 23 | Nonea |
| II-3, female | 66 | 510 | – | – | – | 23 | Noned |
| III-1, female | 48 | 600 | 7 | 3 syncope | Gymnastics, footrace, emotional stress | 23 | Nonea |
| III-2, female | 46 | 560 | 7 | ≈ 15 syncope | Swimming | 23 | Nonea |
| III-3, female | aged 21 years | 590 | 6 | ≈ 45 syncope | Tricyclic antidepressiva? | – | – |
| III-5, female | 36 | 600 | 6 | ≈ 6 syncope | Swimming | 23 | Nonea |
| IV-1, male | 24 | 490 | 6 | – | Swimming, running, climbing | 23 | 3 × syncopebNonea |
- a 50 mg atenolol qd.
- b 12.5, 25, and 37.5 mg qd.
- c 200 mg celiprolol qd.
- d 10 mg bisoprolol qd.
- qd = per day
After the diagnosis of LQTS, all patients (n = 7) received β-blocker therapy. Over a period of 23 years, all six female patients had no cardiac events. Particularly, the highly symptomatic sisters became asymptomatic. The male patient IV-1 was symptomatic under a low-dose atenolol. Recurrend syncope occurred at age 6 (0.6 mg/kg), at age 8 (0.9 mg/kg), and at age 10 (0.8 mg/kg). Under 1.2 mg/kg, he too became asymptomatic (follow-up of 14 years).
ECG characteristics of the family
The QTc of all affected family members was prolonged (Fig. 2, Table 1). Heart rate, PQ, and QRS duration were within normal limits. The T-wave morphology showed a late onset of normal shape without T-U waves or other abnormalities. This pattern most nearly matched to the ‘late-onset normal-appearing T-wave pattern’ previously described for LQT1 carriers (19). In patient III-3, giant T-waves were observed right after resuscitation. The QT/QTc dispersion measured on the 12-lead ECG was not increased in the patients despite symptoms (not shown). In patient IV-1, post-extrasystolic ‘T-wave hump’ augmentation was recorded at age 14 (20).

QTc measurements in five mutation carriers (II-2, III-1, III-2, III-5, and IV-1) showed a small, statistically not significant decrease in QTc from 556 ± 40 ms1/2 (w/o = before β-blocker therapy) to 494 ± 20 ms1/2 during 17 years of treatment (n.s. = not significant).
During long-term follow-up therapy under 50-mg atenolol, the QTc in resting ECG decreased slightly without a significant reduction in all symptomatic patients (Fig. 2). However, QTc of patient IV-1 was less affected by β-blocker therapy during the first 10 years of life (data not shown).
QTc measurements before, during, and after exercise testing before and during β-blocker therapy over 10 years are shown in Fig. 3a–f. In all symptomatic mutation carriers (III-1, III-2, III-5, and IV-1), a rapid increase in QTc was observed during recovery phase. In contrast, this was not seen in the healthy control individual (III-4) and the asymptomatic mutation carrier (II-2).
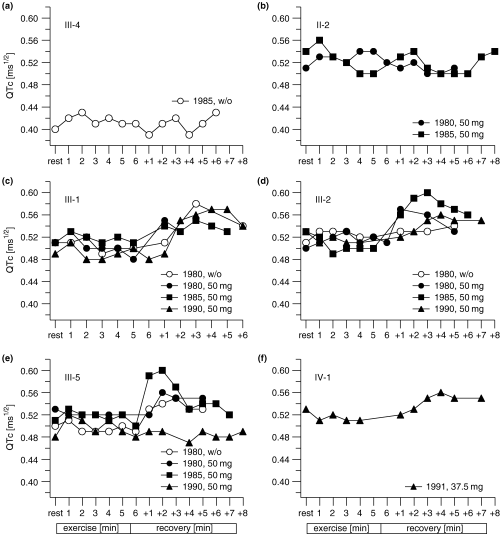
(a–f) QTc measurements during exercise electrocardiogram (0, rest; 1–6 min, exercise; +1–+8 min, recovery) before (w/o) and during β-blocker therapy over 10 years. Symptomatic LQTS (III-1, III-2, III-5, and IV-1) showed an abnormal QT cycle-length relationship, with failure of the QT to shorten normally with increasing heart rate; leading to an increase in QTc just after exercise. However, the QTc in the wild-type carrier (III-4, Fig. 1) and the asymptomatic mutation carrier (II-2) remain relatively constant during the recovery phase.
Genetic analysis
We determined the genotype of the index patient's nuclear family that consists of four symptomatic and four asymptomatic family members. Genetic analysis of all LQT genes except the LQT4 and LQT7 genes identified two amino acid alterations in the KCNQ1 gene (Fig. 4a): a heterozygous amino acid exchange from valine to methionine (V254M) and the same heterozygous amino acid exchange in the C-terminal region of KCNQ1 (V417M). Genetic analysis of the other LQTS genes demonstrated that the variants identified were the only ones present in the probands. For further mutation screening, we used allele-specific PCR techniques (Fig. 4b) and identified both variants in seven LQTS patients. In order to exclude a possible polymorphism, we investigated 100 unrelated healthy individuals in whom the mutations were not detected. Because both variants were found transmitted over three generations, we assumed cis locations. Alignment of both mutations with KCNQ1 cDNA sequences of different species showed that only the V254 residue is highly conserved during evolution; the mutant M417 residue was found to be present in some but not all species (mouse, rat; Fig. 4c).

(a) Sequence analysis of exons 5 and 9 of KCNQ1 gene in family members with LQTS. The arrow indicates a G–A transversion at nucleotides 760 and 1249 that leads to an amino acid exchange from valine (GTG) to methionine (ATG) in codons 254 and 417. (b) Allele-specific polymerase chain reaction for the detection of the V254M and the V417M mutations in six family members. For the identified V254M mutation, primer 5′-GCCCCTACCCTAACCCGGGCA-ACGCACCTGGCGGTGGATGAAGACCTT-3′ detected the mutant 254M allele (209 bp fragment) and primer 5′-CAGCCACCTGGCGGTGGATGAAGACGAC-3′ detected wild-type allele (189 bp fragment). The opposite strand primer was 5′-GCCCATGCCATCGGCCAGCCCTAG-3′. For the identified V417M mutation, primer 5′-CTGCTAGCAAGAAGGCCCTGGCCGGGTGGCAGGTGGGCTACTCACCTT-3′ detected the mutant allele 417M (228 bp fragment) and primer 5′-CCGTGGTGGCAGGTGGGCTACTCACGAC-3′ detected the wild-type allele (208 bp fragment). The opposite strand primer was 5′-CTCTGAGGTCCCAGACCCTGCCACCC-3′. Heterozygous individuals show for the 254 mutation the wild-type (lower band, 189 bp) and the mutant allele (upper band, 209 bp) and for the V417M mutation the wild-type (lower band, 208 bp) and the mutant allele (upper band, 228 bp). Homozygous individuals have only one of the bands (189 bp for V254V and 208 bp for V417V). The gel was composed of 3% NuSieve agarose (FMC, Rockland, ME) and 1% agarose (Biozym, Oldendorf, Germany). (c) Amino acid sequence alignments of the interdomain S4-S5 (V254M) and of the C-terminal part (V417M) of the KvLQT1 α-subunit and orthologous potassium channels are shown in the lower panels. Residue 254 is highly conserved between species, in contrast to residue 417 where the M4177 allele was found to be present in mouse and rat.
Functional characterization of V254M and V417M mutants
Wild-type KvLQT1 channels coexpressed with the regulatory subunit MinK (KvLQT1 + MinK, Fig. 5a,b) displayed typical voltage-dependent, slowly activating, non-saturating IKs-type currents. Expression of the double mutant (V417M-V254M) in presence of Mink resulted in a nearly complete loss of function. This effect was most likely related to the V254M mutant, because coexpression with MinK mediated only a small residual current, whereas currents mediated by V417M + MinK did not show any reduction of IKs amplitude (21) (Fig. 5a,b). Also, activation/deactivation channel kinetics were not changed for V417M + MinK (data not shown).
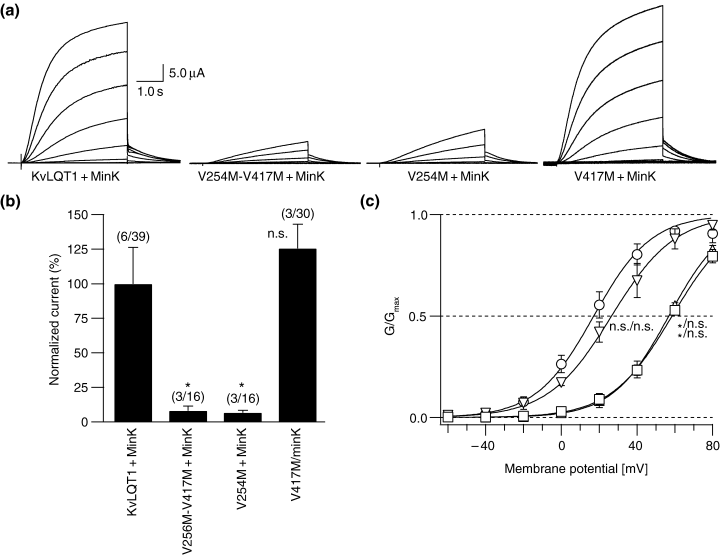
Electrophysiological characterization of the KvLQT1 + MinK complex and its mutants. (a) IKs traces using a protocol starting from a holding potential of −80 mV and increments of 20 mV to finally +80 mV. The tail potential was −50 mV. Leak subtraction was performed using a P/4 protocol provided by the PULSE acquisition software. (b) Normalized mean current amplitudes of the expressed channels from a holding potential of −80 mV. A 4-s test pulse to +40 mV was applied, and the maximum amplitudes were measured using PULSEFIT software and normalized. Values [mean percentage ± SEM] are KvLQT1 + MinK, 100 ± 26.3; V417M-V254M + MinK, 8.2 ± 3.4; V254M + MinK, 6.7 ± 1.7; and V417M + MinK, 125.6 ± 17.5. The holding potential was −80 mV. ‘*’ indicates p < 0.05; n.s. = not significant compared to wild-type values; (n/m) = number of oocyte batches/number of all oocytes measured. (c) Isochronal activation curves derived from tail currents at a potential of −50 mV after stepping to test potentials as described in (a). Midpoint potential of activation and slope were V1/2[mV] = 17.1 ± 40 and slope = 15.3 ± 1.0 (○) for KvLQT1 + MinK, V1/2 = 58.5 ± 0.2 and slope = 16.2 ± 2.0 (□) for V254M-V417M + MinK, V1/2 = 56.8 ± 1.2 and slope = 14.9 ± 2.1 (▵) for V254M + MinK, and V1/2 = 26.5 ± 4.2 and slope = 16.8 ± 2.2 (▿) for V417M + MinK. Curves were drawn using a standard Boltzmann function. Labels indicate statistical data on V1/2 and slope compared to wild-type values ‘*’ indicates p < 0.05, n.s. = not significant. Data are from two to four independent experiments with eight to 21 oocytes.
To assess the heterozygous mutation state found in the patients, wild-type KvLQT1 was coexpressed with non-functional V417M-V254M or V254M subunits (1 : 1 ratio) in the presence of MinK. Figure 6a shows that coexpression of the double mutant eliminated wild-type function in a dominant negative mode, indicating an interaction of the mutant with the wild-type. In addition, coexpression of V254M and wild-type also suppressed the IKs current, suggesting that V254M is the key mutation responsible for the observed electrophysiological alterations.
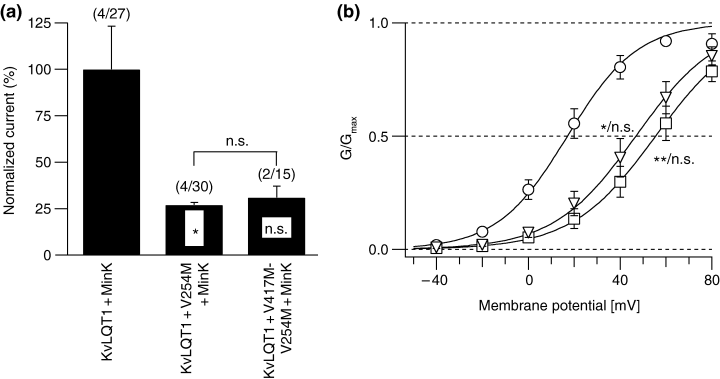
Electrophysiological effect of the V417M-V254M and V254M mutations on IKs current. (a) Normalized mean current amplitude [mean percentage ± SEM] of 1 : 1 coexpression of mutant channels and wild-type KvLQT1 (KvLQT1 + MinK, 100 ± 23.2; KvLQT1 + V254M + MinK, 27.1 ± 1.4; KvLQT1 + V417M-V254M + MinK, 31.1 ± 6.1). ‘*’ indicates p < 0.05; n.s. = not significant compared to wild-type values; (n/m) = number of oocyte batches/number of all oocytes measured. Experimental procedures were as in Fig. 5b. (b) Isochronal activation curves as described in Fig. 5c. Midpoint potential of activation and slope were V1/2[mV] = 17.1 ± 4.0 and slope = 15.3 ± 1.0 (○) for KvLQT1 + MinK, V1/2 = 55.5 ± 6.0 and slope = 18.3 ± 0.4 (□) for KvLQT1 + V254M + MinK, and V1/2 = 47.2 ± 6.2 and slope = 17.9 ± 0.8 (Δ) for KvLQT1 + V254M-V417M + MinK. Experimental and analytical procedures were as described in Fig. 4c. Labels indicate statistical data on V1/2 and slope compared to wild-type values. ‘*’ indicates p < 0.05; ‘**’ indicates p < 0.01; n.s. = not significant. Data are from two to four independent experiments with seven to 21 oocytes.
Voltage dependence of channel activation (Fig. 5c) revealed that V254M induced a marked shift in the midpoint potential comparable to that seen with the double mutation (Fig. 6b), whereas V417M did not show a significant shift. In addition, this suggests that V254M and V417M-V254M subunits may assemble with KvLQT1 wild-type subunits to form heteromeric KvLQT1-type channels with markedly attenuated function.
Discussion
In this report, we describe the long-term follow-up of an LQT1 family effectively treated with β-blockers over a mean period of nearly 18 years. Until now, only a few reports over a two-decade follow-up for genotyped families have been reported (22, 23). Affected family members presented with QT-related symptoms from sudden cardiac death to recurrent syncope before treatment; however, all symptomatic patients became asymptomatic with sufficient β-blocker therapy.
Genetic analysis revealed two amino acid alterations located in cis (V254M-V417M) being absent in the control population but present in all LQT patients of the family. Upon functional analysis, it turned out that the 417M allele had no effect on IKs current and that this presumably represents a rare but neutral variant. In contrast, the 254M allele that frequently has been reported by others (2, 16, 24–26) caused an IKs current reduction through a dominant negative effect that, finally, is responsible for the cardiac phenotype. Thus, the V254M mutation appears to be a common mutation in LQT1 patients, and we considered that the effective treatment with β-blockers was related to this specific mutation.
In the report of Wang et al. (2), the V254M mutation was identified in a large Utah pedigree with more than 70 affected members; data on disease manifestation and treatment effectiveness were not provided by this and other reports (2, 16, 24). In the V254M KCNQ1 family described by Donger et al. the mean QTc of mutation carriers was 504 ± 59 ms½ (n = 6) which is lower than in the family members of the present study (556 ± 40 ms½, n = 6). Cardiac symptoms did not occur before the age of 10 years, in contrast to the family reported here (onset of symptoms: 6.7 ± 0.7 years). However, 57% (4/7) of symptomatic mutation carriers experienced sudden death before 40 years, but data are not comparable because no information on treatment was given in the report. Also, Paulussen and coworkers reported the V254M mutation together with an independent LQT3 variant (A572D) and speculated whether this variant may worsen the cardiac phenotype (26). Because functional data of the A572D were not provided, a combined effect of the two amino acid alterations has to be further proven (26).
In concordance with data from the LQTS registry (27), we did not find a statistically significant reduction in QTc during β-blocker therapy (Fig. 2). Repetitive exercise ECGs over 10 years showed that those with symptomatic LQTS had an increase in QTc that was pronounced during the recovery period and related to a failure of QT shortening with increasing heart rate during exercise (Fig. 3a–f). This is compatible with predictions from experimental models (28) and another clinical report (29) in LQTS. In contrast, the wild-type carrier typically showed a decrease in QTc during exercise that returned to the baseline QTc values in the recovery phase but did not exceed the rest values. Interestingly, this behavior was also found in the asymptomatic LQTS patient, suggesting that the abnormal response to sympathetic activity is more pronounced in symptomatic LQT1 family members.
β-blocker therapy is the current long-term treatment of choice in LQTS, because it has been shown in probands to reduce the number of cardiac events from 0.97 to 0.31 per year during a follow-up of 5 years (27). Especially in LQT1 patients, β-blockers are most efficacious because the arrhythmia triggers are predominantly exercise and physical exertion (30). For carriers with the 254M allele, long-term treatment with β-blocker therapy (all patients received up to 50 mg/day atenolol) seems sufficient. We observed persisting symptoms only in patient IV-1 (until age 8 years), because the body-weight-adjusted dosage was probably too low (0.6–0.9 mg/kg). With a dose of 1.2 mg/kg, he now remained asymptomatic for over 14 years. In patients with LQT1 syndrome (n = 69), it has been reported that on average cardiac events can be reduced from 57% to 19% during 5 years of β-blocker therapy (27). Severe failure of this therapy, i.e. aborted cardiac arrest or LQTS-related death, was observed in four out of 13 LQT1 patients (31%). This obvious difference with our study (showing no cardiac events during sufficient therapy) is likely to be related to different LQT1 genotypes. Chen et al. described an LQTS family with a KCNQ1 mutation G568A and atenolol at 4 mg/kg plus a pacemaker. Both regimen were not effective in preventing ventricular tachycardia and sudden death in the proband (31). In addition to the LQT genotype, other factors are known to modify cardiac repolarization (e.g. drugs with action potential prolonging properties, so-called drug-induced LQTS). Recent studies have identified several medications that prolong action potential duration and trigger malignant arrhythmias, especially in patients with congenital LQTS (32). Most notably, the deceased patient III-3 with LQTS received a tricyclic antidepressant (amitriptyline) 1 week before sudden death. Amitriptyline is a known antidepressant drug that in some reports has been associated with torsades de pointes tachycardia (33). We assume that this concomitant medication upon the repolarization prolongation may have triggered severe cardiac arrhythmia, retrospectively.
For LQTS patients, several antiarrhythmic options are available. However, it is very important to administer an appropriate antiarrhythmic option for patients with a common specific genotype. This report provides evidence of the efficacy and long-term safety of β-blocker therapy in V254M LQT1 carriers. Over 18 years, affected family members were without recurrence of symptoms. Thus, this report provides important information for clinicians treating LQT1 patients.
Acknowledgements
We are indebted to the family members for their participation in this study. We also thank Sabine Lange and Ellen Schulze-Bahr for their excellent technical assistance. This work was supported by grants from the IMF (Innovative Medizinische Forschung, We-1–2-II/97–17), University of Münster, Germany, the Dr Adolf Schilling Foundation, Münster, Germany, the Deutsche Forschungsgemeinschaft (SFB 556-A1, Schu1082/3–1), Bonn, Germany, and the Fondation Leducq, Paris, France.




