Concordance of phenotypic expression and gender identity in a large kindred with a mutation in the androgen receptor
Abstract
A 14-year-old female presented to the Pediatric Endocrine Clinic, Universidade Federal o Parana Curitiba, Brazil, for obesity. A few years later, despite normal breast development, the patient had failed to menstruate and lacked pubic and axillary hair. Laboratory analyses revealed high levels of testosterone. Karyotype analysis was XY. Direct sequencing of her genomic DNA showed a G to T transition at nucleotide 2089 at exon 2 in the androgen receptor gene, resulting in a substitution of Phe for Cys at position 576. This mutation disrupts the first Zn finger critical to DNA binding and transcriptional activity and results in complete androgen-insensitivity syndrome (CAIS). This individual was part of 700-member multigenerational kindred of German origin living in small villages in Southern Brazil. Family members who gave informed consent were screened using a polymerase chain reaction-based method. Nineteen CAIS-affected individuals and carriers were identified. All presented with infertility and lack of or sparse pubic hair. The prevalence of common AIS within the kindred greatly exceeds that of the general population and is due in part to their isolated familial and community structures. All individuals are genuinely feminine in their appearance, sex behavior, gender identity, and integration within their communities. We conclude that CAIS leads to complete feminization of XY individuals and results in individuals who are psychologically and socially established and integrated as women within the familial and cultural contexts of their communities.
Androgen-insensitivity syndrome (AIS) is an X-linked, recessive, heritable disorder in which karyotypic males exhibit partial or complete resistance to testosterone required for masculinization (1–5). The clinical presentation of AIS ranges from a typically male phenotype with decreased body hair and/or oligospermia to a typically female phenotype with primary amenorrhea and lack of pubic and axillary hair; the latter phenotype is categorized as complete AIS (CAIS). The range of clinical abnormalities in AIS is the result of mutations throughout the androgen receptor (AR) gene (1–7). Some mutations lead to receptors with reduced hormone-binding affinity. In these cases, doses of testosterone administered exogenously can exert a partial effect with modest response (8–10). In others, the molecular defect is at a locus whose function cannot be bypassed to recover or activate the receptor (11).
The assignment of sex and the sex of rearing for AIS patients is generally based on the physical appearance and functioning of the genitalia. The usual practice is to assign a female sex to CAIS-affected individuals. In recent years, the assignment of sex based on physical appearance has been re-evaluated within the social, cultural, and familial environments of affected individuals, and several societies and groups have been established to offer support to affected individuals and their families (12–14).
We were presented with the unusual opportunity to study the clinical, molecular, gender identities, and psychological outcomes of individuals who were discovered to have CAIS. These individuals were part of a kindred of more than 700 members distributed across four generations. CAIS-affected individuals in the kindred remained insulated from their disease, and despite the discordance in genotype–phenotype had well-developed self-identities and had established social, familial, and community roles as females. This work first appeared in abstract form (15).
Materials and methods
Construction of pedigree
The index case was identified and genomic DNA sequenced. Members of the index case's family knew of other family members who had infertility problems and amenorrhea, although they were neither aware of the extent of their family nor the whereabouts of other families. Three female family members volunteered to contact distant relatives, and a family pedigree was constructed with their guidance.
Informed consent was obtained from all subjects or their parents. Follow up of the family was maintained through contact with local physicians, referrals, and female relatives throughout the kindred.
Mutation analysis
Blood was drawn from each of the individuals. Only two in each of the families refused to have blood drawn. Genomic DNA extracted from blood was prepared for polymerase chain reaction (PCR) amplification, according to published methods (16).
Because of the family size, five individuals were selected for direct sequencing. These were based on clinical presentation, linkage to other branches of the family, and to serve as controls: (i) the index patient (IV-10, indicated by arrow in Fig. 1); (ii) an unaffected relative (III-45) to act as a negative control for polymorphisms; and (iii) two suspected CAIS-affected relatives (II-6 and III-54). Two hundred to four hundred nanograms of genomic DNA was used in each 50–100 µl PCR. Each AR exon was amplified individually in triplicate by PCR using primer sequences derived from published data (13). PCR products were cloned into pGEM-T (Promega, Madison, WI) and sequenced on an ABI 373 automated DNA sequencer on both strands. The non-coding strand was sequenced in the antisense direction. In all cases, a second sample of DNA was amplified and sequenced to confirm mutations. In this manner, we were able to pinpoint the mutation in each of the individuals and to ascertain whether the same mutation or a de novo mutation was presented by each of the families.
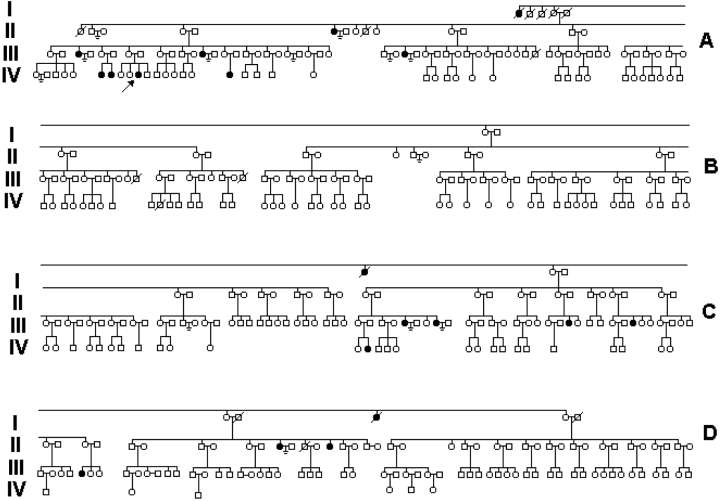
Partial family tree showing nuclear families with 440 members distributed across four generations, including all 19 individuals with complete androgen-insensitivity syndrome. Index case is indicated by arrow.
PCR-based screening protocol
A rapid PCR-based method was developed to identify affected individuals and carriers. Using a specific set of primers that matched the wildtype or the mutated gene (differing by one base), PCR produced a product when the wildtype primers matched the wildtype sequence but not when the wildtype primers were applied to an affected individual that bore the mutation. Similarly, a PCR product from those affected with the mutation was produced only with primers that matched the mutated sequence. Because the mutation was a single-base transition from G to T, the primers were based at the 3′ terminal region of the mutation at the locus of the mutation itself.
Each sample of DNA was amplified with two pairs of primers. A common reverse primer rpX2CTL (5′-TGTGCAAGACCCTTTACCTT-3′) was used in combination with forward primers that allow discrimination of the nucleotide at position 2089. Forward primer fp2089G (5′-CACTATGGAGCTCTCACATG-3′) amplifies a 75-bp product if a G (wildtype) is at nucleotide 2089 and forward primer fp2089T (5′-CACTATGGAGCTCTCACATT-3′) (wildtype) is at nucleotide 2089, and a forward primer fp2089T (5′-CACTATGGAGCTCTCACATT-3′) amplifies a 75-bp product if a T is at nucleotide 2089. Note that primers fp2089G and fp2089T differ only in the 3′ terminal nucleotide (underlined), which corresponds to nucleotide 2089 in the AR gene (location of G to T point mutation). Differential screening of samples using these primers is possible as Taq DNA polymerase lacks a 3′−5′ nuclease proofreading activity (i.e. in combination with the common reverse primer, CAIS-affected individuals prime only a PCR product with fp2089T; unaffected individuals with fp2089G and carriers produce PCR products with both primers). An additional positive control reaction was performed for each sample, of the entire 210-bp length of exon 2 with forward primer fpX2CTL (5′-TTCAGT GACATGTGTTGC-3′) in combination with the common reverse primer. One hundred nanograms of genomic DNA was used in each 20 µl PCR using the following cycling conditions: 94 °C for 2 min; 25 cycles of 92 °C for 15 s; 74 °C for 15 s; 72 °C for 15 s; 72 °C for 2 min. PCR reactions were analyzed by electrophoresis on 15% acrylamide (acrylamide : bis-acrylamide 38 : 1) ×1 TBE (0.1 m Tris–HCl, pH 8, 0.083 m boric acid, 20 mm ethylenediaminetetraacetic acid) gels.
Social and psychological evaluation
Semistructured, informal, and open-ended interviews including life histories of affected individuals within the family were undertaken. CAIS-affected patients were age-paired with controls. Those who preferred interviews, or in some cases those who were induced to do so, described their own or their child's sex preferences. Adolescent and adult CAIS-affected individuals (n = 8) as well as paired controls (sisters and cousins, adolescent girls, and adult women paired for age) from the same kindred provided oral answers to questions about their gender identity and sex preferences. Parents provided oral answers to questions about their child's typical activities and sex-typed play (n = 3) (17).
Other methods
In some cases where testes were removed in affected individuals, histopathology was classified according to predominant cell types in each testis.
Results
The index patient (noted in Fig. 1 as IV-10, indicated by arrow) was a 14-year-old girl who presented to the Pediatric Endocrine Clinic in Southern Brazil for the treatment of obesity. The patient lacked pubic and axillary hair and had amenorrhea despite her normal breast development. Subsequent analyses revealed an XY karyotype and high levels of testosterone (21 nmol/l). The level of luteinizing hormone was 8.21 IU/l.
Pedigree and family characteristics
The index case is a member of an unusually large family currently composed of more than 700 people (Fig. 1). It maps four generations to the first German immigrants from the Rhine Valley region who settled in the Iguassu Falls region in Southern Brazil around 1890. The predominant sociocultural influence is German; German language and culture are practiced at home. They have fair complexions, hair, and eye coloring. Because of the cultural and geographical isolation and socioeconomic status (their occupational class is predominantly agricultural), the family never sought a reason for the infertility, until a diagnosis was sought in this study. Because of their Catholic upbringing, children and close nuclear family ties are highly valued and sibling sets are large (average = 10 for completed nuclear families in generations I and II). There are no recent cases of consanguineous marriages.
Mutational analysis of the AR gene
Direct sequencing shows a substitution of T for a G in exon 2, at nucleotide 2089, corresponding to a point mutation of phenylalanine for cysteine at amino acid position 576 of the AR. The cysteine is placed at the first Zn finger of the DNA-binding domain of the receptor critical to receptor function (1, 16, 18–22). Despite the fact that the receptor is still able to bind hormone, disruption of the Zn finger and DNA binding leads to loss of transcription activity of the receptor.
When other family members were screened for the presence of the mutation using the PCR-based protocol, CAIS-affected individuals produced a band with primer pair T2089 and not with primer pair G2089, whereas unaffected individuals produced a band with G2089 and not with T2089. Carriers carry a normal and mutant gene and, therefore, produce a product with both sets of primers (Fig. 2). A total of 19 members were identified. The mutation is represented in the first generation and has been vertically transmitted throughout the pedigree (Fig. 1).
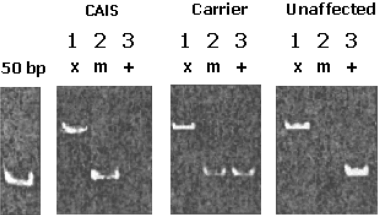
A representative polymerase chain reaction (PCR) analysis of complete androgen-insensitivity syndrome (CAIS)-affected individuals, CAIS-unaffected individuals, and carriers is shown. PCR amplification was performed using three sets of primers for each sample and then analyzed by acrylamide gel electrophoresis as described in Materials and methods. Primer set G2089 detects the wildtype sequence, so a band is amplified from unaffected individuals and carriers but not from CAIS-affected individuals. Primer set T2089 detects the mutant sequence, so a band is obtained from affected individuals and carriers but not form unaffected individuals. Primer set X2CTL was an internal positive control, which amplifies the entire length of exon 2. Lanes 1, 2, and 3 correspond to control (x), mutant (m), and wildtype (+) alleles, respectively.
Clinical features of affected individuals
The clinical features of 17 surviving members of the family with CAIS were examined. The two additional members with CAIS died prior to clinical examination but were reported to have amenorrhea and sparse secondary sexual hair. The clinical presentations of seven members representative of CAIS-affected individuals are summarized in Table 1. Serum testosterone (prior to surgery) ranged from 12.5 to 26.0 nmol/l, and all patients spontaneously feminized at puberty. The only health problem associated with CAIS in these kindred was obesity, which was a common occurrence even in unaffected members of the families. Therapeutic measures for some included hormonal replacement with conjugated estrogens (1.25 mg/day). Once informed, the CAIS-affected individuals opted either for vaginal enlargement or for repeated coital dilation (Table 1). Histopathology of the tumors is summarized in Table 1. No evidence of seminiferous tubules or epididymis was found in any of the patients. Some of the adult patients refused orchidectomy, whereas parents of those younger than 12 years of age decided to postpone orchidectomy until they become adults.
| Hair | Vaginal Depth(cm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Pubic | Axillary | Prior to sexual activity | Current | Age of first penile penetration (years) | Method of vaginal enlargement | Marital status | Testes tumor | Bone densitometry before orchidectomya |
| 15b | No | No | 2 | 14 | Never | Sigmoid neovagina | Single | No | Normal |
| 19 | No | No | 4 | 9 | 14 | Passive dilationc | Single | Unoperated | Not done |
| 28 | Sparse | No | 3–4 | 8 | 15 | Passive dilationc | Engaged | Bilateral hamartoma | Normal |
| 30 | No | No | 3–4 | 10 | 16 | Passive dilation | Married | No | Not done |
| 32 | Sparse | No | 4 | 6 | 18 | Passive dilation | Single | Harmatoma and seminomad | Normal |
| 34 | Sparse | No | 2–3 | 16 | 17 | Skin graft neovagina | Married | No | Normal |
| 48 | No | No | ? | ? | 18 | Passive dilation | Married | Normal testes | Normal |
| 51 | No | No | ? | 10 | 19 | Passive dilation | Married | Unilateral seminomaa | Normal |
- Testosterone levels prior to orchidectomy were >16.5 nmol/l in all patients, and estrogen replacement was started after orchidectomy. Hamartomas composed of multiple foci with tubules lined by Sertoli's cells with the stroma containing hyperplasic Leydig's cells were found in two patients. These benign tumors occur relatively much more commonly than do seminomas among complete androgen-insensitivity syndrome-affected patients (37).
- a Seminoma was removed at the age of 32, followed by 1 year of chemotherapy (1981–1982). Contralateral testis, removed at age of 51, with normal histopathological exam.
- b Index patient.
- c Besides repeated coital dilation, chronic use of flexible plastic cylinders were used.
- d Unilateral seminoma and contralateral harmatoma.
Profile of affected individuals
Affected individuals demonstrated sex role behaviors, marriage, and family practices that were fully female, the sex established at birth according to usual medical practices. There was no ambiguity in sexual preference, assignment, or community acceptance.
All CAIS-affected adolescent and adult patients were fully satisfied with their female gender, demonstrated sex self-identities and interest in their feminine appearance, sex role behaviors, gender personalities, and engagements with only heterosexual partners identical to those of their sisters or cousins. Marriage and plans for child adoption were common to all adolescent and adult CAIS-affected individuals. Some expressed an interest for having an intimate life with one or more heterosexual partners before taking any definitive steps leading to marriage. As expected, female-typical play was clearly evident during the assessment of three toddlers and answers presented by their parents (17).
From the 17 surviving CAIS-affected patients, five are still younger than 18 years of age (all in IV generation); two of them are teenagers and have had boyfriends. The 11 living adults range in age from 18 to 67 years, seven are married, three are engaged, and one remained unmarried (one is a nun). The oldest affected member (I.1, Fig. 1) was 74 years old and died during the course of this investigation. She had never married and apparently lived in good health. The causes of death, at 46 and 51 years, of two additional family members described with the clinical features of CAIS were unknown.
At the end of this investigation, most of the carriers expressed fear of having a child with CAIS, and some of them would approve abortion. During this investigation, one carrier (individual IV.7) had an unplanned pregnancy for which prenatal diagnosis and abortion were out of the question, and she finally had a second CAIS-affected child.
Not all of the CAIS individuals were informed of their diagnosis. Parents decided whether or not to reveal the discordance in genetic and phenotypic sex based on the predicted psychological impact on the individual. This decision resulted in nine of 17 CAIS patients not being aware of their diagnosis. Three of these nine patients are older than 40 years of age.
Discussion
Analysis shows a Cys–Phe point mutation at codon 576 that completely disrupts the first zinc finger of the AR. This mutation destroys the AR DNA-binding activity and leads to CAIS (1, 4, 16, 20–22). Because of the geographic isolation and the cultural insularity of families, the number of affected individuals within this kindred is high. Only one other study has reported mutations in a kindred family, but not of this size nor with the geographic or cultural isolation seen here (23).
Mutations at position 576 have been reported previously, notably a Cys to Arg transition (24, 25) and a similar mutation of Cys to Phe at 576, that appeared in abstract form (26). While mutations have been extensively studied and identified by Wilson and colleagues (1, 20–22), only one similar point mutation in exon 2 ZF1 has been described before in the literature (27) but in codons 579 (Cys)(TGC) mutated to Phe (TTC) and 582 Phe (TTC) mutated to tyrosine (TAC). In addition, a point mutation in the second Zn finger of the DNA-binding domain of the AR was also found to cause CAIS in two siblings with receptor-positive androgen resistance (16). To the best of our knowledge, this study represents the largest kindred family in which mutation in this specific region of the ARX has been reported.
Even though the AR has been extensively studied (1, 3, 27), a clear relationship between clinical presentation and the binding parameters of the AR and the nature of the mutation have not been established (4–7, 28). Mutations in exons 2 and 3 (the DNA-binding domain (DBD)) usually (16, 29) but not always (30) lead to CAIS. The DBD has the most conserved amino acid sequence among the steroid–thyroid–retinoid receptor super family (25, 26). In this study, a clear relationship exists between the nature and position of the mutation and the resulting phenotype. Cys 576 is one of eight invariant residues involved in coordinating two zinc atoms that comprise the zinc fingers of the DBD (24, 31). Cys 576 is located at the P box in the first zinc finger, and its substitution with Phe would severely affect the structural integrity of the DBD. It is a mutation that could never be overcome by current therapies, because there are no other pathways by which AR transcriptional activity can be activated.
The PCR method clearly discriminates among CAIS-affected individuals, CAIS-unaffected individuals, and carriers. PCR-based diagnosis provided a much clearer picture of both carrier and unaffected female relatives. Importantly, the PCR-based method is rapid, accurate, and can be used prenatal diagnosis and genetic counseling. From DNA isolation, it is possible to obtain results in 3–4 h. Numerous techniques have been used for such analysis (23, 32); these methods, however, are not 100% sensitive, require optimization, are prone to false interpretation, and are time-consuming multistep procedures.
While infertility was known among members of this family, the majority of the kindred were unaware of the basis of their presentation. The social, interpersonal, and marriage practices clearly showed that CAIS-affected individuals accommodated this infertility. Most of them are sexually active with male partners, and repeated coital dilation has been successful in creating a vagina without surgical intervention. The two CAIS patients who underwent vaginal enlargement and were sexually active expressed greater satisfaction. They did not have any complication such as increased vaginal secretions, dyspareunia, or prolapse of the neovagina.
CAIS-affected individuals in this family have self-identities, sex roles, and gender personalities that are fully female. There is no ambiguity in sexual perception, preference, assignment, or sex-type activity. The marriage and family practices of CAIS-affected individuals are fully consistent with assignment of the female sex. Within their household, economic, and community structures, all CAIS-affected individuals integrate fully as females. Prior to their diagnosis with CAIS, their very feminine phenotype did not single these women as having a problem other than infertility. While our first impression was that the deep cultural and social customs embedded within these community would render the new medical information irrelevant, some affected individuals and carriers reported to have experienced stigmatization as individuals requiring complex medical intervention after their diagnosis became known. However, several female relatives played key roles in genetic counseling and in collecting and disseminating information in the kindred. The importance of such women in collecting and coordinating information, recreating a new sense of the family around the medical issue, and helping to translate scientific diagnosis into laymen's language has been reported before (33). The role of these women in providing counseling after diagnosis supports the idea of ‘truth telling’ to CAIS-affected individuals and their families. A general consensus has emerged that strongly favors disclosure in association with appropriate support systems rather than concealment and possible self-discovery with no available support systems (34).
This study supports the premise that the lack of the AR responsiveness is an important determinant in female-typical psychological development (35) and that subjects with CAIS maintain their gender identities and sex assigned to them in infancy (33).
In conclusion, this work shows that cultural background and familial support in a Western society plays an important role in the acceptance of reproductive abnormalities even when the societal abnormalities become known later in life (36). No matter what the society or societal settings, individuals with CAIS are comfortable with their gender identity, roles, gender personality, and acceptance within the community as females. Despite the fact that they are technically intersex, because their genotype is inconsistent with their phenotype, there is no discordance between female sex assignment and their female sex roles.
Acknowledgements
We thank Dr R.D. Feigin and Dr B. O'Malley, Department of Cell Biology, Baylor College of Medicine and Texas Children's Hospital, Houston, TX, USA, for their generous support of this work.




