Role of macrophage polarisation in skin wound healing
Abstract
Skin wound healing is a complex pathophysiological change that is driven by macrophages and their secreted related factors. Depending on the stimuli, macrophages can be polarised into two subtypes of macrophages with completely different phenotypes and functions, namely M1 and M2. The aim of this study was to explore the role of M1 and M2 macrophages in skin healing in order to develop new drugs for the treatment of refractory wounds. Primary bone marrow-derived macrophages (BMDMs) were isolated from rats and expanded in vitro using macrophage colony stimulating factor. In addition, the BMDMs were polarised into the M1 and M2 subtypes using lipopolysaccharides (LPS) and interleukin-4 (IL-4), respectively. Cytokine levels in the culture supernatants were measured by an enzyme linked immunosorbent assay. Epidermal wounds were made on the dorsal surface of rats, and treated with M1 or M2 cell suspensions or phosphate buffered saline. Wound healing was recorded on days 1, 3, 7, 10, and 14 after stamping, and the wound healing rate was measured by haematoxylin-eosin and Masson staining. A total of 3 to 4 × 107 bone marrow cells were extracted from each rat femur. The BMDM culture had 87.1% CD45+ cells, 89.2% CD68+ cells, and 86.5% CD45+CD68+ cells. Furthermore, IL-12 (P < .05) and IL-10 (P ≥ .05) levels, respectively, increased and decreased in the culture supernatants of the M1 cells after LPS stimulation compared with those in the M0 (unstimulated) group. Likewise, IL-4 stimulation led to a significant increase in IL-10 levels (P < .01) in the conditioned media of M2 cells, while that of IL-12 decreased slightly (P ≥ .05). In the rat model, the infusion of M2 cells accelerated wound healing and tissue regeneration, whereas the M1 cells delayed the recruitment of inflammatory cells, granulation growth, and collagen deposition, which impaired wound healing. Macrophage polarisation and activation are critical for skin wound healing. While exogenous M1 cell infusion delayed wound healing, the M2 cells promoted wound healing in a rat model.
1 INTRODUCTION
Skin wound healing typically proceeds through the haemostasis (vasoconstriction and clotting), inflammatory, proliferative (epithelialization and granulation), and reconstruction (epidermal scarring) phases.1 In this complex repair process, macrophages and their secreted related factors play important roles.2 The inflammatory phase sets in within a few hours of trauma and is initiated with the infiltration of leukocytes into the wound site in response to injured cells, pathogens, and foreign bodies, as well as chemotactic factors released by the resident mast cells and granulocytes. The peripheral monocytes are recruited to the peri-wound tissues by various inflammatory and chemotactic factors, where they differentiate into macrophages. The cytokines secreted by the activated macrophages play important roles in the inflammatory, proliferative, and reconstruction phases of skin wound healing.3
Macrophages are polarised into the M1 and M2 phenotypes, which differ on the basis of cell-surface markers and functions in response to distinct stimuli. The classically activated M1 cells have proinflammatory and phagocytic functions.4 In 2003, Gordon et al reported a subtype of macrophage with a phenotype different from classical activation pathway macrophages in the presence of interleukin-4 (IL-4), called alternative activation pathway macrophages.5 Although the existence of both phenotypes is widely recognised, their regulatory roles in skin healing are still controversial.6 In this study, we explored the roles of M1 and M2 macrophages in skin wound healing using a rat model in order to provide a theoretical basis for the development of new drugs for refractory wounds.
2 MATERIALS AND METHODS
2.1 Experimental animals and materials
Five clean-grade healthy adult (Sprague–Dawley) SD rats (250-350 g), phosphate buffered solution (PBS), foetal bovine serum (FBS), PRMI-1640 medium, recombinant murine macrophage colony stimulating factor (M-CSF), tryple express, red blood cell (RBC) lysis buffer, FITC-labelled mouse anti-rat CD45 antibody, purified mouse anti-rat mononuclear phagocyte, anti-mouse lgG1 APC, and medical-grade ethanol were used for the experiments.
2.2 Experimental methods
2.2.1 Acquisition of bone marrow-derived macrophages
Bone marrow-derived macrophages (BMDMs) were isolated from healthy adult (Sprague–Dawley) SD rats (250-350 g). The animals were euthanized by cervical dislocation and soaked in 75% alcohol for 5 min for disinfection. The cadavers were dissected, and the knee joints were exposed layer by layer. After cutting the patellar ligament, iliotibial band, sartorius muscle, medial and lateral collateral ligaments, and cruciate ligament, the lower end of the femur was completely dissociated and the tibial surface of the femur was exposed. The bone marrow cavity was accessed from the femoral intercondylar fossa along the femoral shaft, and the contents were flushed with 2 mL PBS containing 2% FBS using a 5 mL syringe. The cell suspension was passed through a 400-mesh stainless steel filter to remove tissue and bone blocks, and the RBCs were lysed with three volumes of lysis buffer on ice for 10 min. The bone marrow cells were collected by centrifuging at 450g for 8 min at 4°C, washed twice with sterile PBS, and resuspended in complete medium at a density of 2 × 106 cells/mL. After culturing for 72 h at 37°C, the floating cells were discarded, and the adherent cells were cultured further in fresh medium. On the 7th day, the adherent cells were washed 2 to 3 times with sterile PBS (without Mg2+ and Ca2+), and dislodged by incubating with 2 mL of Tryple Express digestion solution for 7 min at 37°C. The reaction was stopped once the cells started to detach by adding complete medium, and the harvested cells were washed twice with PBS.
2.3 Identification of the BMDMs
The cell density of the suspension was adjusted to 104 to 105 cells/mL using a blood count plate. The cell suspension with adjusted density was loaded into four Eppendorf (EP) tubes. 1000 μL per tube. The numbers are A, B, C, and D, respectively. Three kinds of antibodies (FITC Mouse Anti-Rat CD45, total Mouse Anti-Rat Mononuclear Phagocyte [anti-rat68] and Anti-Mouse IgG1 APC) were extracted from a refrigerator at 4°C. It was placed in a vortex oscillator and briefly shaken for 3 seconds, centrifuged at 1500 r/min for 20 seconds, and then the adherent antibodies were collected. EP tube A was the blank control group without any antibody. Add FITC Mouse Anti-Rat CD45 to EP tube B; total Mouse Anti-Rat Mononuclear Phagocyte and Anti-Mouse IgG1 APC were added to EP tube C simultaneously. The above three antibodies were added to EP tube D simultaneously. The cells were incubated at 4°C for 15 minutes in the dark. The cell suspension was blown evenly by using a pipetting gun. The positive rates of CD45, CD68, and double positive rates were detected by flow cytometry.
2.4 Polarisation of BMDMs
The BMDMs conditioned medium was prepared. The composition of M1 conditioned medium was PRMI-1640 + 10%FBS + lipopolysaccharides (LPS) (100 ng/mL), and the composition of M2 conditioned medium was PRMI-1640 + 10%FBS + IL-4 (10 ng/mL). BMDMs cells were prepared successfully, centrifuged at 450g × 8 min, resuspended in PBS at 4°C, and then divided into two equal parts. The cell suspension was centrifuged again under the above conditions, and the cell precipitation was resuspended in the cell culture dish using M1 and M2 conditioned medium, respectively. After 24 hours, the polarisation of BMDMs was identified by the IL-12 ELISA kit and the IL-10 ELISA kit. The BMDMs cultured in a normal medium environment were recorded as M0.
2.5 Regulation of wound healing by M1 and M2
Five SD rats (230 ± 10 g) were anaesthetised with ether and disinfected with 75% ethanol. The skin on the backs of rats was pulled, and the posterior midline was the longitudinal axis. The double layer of skin was placed at the stamping hole position of the sterilised DELI punch. The upper stamping position was located at the focal point connecting the scapular line and the lower scapular line, and the lower stamping position was located at the focal point connecting the scapular line and the iliac crest. Two wounds with a diameter of 6 mm were made by stamping each time, and four wounds were made by stamping twice and numbered 1 to 4 in the clockwise direction. The animals were transferred to autoclaved cages after the procedure, and the bedding material, feed, and drinking water were sterilised to prevent wound infection. Immediately after wounding, the edges of three random wounds in each animal were respectively injected with 1.0 mL M1 cell suspension, M2 cell suspension, and PBS using 5UI insulin needles, and the remaining wound was untreated as a blank control. Wound healing was observed on days 1, 3, 7, 10, and 14 after stamping and photographed. The wound healing rate was measured at each time point, and the tissues were excised for HE staining and Masson staining. Five animals were analysed for each time point.
3 RESULTS
3.1 Identification of the BMDMs
A total of 3 to 4 × 107 bone marrow cells were obtained from each femur. The cells were spindle-shaped and gradually extended with prolonged culture. As shown in Figure 1, the bone marrow cells started forming pseudopodia after 24 to 48 hours of culturing, and their shape changed from oval/round to triangular/polygonal. Furthermore, 87.1% of the cultured BMDMs were CD45+, 89.2% were CD68+, and 86.5% were double positive.

3.2 Polarisation of BMDMs
Compared with the M0 cells, LPS stimulation significantly increased the level of IL-12 (P < .05), and slightly decreased that of IL-10 (P ≥ .05) in M1 cells. After IL-4 stimulation, the content of IL-10 increased significantly (P < .01), and that of IL-12 decreased slightly (P > .05) in the M2 cells.
3.3 Regulation of M1 and M2 on wound healing
3.3.1 Evaluation of wound healing rate
The average unhealed areas in the M1, M2, and PBS-treated and untreated wounds were measured 1, 3, 7, 10, and 14 days after wounding (Table 1). t-test was used to verify whether there were statistical differences between experimental wounds (M1, M2, PBS) and blank control wounds, the results are shown in Table 2.
| Postoperative days | Groups | |||
|---|---|---|---|---|
| M1 | M2 | Phosphate buffered solution | Blank control | |
| 0 | 28.04 ± 4.42 | 27.94 ± 3.02 | 28.28 ± 3.10 | 27.47 ± 3.57 |
| 3 | 27.56 ± 2.55 | 12.48 ± 2.71 | 19.44 ± 2.61 | 20.23 ± 1.70 |
| 7 | 19.29 ± 1.09 | 5.48 ± 1.82 | 14.65 ± 2.79 | 15.21 ± 1.44 |
| 10 | 12.51 ± 1.12 | 3.14 ± 1.77 | 6.97 ± 0.96 | 7.12 ± 1.34 |
| 14 | 3.51 ± 0.18 | 0.27 ± 0.12 | 1.42 ± 0.35 | 1.34 ± 0.21 |
| Days | Groups | ||
|---|---|---|---|
| M1 versus blank control | M2 versus blank control | Phosphate buffered solution versus blank control | |
| 0 | 0.828125 | 0.827806 | 0.711643 |
| P ≥ .05 | P ≥ .05 | P ≥ .05 | |
| 3 | 0.000687 | 0.000633 | 0.586186 |
| P < .01 | P < .01 | P ≥ .05 | |
| 7 | 0.000987 | 0.000014 | 0.700454 |
| P < .01 | P < .01 | P ≥ .05 | |
| 10 | 0.000124 | 0.003902 | 0.843843 |
| P < .01 | P < .01 | P ≥ .05 | |
| 14 | 0.000000 | 0.000009 | 0.672787 |
| P < .01 | P < .01 | P ≥ .05 | |
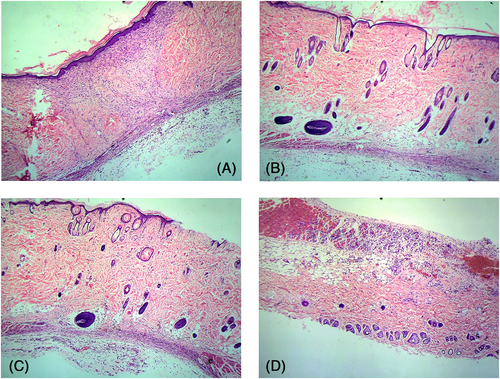
3.4 Results of the HE staining
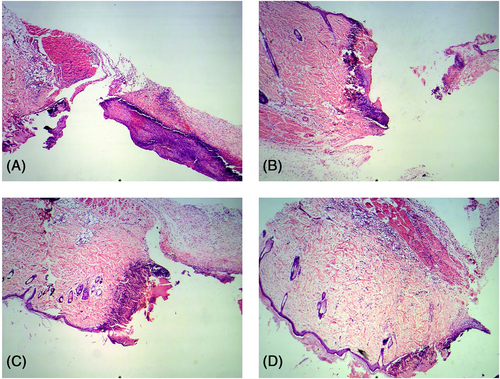
Postoperative day 1: Obvious peri-invasive injuries were observed in the treated and untreated wounds. The epidermis, dermis, subcutaneous tissue and some superficial muscularis were clearly visible in the wound margins. Neutrophil infiltration was observed in all wounds, and the degree of infiltration was similar in the experimental and control groups. In contrast, macrophages were observed across the subcutaneous, dermal, and superficial myometrium layers in the wounds treated with the M1 and M2 cell suspensions (Figure 2).
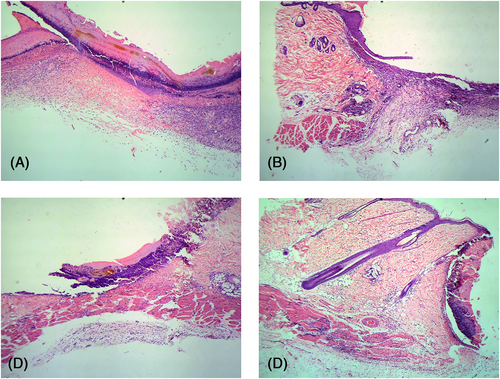
Postoperative day 3: The infiltration of inflammatory cells in the PBS group and blank control group was significantly aggravated, and basal cell regeneration was observed at the wound margin, but there was no significant difference between the two groups. The infiltration of inflammatory cells in the M1 group was more intense than that in the above two groups. The infiltration of inflammatory cells in the M2 group was enhanced compared with 1 day after the operation. Obvious new granulation tissue was observed at the wound margin and base (Figure 3).
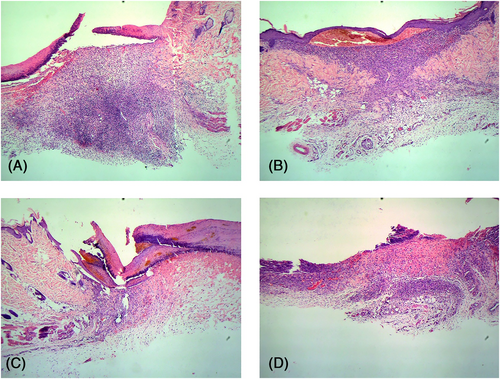
Postoperative day 7: Extensive granulation tissue was seen in the M2-treated wounds, along with numerous exogenous macrophages around the new tissues. In the other groups, infiltration of inflammatory cells continued and limited granulation repair was observed (Figure 4).
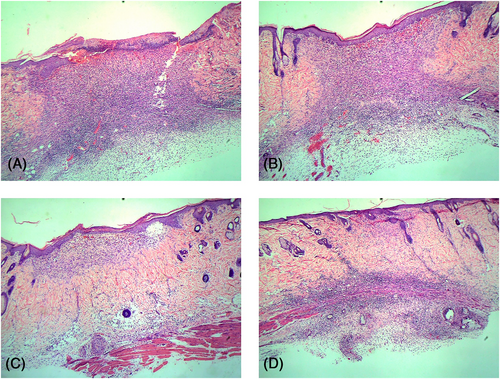
Postoperative day 10: Fewer inflammatory cells were observed in the PBS-treated and untreated wounds, and pink-coloured new granulation tissue appeared around the wound. In contrast, the M1-treated wounds still had significant inflammatory exudation with miniscule granulation tissue repair. On the other hand, treatment with M2 cell suspension decreased the number of inflammatory cells and exogenous macrophages at this time point, and numerous pink-coloured new basal tissues and capillaries were observed at the infiltration site of macrophages, which indicated peak wound repair (Figure 5).
Postoperative day 14: The invasive pericyte infiltration ceased and epithelialization was complete in the M2-treated wounds. In the PBS-treated and untreated wounds, the infiltrating cells showed obvious signs of fading, and the epithelialization was nearing completion. However, the infiltration of inflammatory cells was unabated in the M1-treated wounds, although granulation tissue and neovascularization could be observed (Figure 6).
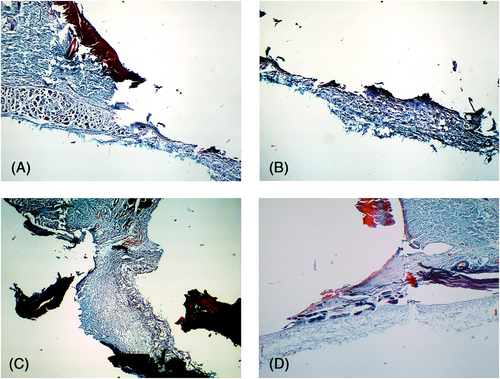
3.5 Results of the MASSON staining
Postoperative day 1: Obvious breakage and missing skin tissues were seen at the wound edges of each group. The remaining tissue structure was disordered, collagen was absent, and some structures were twisted and folded because of mechanical stress (Figure 7).
Postoperative day 3: The wound base had gradually levelled in the PBS and blank control groups and showed pale blue staining that was indicative of incipient collagen repair. In the M2-treated wounds, the incision margin and staining intensity of the wound base were obvious, indicating significant collagen production. Furthermore, the scattered neovascularization observed in the wound base was also indicative of accelerated tissue repair. In contrast, no significant change was observed in the M1-treated wounds compared with day 1, and the lesion structure was more disordered and inflammatory exudation was obvious (Figure 8).
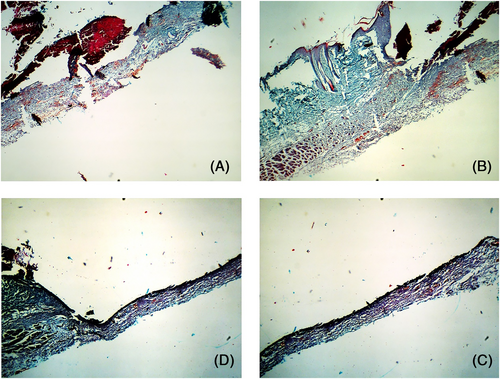
Postoperative day 7: Collagen repair was significant in all wounds and had peaked in the M2-treated wounds, which showed new interwoven collagen fibres. However, the Masson-stained area in the M1-treated wounds was relatively thinner and lighter in intensity, indicating weaker collagen deposition and tissue repair (Figure 9).
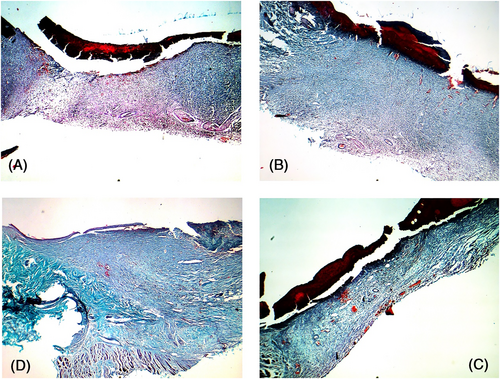
Postoperative day 10: Collagen repair had peaked in the PBS and blank control groups, and hyperplasia was observed in the blue-stained region in the dermis. The dermis was more intensely stained in the M2-treated wounds, the arrangement of collagen fibres was regular, and new capillaries were observed, which indicated that the collagen repair was nearly complete. Although collagen repair had started in the dermis of the M1-treated wounds, the new collagen fibres were disordered (Figure 10).
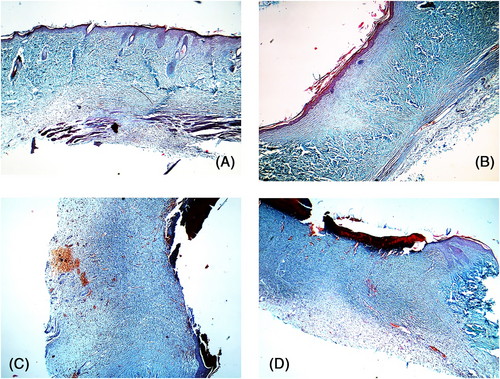
Postoperative day 14: The collagen repair phase was nearly complete in the PBS and blank control groups, with regularly arranged collagen fibres and normal skin structure. The M1-treated wounds showed numerous disordered collagen fibres, which were similar to those seen in the PBS group 10 days after operation. In contrast, the collagen in the dermis of the M2-treated wounds was clear and ordered, and the healing process was complete (Figure 11).
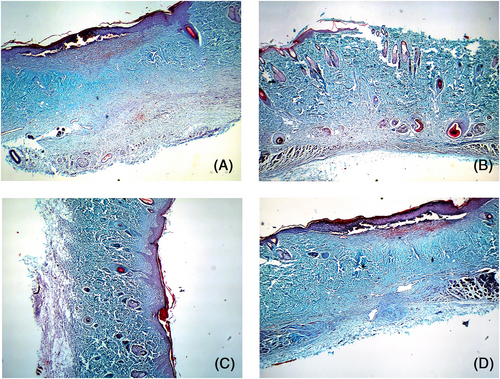
4 DISCUSSION
Macrophages are a highly heterogeneous population of cells that are critical to the wound healing process.7 The proinflammatory factors and chemokines released by the M1 macrophages mediate immune responses and are essential for immune surveillance. On the other hand, the M2 macrophages have a weaker antigen presenting ability and secrete TGF-1, which not only attenuates immune responses but also promotes wound healing. Studies have proven that M1 and M2 are essentially a continuous state of cell (M0) in function, and there is a sequential evolution process. This also explains the alternating infiltration of M1 and M2 cells during wound healing.4 In this study, we observed that the exogenous M2 cells accelerated and enhanced wound healing, whereas the M1 cells delayed the recruitment of inflammatory cells, tissue granulation, and collagen deposition, resulting in overall poor healing.
In healthy skin tissues, the content of macrophages is small, about 1 to 2/mm2, and they are not activated.8 These macrophages mainly perform the function of immune surveillance, and almost do not secrete cytokines. Following a skin injury or infection, the peri-invasive macrophages migrate to the affected site in response to chemokines and inflammatory mediators (such as foreign bodies and microorganisms).9 The adhesion factors secreted by the injured vascular endothelium and the platelet-derived growth factor (PDGF) and transforming growth factor (TGF) secreted by the fibroblasts activate the infiltrating macrophages. Activated macrophages have the function of secreting inflammation-related cytokines (MCP-1), TGF, PDGF, and so forth.10 The above factors can continue to chemotaxis and activate more macrophages, starting the positive feedback chemotaxis and recruitment of M1, rapidly magnifying the number of activated macrophages in the wound, thus ensuring the effect of non-specific immunity.11
Some experiments have confirmed that when the experimental animals were injected with LPS to induce macrophages in M1, the time of tumor necrosis factor (TNF) increase and peak appeared to be advanced.12 TNF can induce changes similar to the early inflammatory response and increase the expression of adhesion molecules in vascular endothelial cells and leukocytes. A moderate M1-mediated inflammatory response exerts a protective role by removing foreign bodies and damaged tissues, killing pathogens, and mobilising metabolic substrates. However, an uncontrolled M1 response with the continuous release of inflammatory mediators can exacerbate tissue damage. Consistent with this, we found that the M1 cell suspension impaired skin wound healing in rats, and slight redness, swelling, and exudation were observed even 14 days after wound induction. The healing process was delayed because of prolonged neutrophil infiltration, collagen production was low, and the new collagen fibres were fine and disordered. Overall, the M1 macrophages can improve the inflammatory response but prolong its duration, eventually leading to delayed wound healing.
The M2 is also known as an “anti-inflammatory macrophage” in some damage repair studies because of its low expression of inflammatory factors. As an inducer of alternative activation of macrophages, IL-4 can transmit alternative activation signals to the nucleus of macrophages through the Janus kinase family-SATA6 (JAK-SATA6) signalling pathway and the insulin receptor family-phosphoinositol 3-kinase (IRS2-PI3K) signalling pathway.13 These alternative signals specifically activate the Argl gene, which encodes the hydrolase Arginase l, a key factor in the regenerative effects of M2 macrophages.14 Argl is a hydrolase that hydrolyzes L-arginine to urea and L-ornithine, which are further converted to l-proline and become an important raw material for collagen production. Skin collagen predominantly consists of type I and type III collagen fibres. Type III collagen surrounds type I collagen, which is the key to determining the thickness of collagen fibres. Collagen synthesis increases during skin injury repair, which leads to a surge in the demand for l-proline. Insufficient supplies of l-proline can decrease the content of type III collagen, resulting in thinner skin on the repaired wounds and delayed healing. At the beginning of the healing procedure, M2 macrophages were given in sufficient amounts to the experimental wounds, which indirectly provided important raw materials for collagen synthesis. The MASSON staining method was used to evaluate the collagen deposition rate and quality of each experimental wound. It can be seen that the collagen deposition rate of the M2 group was fast, and the collagen fibres were stained deeply with a clear texture and orderly arrangement.
In this study, LPS and IL-4 were used to induce macrophages to transform into M1 and M2. LPS is a component of the cell wall of gram-negative bacteria, and a pathogen associated molecular pattern (PAMP) that polarised macrophages to the M1 via pattern recognition receptor (PRR)-mediated signal transduction. Toll-like receptor 4 (TLR4) is a type of PRR mainly expressed on macrophages. When LPS binds to CD14 molecules on the surface of macrophages through LPs-binding protein (LBP), TLR4 on the cell surface is stimulated to form homologous dimers and initiate TLR4 activation signals. Activation activates myeloid differentiation protein 8 (MyD88)-dependent intracellular signal transduction, and with the involvement of serine/threonine kinase-IL-1 receptor-associated kinase (IRAK) and TNF receptor-associated factor 6 (TRAF6), Through signalling cascades, such as NF-B, signals are transferred into the nucleus of the macrophage and relevant target gene transcription is initiated, thus inducing macrophages to be M1. IL-4, a glycoprotein of molecular weight 18 to 19 KU, is produced by antigen- or mitogen-stimulated CD4+ T cells. When IL-4R, an IL-4 receptor on the surface of macrophages, recognises the presence of IL-4, IL-4R quickly binds to IL-4 to form a type I receptor. By activating JAK1 and JAK3 in the JAK kinase family, insulin receptor substrate (IRS) is recruited and forms a complex with it. The complex binds to Signal Transducers and Activators of Transcription 6 (STAT6) and promotes the tyrosine phosphorylation of STAT6. Phosphorylated STAT6 enters the nucleus through the nuclear envelope and transmits signals to relevant nuclear transcription factors, thereby inducing macrophages to become M2.
To summarise our findings, M-CSF can induce the differentiation of bone marrow cells into macrophages in vitro, which can then be polarised to the M1 and M2 by LPS and IL-4, respectively. Exogenous M1 infusion delayed wound healing in the rat model, whereas exogenous M2 infusion promoted the same. Our findings confirm that the activation and polarisation of macrophages are closely related to wound healing. However, the underlying molecular mechanisms remain to be elucidated and will be the focus of our subsequent studies.
Acknowledgements: All applicable institutional and/or national guidelines for the care and use of animals were followed. We would like to thank TopEdit (www.topeditsci.com) for the English language editing of this manuscript.
ACKNOWLEDGMENTS
All applicable institutional and/or national guidelines for the care and use of animals were followed.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
This study has been approved by Medical Ethics Committee of the Affiliated Hospital of Qingdao University (QYFYYXLL30016).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




