Within-patient randomised clinical trial exploring the development of microskin implantation in the treatment of pressure ulcers
Abstract
Pressure injury often seriously affects the life quality of aged patients, especially the long-term bedridden casualties. Widely adopted by different disciplines, negative pressure suction has its role in pressure injury. Microskin implantation has been demonstrated powerful in increasing the expansion ratio of donor area-derived skin and accelerating wound healing by forming “skin islands”. The study was designed to evaluate the efficacy and safety of additional use of bedside microskin implantation in the palliative care of pressure injury of aged patients who cannot tolerate surgical treatment as a supplement for standard negative pressure suction. An open-label within-patient RCT was conducted in aged patients with pressure injury. Sixteen patients were enrolled. After granulation tissues formed, half of a pressure injury was randomised to receive the negative pressure suction as the control group, and the other half exposed to additional bedside microskin implantation as the experimental group. Efficacy was evaluated within 1 month after treatment, and the primary endpoints included the wound healing rate and pressure ulcer scale for healing (PUSH) scores. The secondary outcomes included survival rate of implanted microskin, pain intensity assessment, satisfaction surveys from patients or their family, and pressure ulcer healing complications. Sixteen patients completed the study. After 14 days of operation, 5.63 ± 1.78 out of 10 pieces of implanted microskin survived and formed neonatal epithelium. The wound healing rates of the control group and the experimental group at 1 month were (26.17 ± 9.03%) and (35.95 ± 16.02%), respectively (P < .01). The mean PUSH score before the surgery was 12.38 ± 2.23. At 1 month after surgery, the mean difference of PUSH score from baseline was 2.13 ± 0.96 in the control group and 2.81 ± 0.83 in the experimental group (P < .01). The treatment of microskin implantation did not cause additional pain or complications to the patients. Accompanied by a better ulcer status, the majority of patients or their guardians have a high degree of acceptance towards the microskin implantation. Bedside microskin implantation could accelerate wound healing with lower PUSH scores. As a complementary palliative treatment, supplementary microskin implantation is effective and well tolerated.
1 BACKGROUND
With social progress and economic development, population aging is one of the main problems of the current social population structure.1, 2 Meanwhile, the prevention and treatment of aging-related diseases and accompanied complications have also gradually become a hot spot in medical research.3, 4 Due to poor health conditions of the elderly, accompanied by various underlying diseases and chronic diseases, the aged individuals often spend a lot of time in bed.5-7 Pressure injury is a common clinical disease caused by continuous pressure on the skin tissues with bone protrusions. Patients who have been bedridden for a long time are prone to pressure injury if they do not turn over frequently.8 The initial manifestation of pressure injury is congestion in the compressed area, followed by blisters, ulcers, or even local necrosis, and so forth, which may lead to amputation or even mortality of patients.
Timely treatment of pressure injury is very important for elderly patients, which could effectively prevent further expansion of the wound, reduce the probability of infection, and effectively prolong the prognosis of the patients.9 For patients with indications for surgery, surgical treatment is the most efficient method to seal the wounds, like flap grafting and autologous skin grafting. However, due to the existence of underlying diseases such as hypertension or diabetes, some elderly individuals may not be able to tolerate surgery or anaesthesia. Meanwhile, along with aging, the skin repair and regeneration capabilities are reduced, and traumatic operations, such as full-thickness skin grafts or flap operations, may lead to the formation of new hard-to-heal wounds.10 In addition, some elderly patients and their families may prefer conservative treatment to surgery based on factors like humanistic welfare or hospice care. In view of the above factors, non-surgical treatment or palliative care is very meaningful for the elderly suffering pressure injury.11 Finding a safe and feasible treatment of pressure ulcer that is suitable for those elderly patients may help improve the life quality of elderly patients, shorten hospital stays, and save treatment costs.12
In current clinical treatment, an effective treatment of pressure injury in elderly patients is the application of negative pressure suction after debridement.13, 14 The negative pressure suction can effectively reduce the level of exudation and inflammation in the pressure ulcer, with the growth of granulation tissue greatly promoted. But the re-epithelialization after negative pressure suction still simply relies on the crawling of the peri-wound epithelium to seal the wound, which could not effectively solve the problem of taking too long to seal the wounds. There are still underlying potential possibilities to get faster wound healing rates of the wound. Microskin transplantation is a technique commonly used in the treatment of large-area burn patients.15 By planting the skin in the form of microskin on the wound surface, a large number of “skin islands” can be formed, whose proliferation and migration can significantly accelerate the wound healing. Inspired by this, we applied this technique for pressure treatment. To further improve the survival rate of skin grafting, we put the sterile syringe needle obliquely through the granulation tissue to the fibrous plate, and then implanted the microskin with the dermis side down along the puncture tract, which was named as the microskin implantation technique.16 This prospective study aimed to explore the efficacy and safety of bedside microskin implantation combined with negative pressure suction in the treatment of pressure injury in elderly patients, and aimed to provide new treatment methods and ideas for the palliative care of pressure injury.
2 MATERIALS AND METHODS
2.1 Clinical data
A prospective, open-label, within-patient RCT was conducted in the Fourth Medical Center and Sixth Medical Center of PLA General Hospital, Beijing between August 2016 and July 2021. The within-patient methodology excludes patient-related confounders. Elderly patients suffering with pressure injury were eligible to participate and 16 patients were finally enrolled in the study. The study was approved by Ethical Review Committee of PLA General Hospital. Patients were informed with the contents of the experiment with informed consent signed before undergoing treatments. This study was performed in accordance with the Declaration of Helsinki, guidelines for Good Clinical Practice, and the CONSORT statement for reporting within-person randomised trials.
2.2 Inclusion and exclusion criteria
Inclusion criteria: Subjects would be included in the study if they meet the following criteria: age greater than 60 years; at least one pressure ulcer was in stage III or IV according to European Pressure Ulcer Advisory Panel/National Pressure Ulcer Advisory Panel (NPUAP) guidelines; there is a contraindication to surgery; the patient or their family refuses to undergo surgery.
Exclusion criteria: Patients were excluded as following conditions: excessive smoking (22 or more cigarettes per day); receiving other treatments that may interfere with normal wound healing, such as radiation therapy, corticosteroids, or chemotherapy; suffering from peripheral vascular disease, malignant tumours, or acute infections; diabetes with poor blood sugar control.
2.3 Randomization and allocation concealment
Stratified randomization schedule was conducted by a statistician of the Fourth Medical Center of PLA General Hospital using the software of Statistical Analysis System (SAS) package (Version 9.1.3; SAS Institute Inc., Cary, NC). Using the self-control method, the selected pressure injury of enrolled patients was equally allocated into two parts, then randomly assigned to the control group or the experimental group for treatment and observation within 1 month. The randomization allocation was revealed to the doctors just before application of the treatment. The allocation concealment was blinded to the outcome assessors, and data analysts. All clinical treatment effect assessments performed by the same clinician. This study was set up as an open-label study because of the highly distinguishable features of the two treatments.
2.4 Intervention
Since the patient's admission, the duration of bed rest was strictly controlled with posture changed every 2 h, to avoid further formation or deterioration of pressure injury, supplemented by an air mattress or suspension bed. The nutritional status of patients was improved and underlying diseases were symptomatically treated. In addition, medications for pain control and infection prevention were also applied. As for pain control, patients were given ibuprofen 200 mg (1 tablet) each time for every 8 h as needed. As for infection prevention, povidone iodine (Betadine solution) followed by cleanse with saline gauze was used to disinfect the wound before further intervention. After debridement at the bedside, the pressure injury was sealed with a transparent dressing sufficed to cover the wound area and attached with a suction drain (−80 ~ −100 mmHg). The dressing and suction drain were replaced every 5 days or after contamination.
Further microskin implantation experiments were performed when the ulcers met the following conditions: (1) the formation of qualified granulation tissue, (2) no obvious necrotic tissue on the wound, (3) no purulent exudation and odour, (4) the emergence of new epithelium around the wound. Then, the ulcers were first disinfected and then initiated for further randomization and interventions (Figure 1). After taking pictures of the wound with a digital camera, we measured the area of the wound with ImageJ software and then equalised the wound into two parts. Although we may not be able to ensure the complete consistence of the wound in shape, we will pay attention to the fact that the areas on both sides of the wound were the same, and the basic situation were similar. As for the randomised-selected experimental group, after local anaesthesia with 10 mL lidocaine solution (2.5 g/L), the ~10 × 10 mm razor-thin graft was obtained from the outer side of thigh, which was then bandaged properly. Then, the obtained graft was cut into 10 small skin pieces of ~2 × 5 mm size, namely the microskin, then wrapped in saline-soaked gauze. A sterile 18G syringe needle was pierced obliquely into the granulation tissue to prepare the tunnel for microskin grafting. After the 30G syringe needle was blunted by hemostatic forceps, it was then used to obliquely implant the microskin pieces with the dermis side down into the granulation tissue along the previously prepared tunnel, with an interval of approximate 1–2 cm. Vaseline gauze of 1 × 1 cm were covered on the planting sites to ensure that the implanted microskin stayed in place to avoid interference from negative pressure suction and dressing replacement. After the bedside microskin implantation in the experimental sides, the whole ulcers were covered fully with dressings and negative pressure suction following protocols mentioned above.
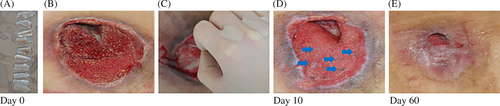
In the baseline period, 14 days, and 1 month after the operation, the efficacy and safety of microskin implantation were assessed. Outcomes of wound-healing rate, pressure ulcer scale for healing (PUSH) scores, pain intensity, questionnaire of satisfaction, and wound-healing complications within the 1 months were analysed by professionals blinded to the group allocation. In addition, if a patient had more than 1 ulcer, only the largest pressure ulcer was included in the study, all the other ulcers were treated by only the negative pressure suction. The study protocol was outlined in Figure 2.
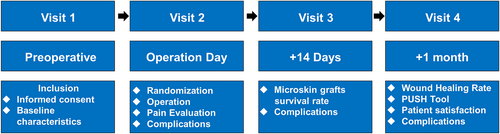
2.5 Treatment effect evaluation
2.5.1 Primary outcomes
The curative effect was evaluated by the wound-healing rate and PUSH scores. The wound-healing rate between the groups were quantified by using digital images taken 1 month after treatment and evaluated with ImageJ software. With respect to area, exudate, and the type, the PUSH scores were developed by NPUAP as a quick, reliable tool to monitor the change in pressure ulcer status.17
2.5.2 Additional outcomes
Additional outcomes included the survival of microskin, Wong-Baker FACES pain rating scale (WBFPS), a questionnaire of satisfaction from patients or their families, and wound-healing complications within 1 month after treatment. The pain intensity during the procedure was assessed by doctors using the 10-point WBFPS (0, absence of pain; 10, the worst pain imaginable).18 A questionnaire was administered on 1 month postoperative to investigate the acceptance towards the microskin implantation. Wound-healing complications were recorded to assess safety. Complication was defined a priori as the presence of at least one of the following conditions: blood oozing or wound infection. Blood oozing was defined as bleeding in the donor area or pressure injury area requiring additional treatment. Wound infection was defined as occurrence of any one of the following symptoms: warmth, erythema, purulent discharge, or positive culture swab. Also, other indications for wound infection were noticed: (1) impaired healing despite treatment, (2) edematous, friable, or discoloured (i.e., pale and dusky, as opposed to healthy beefy-red) granulation tissue, (3) pocketing or recessed areas of wound, (4) wound breakdown, (5) foul odour, and (6) increased pain.
2.6 Statistical analyses
Descriptive statistics were used for demographic and perioperative data. Normally distributed data are presented as mean ± standard deviation and were tested using the paired t test or one-way ANOVA test. Non-normally distributed or ordinal data were presented in quartiles, whose paired data were tested using the Wilcoxon signed-rank test for continuous outcomes, and the McNemar's test for paired dichotomous values. The analysis was performed in a blinded fashion. For all statistical tests, the 0.05 level of confidence was accepted as a significant difference.
3 RESULTS
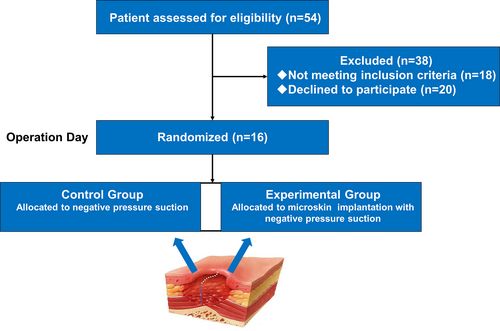
As shown in the Figure 3, 54 patients were initially recruited in the study. Of these 54 participants, 18 individuals did not meet the inclusion criteria, and 20 refused to participate in the study. Therefore, 16 patients finally participated in the clinical trial.
Characteristics of all included participants at the baseline were summarised in Table 1. All patients were Chinese with 4 women and 12 men. At the baseline, the mean age was 75.50 ± 10.17 years old. The stages of pressure injury were III (1 man and 1 woman, 12.50%) and IV (11 men and 3 women, 87.50%), respectively.
| No. | Age (years) | Gender | Ulcer amount | Ulcer status | Comorbidities (major conditions listed) | Wound size (cm2) before grafting | Wound size (cm2) of the Control Group | Wound size (cm2) of the experimental group | Wound healing rate (%) (control group/experimental group) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | Male | 2 | IV | Paraplegia | 111.2 | 42.7 | 39.3 | 23.23/29.40% |
| 2 | 62 | Male | 3 | III | Paraplegia | 63.0 | 20.7 | 15.4 | 34.31/51.10% |
| 3 | 65 | Male | 1 | IV | Vegetative state | 196.6 | 76.2 | 70.3 | 22.53/28.45% |
| 4 | 66 | Male | 6 | IV | Paraplegia | 176.8 | 67.6 | 61.6 | 23.48/30.31% |
| 5 | 66 | Male | 4 | IV | Paraplegia | 77.4 | 26.4 | 21.1 | 31.90/45.44% |
| 6 | 73 | Male | 4 | IV | Cerebral haemorrhage | 97.8 | 41.0 | 39.7 | 16.25/18.85% |
| 7 | 73 | Male | 3 | IV | Paraplegia | 574.8 | 249.3 | 242.6 | 13.24/15.59% |
| 8 | 75 | Female | 3 | IV | Paraplegia | 73.6 | 21.1 | 11.4 | 42.59/69.14% |
| 9 | 75 | Male | 1 | IV | Paraplegia | 90.8 | 30.1 | 24.3 | 33.69/46.40% |
| 10 | 77 | Male | 1 | IV | Paraplegia | 165 | 64.1 | 58.0 | 22.27/29.68% |
| 11 | 79 | Female | 1 | IV | Cerebral thrombosis, hip replacement | 49.0 | 14.4 | 8.9 | 41.33/63.62% |
| 12 | 81 | Male | 1 | IV | Brain atrophy | 145.2 | 62.4 | 60.9 | 14.04/16.07% |
| 13 | 86 | Female | 2 | III | Left femoral neck fracture | 89.6 | 32.0 | 27.8 | 28.56/37.85% |
| 14 | 87 | Male | 1 | IV | Lumbar fractures | 192.4 | 77.9 | 73.4 | 19.02/23.68% |
| 15 | 88 | Male | 1 | IV | Parkinson's disease | 179.4 | 70.9 | 65.6 | 21.00/26.84% |
| 16 | 95 | Female | 1 | IV | Parkinson's disease | 97.6 | 33.5 | 27.9 | 31.34/42.75% |
3.1 Primary outcome
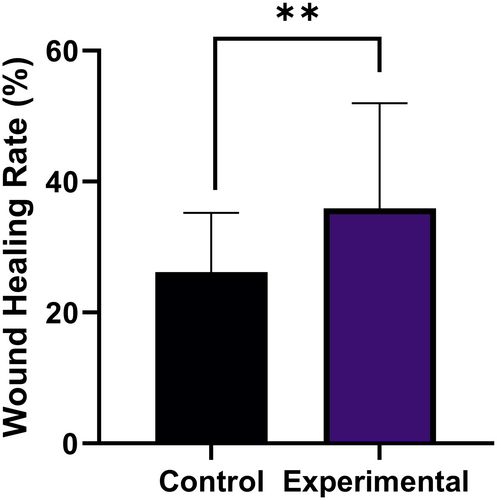
At 1-month follow-up, the wound-healing rate was (26.17 ± 9.0%) versus (35.95 ± 16.02%), respectively in the control sides and the experimental sides (P < .01, Figure 4).
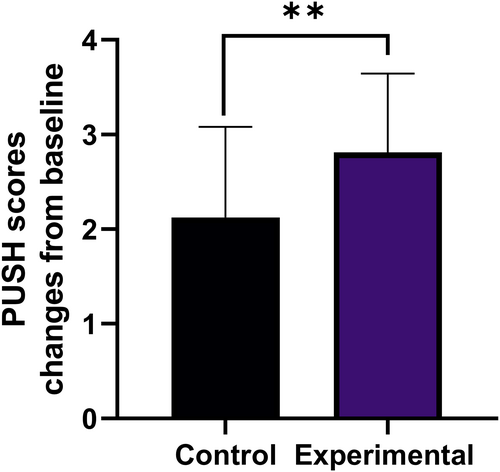
At the baseline, the mean PUSH scores were 12.38 ± 2.23. At 1 month after surgery, the mean difference of PUSH score from baseline was 2.13 ± 0.96 in the control group and 2.81 ± 0.83 in the experimental group (P < .01, Figure 5).
3.2 Additional outcomes
3.2.1 Survival rate of implanted microskin
Evaluated 14 days after the procedure, the survival rate of the implanted microskin was 5.63 ± 1.78 out of 10.
3.3 Pain
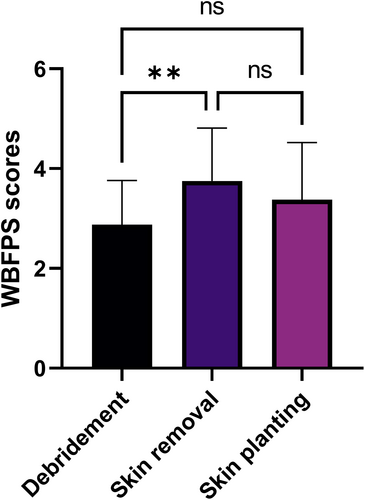
In view of the fact that some patients lost the expression ability due to underlying diseases, WBFPS were applied to evaluate the degree of pain based on the patient's expression during the operation. According to the WBFPS scoring standard, the degree of pain was evaluated first at the time of debridement, then the time of skin removal, and finally the time of implantation of microskin into granulation tissues. The patient's pain level during skin removal (3.75 ± 1.07) was slightly higher than that of debridement (2.88 ± 0.89, P < .01). However, there was no statistical difference in the pain scores between debridement and skin planting (3.38 ± 1.15, P > .05, Figure 6).
3.4 Patient satisfaction
Patient satisfaction outcomes ascribed to the experimental sides were summarised in Figure 7. A majority of patients or their families (11 cases) believed that the bedside microskin implantation was going to make more of a difference than the ordinary treatment. In the experience of three cases, application of the bedside microskin implantation was somewhat bothersome, two of them were troubled by the concerns about the necessity of the treatment and the possible hazards associated with skin removal operation.
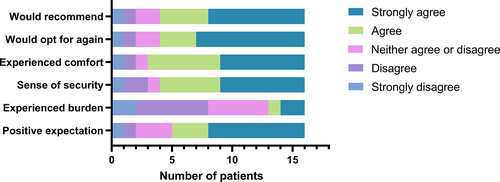
A majority of cases (12, 75.00%) stated that the bedside microskin implantation felt secure and effective and would opt for this treatment again if necessary.
3.5 Complications
During the operation in the experimental side, there may be slight bleeding when puncturing the granulation tissue with syringe needles, which can be properly solved by gauze compression, and no more bleeding was seen after the placement of the negative pressure drainage.
After the ~10 × 10 mm split-thickness autologous skin was obtained, all the skin donor sites healed effectively within 14 days after the operation, with no adverse events or delayed healing recorded.
During the 1-month follow-up, there were neither no complications such as bleeding or wound infection in both the experimental sides and control sides of the pressure injury, nor other injuries related to the intervention or medical device.
4 DISCUSSION
Posing extensive damages to the skin and body, pressure injury is widespread chronic wounds, whose recovery time often exceeds 3 months.19, 20 The incidence of pressure injury will possibly grow rapidly in the future due to the increased life expectancy and aging society. Meanwhile, the average age of patients with pressure injury has gradually increased, with worse general condition and decreased physical function. For grade III-IV pressure injury with surgical indications, surgical debridement and surgical reconstruction can greatly help shorten the time of wound healing and hospitalisation.21-23 Some elderly patients with pressure injury may not be able to tolerate surgical treatment due to the high risks of surgery and anaesthesia. Furthermore, the ability of wound healing slows down with aging, thus the healing status of wounds in elderly patients with pressure injury after surgery has become a new problem that plagues doctors' treatment choice.24, 25
Currently, the application of negative pressure suction is widely-accepted as a good non-surgical treatment or preoperative preparation for pressure injury.26 With the application of negative pressure suction on wounds, a growing body of evidence has demonstrated its superiority in the aspects of improving blood flow, decreasing oedema, diminishing lateral and shear stress on wound site, and increasing lymphatic clearance.27 Significant associations with lower rates of wound complications and better efficacy have been shown in meta-analyses and reviews for negative pressure suction in wound healing of pressure injury, but outcomes vary for the treatment of the elderly, awaiting further improvements.28
Mainly for patients with extensive burns, microskin transplantation is developed for patients' shortage of sufficient skin donor areas.29 By cutting the patient's autologous skin into very small particles followed by uniformly transplanted on the wound surface, microskin transplantation can increase the expansion ratio of skins up to 20 times, by forming numerous “skin islands” on the wound surface.30 Based on the “skin islands”, epidermal cells and fibroblasts can proliferate and crawl, thus accelerate the closure of wounds. Several clinical trials were conducted and had demonstrated the efficacy of microskin transplantation on burn wound healing.15 In view of this, we considered the possibilities of microskin transplantation in the treatment of aged patients with pressure injury.
Skin with a proliferating and migrating mood in the wound edge was often wasted during the debridement of the pressure injury. To solve this problem, we made full use of the wound edge skin in the form of the microskin, and sticked them on the wound surface, which achieved certain therapeutic effects. However, due to the poor condition of the wound surface, the microskin adhered to the wound surface often had a relatively low survival rate. Therefore, we further improved the transplantation method and explored whether the implantation of microskin into the wound fiberboard could improve the survival rate of the microskin and the healing rate of pressure injury.16 The average survival rate of skin grafts was 5.63 ± 1.78 out of 10. The relatively small standard deviation indicated that the survival rate of microskin implantation was relatively stable. From our clinical experience, the quality of the obtained skin grafts and wound granulation tissue were also the key to determine the survival rate of the microskin after implantation. In addition, the basic status of the patients should also be paid attention to, such as the nutritional status.
As the first randomised controlled trial to evaluate the efficacy and safety of microskin implantation for treating pressure injury in China, the present study showed that the microskin grafts after implantation had a relative high quality of survive and the activity of expanding and sealing the wound surface. The additional use of bedside microskin implantation would accelerate wound healing without extra wound-healing complications or security issues. With accelerated wound-healing rate, aged patients could reduce their time trapped in bed, thereby reduce related complications and improve life quality and survival time.11 Meanwhile, faster healing process and well-healed wounds can also reduce the psychological burden of the elderly and their families, as well as the financial pressure from nursing costs, hospitalisation costs to the missed work costs, and so forth Microskin implantation did not expose additional pain or discomfort to the patients, and was accepted by the majority. The additional microskin implantation is proved to be a reliable and worthwhile palliative care method for aged patients with pressure injury.
The study has some limitations inherent to open-label studies, which include possible performance and observer bias. Nonblinded questionnaires completed by participants are subject to the same types of bias. Ideally, both patient and observer would be blinded, but this proved impossible logistically. Furthermore, the sample size of this clinical trial is small, and further multi-center trials with larger sample sizes and longer follow-up are urgently needed for higher credibility. In clinical practice, the transplantation details need further improvement and clarification, from the transplantation methods, spacing, frequency, to the skin source, so as to summarise a more complete and efficient treatment plan. Finally, this study did not have a long-term follow-up. Therefore, the efficacy of longer term after treatment is needed to be investigated in future studies.
5 CONCLUSION
In this within-patient RCT, additional microskin implantation was compared with the solely application of negative pressure suction in the treatment of pressure injury of the aged. This study provides new insights into the usage of microskin implantation as supplements for negative pressure suction drains with regard to better wound-healing progress in the management of pressure injury of the aged.
AUTHOR CONTRIBUTIONS
Chuan'an Shen conceptualised the study and designed experiments. Ming Zhang and Jiachen Sun wrote the manuscript. Tianjun Sun help complete the main work in the revision of the manuscript. Ming Zhang, Minhui Zhu, Zhiyuan Shi, Lu Zhang, Xingtong Wang, Yaoyao Song, Xiangbo Ye, and Wanli Chu performed the surgeries for the patients. Yuanxin Deng critically reviewed the manuscript.
ACKNOWLEDGEMENTS
Thanks to the involved patients for the study.
FUNDING INFORMATION
This study was supported from Military Logistics Scientific Research Project Health Special Project (22BJZ35, 21BJZ29), the 13th Five-year Plan for Key Discipline Construction Project of PLA (A350109), the Program of National Natural Science Foundation of China (82072169), and Beijing Haidian District Health Development Scientific Research Cultivation Program (HP2021-04-80 502).
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICS STATEMENT
This study was approved by the Ethics Committee of General Hospital of PLA.
INFORMED CONSENT
We obtained the written informed consent from all the patients participated in this study.
Open Research
DATA AVAILABILITY STATEMENT
Further information and requests for reagents may be directed to and will be fulfilled by the Lead Contact: Chuanan Shen ([email protected]).




