Improved healing of chronic diabetic foot wounds in a prospective randomised controlled multi-centre clinical trial with a microvascular tissue allograft
Funding information: MicroVascular Tissues, Inc.
Abstract
This study assesses the impact of a processed microvascular tissue (PMVT) allograft on wound closure and healing in a prospective, single-blinded, multi-centre, randomised controlled clinical trial of 100 subjects with Wagner Grade 1 and 2 chronic neuropathic diabetic foot ulcerations. In addition to standard wound care, including standardised offloading, the treatment arm received PMVT while the control arm received a collagen alginate dressing. The primary endpoint was complete wound closure at 12 weeks. Secondary endpoints assessed on all subjects were percent wound area reduction, time to healing, and local neuropathy. Novel exploratory sub-studies were conducted for wound area perfusion and changes in regional neuropathy. Weekly application of PMVT resulted in increased complete wound closure at 12 weeks (74% vs 38%; P = .0003), greater percent wound area reduction from weeks four through 12 (76% vs 24%; P = .009), decreased time to healing (54 days vs 64 days; P = .009), and improved local neuropathy (118% vs 11%; P = .028) compared with the control arm. Enhanced perfusion and improved regional neuropathy were demonstrated in the sub-studies. In conclusion, this study demonstrated increased complete healing with PMVT and supports its use in treating non-healing DFUs. The observed benefit of PMVT on the exploratory regional neuropathy and perfusion endpoints warrants further study.
1 INTRODUCTION
The economic burden of diabetic foot ulcers (DFUs) totals over $60 billion U.S. dollars annually.1-5 As a complication of non-healing or infected diabetes-related wounds, three lower extremity amputations occur every minute worldwide.6 Despite availability of advanced wound therapeutics, the 5-year mortality after amputation from a DFU remains over 45%.7 Given that roughly 40% of patients with a DFU have a wound recurrence within 1 year after ulcer healing, and 65% within 5 years, critical focus is needed on achieving wound closure as well as wound remission without recurrence.1 Prior history of a diabetic foot ulceration is the single greatest predictor of recurrent DFUs in the context of peripheral neuropathy, bony prominences, repetitive microtrauma, and reduced tensile strength in the scarred tissue.1, 8, 9 Non-surgical and surgical methods have been used to reduce risk, although long-term outcomes of non-surgical methods are not encouraging as they depend on patient engagement with routine foot care and shoe gear use.10-13 Surgical interventions to improve sensation, reduce offending bony deformities and improve biomechanics may be beneficial; however, the data supporting long-term outcomes is limited.
Microvascular dysfunction and peripheral neuropathy are two of the most common complications of diabetes mellitus, leading to ulceration. Hyperglycaemia itself leads to vascular changes including endothelial dysfunction, hyperpermeability, decreased blood flow, and tissue hypoxia. Vascular defects involving the vasa nervorum contribute to diabetic neuropathy,14-19 which causes the insensate foot and undetected injury.1, 20-22 Restoring the microvasculature in the non-healing diabetic wound environment is essential for complete healing. The microvasculature is composed of small blood vessels (arterioles, capillaries, and venules), extracellular matrix proteins that form the basement membrane and vessel structure and serve as a reservoir for modulation of cellular activity, and inherent cells (multipotent cells, endothelial cells, pericytes, fibroblasts, and smooth muscle cells).23, 24 A healthy microvascular tissue structure provides nutrient and oxygen delivery and removal of waste metabolites, which is critical for tissue function and survival.23-25 Formation of a new vascular and neural network following skin and soft tissue injury is critical for wound resolution beneath complete epithelialisation of the skin defect. New capillaries can sprout from the pre-existing vasculature at the time of injury and organise into functional vascular networks, so microvessel elements have been hypothesised to have the ability to promote angiogenesis and vascular repair as well as orchestrate signals of the wound healing cascade.23-29 This capability has been shown in laboratory studies demonstrating high endothelial proliferation rates and pro-angiogenic effects of microvascular tissue fragments derived from allogeneic cell and tissue sources.28-30
Processed microvascular tissue (PMVT) is a microvascular tissue structural allograft (mVASC®, MicroVascular Tissues, Inc. [MVT], San Diego, CA) comprised of structural elements (small blood vessels and extracellular matrix), inherent non-viable cells, and associated biological signalling factors harvested from the subcutaneous tissue of the thighs, abdomen, and buttocks of cadaveric human donors. PMVT is isolated through a proprietary process that involves cutting, cleaning, extraction, lyophilisation, and sterilisation of the harvested tissue. Characterisation of PMVT confirmed that structural and biologic factors intrinsic to microvascular tissue, including angiogenic and neurotrophic factors, are preserved.30 Preclinical studies of PMVT demonstrated induction of angiogenesis and significantly increased healing rates in rodent models of pressure injury and ischaemia.30, 31 In an SCID mouse model of hindlimb ischaemia, mean perfusion rates increased from 35% to 82% at Day 14 with the PMVT allograft treatment compared with an increase from 31% to 59% for the control.30 Initial clinical applications of PMVT as a topical intervention in complex, recalcitrant, or senescent wounds of other aetiologies have demonstrated its ability to stimulate durable wound closure out to 9 months, even in cases where other advanced biologic therapies had failed.30, 32, 33
In a small case series of patients with DFUs that had failed to heal with previous advanced wound care treatment, weekly application of PMVT allograft resulted in wound closure, improved wound perfusion, and improved lower extremity peripheral sensation.32 This provided the basis for the treatment regimen and analytic techniques used in the randomised controlled trial presented here. The purpose of this clinical study, named the HIFLO (Healing in Diabetic Foot Ulcers with Microvascular Tissue) Trial, was to evaluate the hypothesis that this PMVT allograft would improve wound healing and quality of tissue repair in non-healing DFUs compared with a control arm using a typical collagen alginate (CCA) dressing.
2 MATERIALS AND METHODS
2.1 Clinical study design and materials
The HIFLO Trial was an IRB-approved prospective, single-blinded, multi-centre, randomised controlled clinical trial evaluating patient outcomes after weekly application of PMVT allograft in addition to a standardised diabetic foot ulcer protocol in the treatment of 100 patients with Wagner Grade 1 and 2 DFUs of ≥4 weeks duration, compared with standard wound care with a typical CCA dressing control (ISRCTN #24783859, Western IRB study #1175398 protocol #20171089, and South Shore reference #17-013). The study was conducted at six clinical sites within the United States in accordance with Good Clinical Practice (GCP) requirements. Subjects were eligible for study inclusion if they met all inclusion and exclusion criteria (Table 1). A 14-day run-in period was used to validate the non-healing nature of each subject's index wound, during which all screened subjects received standard wound treatments including wound cleansing, focal debridement, primary collagen calcium alginate dressing (Fibracol™ Plus Collagen Wound Dressing with Alginate, Acelity, St. Paul, MN), secondary three-layer dressing (DYNA-FLEX Multi-Layer Compression System, Acelity, St. Paul, MN) with felt padding, and offloading of the foot. Subjects with <20% reduction in wound area after this run-in period were deemed eligible for study inclusion. A sealed envelope technique, in which the envelopes contained a random allocation sequence, was used to perform a 1:1 randomisation of eligible subjects to the control arm or PMVT. Randomisation was performed in 10-subject blocks to achieve balanced assignment of treatments, and clinical investigators were only informed of the randomisation assignment at the time of each individual patient's initial treatment visit. No changes were made with respect to study methods or eligibility criteria after commencement of the trial.
| Inclusion criteria |
|---|
|
|
|
|
| Exclusion criteria |
|---|
|
|
|
|
|
|
|
|
PMVT is a structural allograft consisting of microvascular tissue fragments harvested from eligible non-diabetic donors younger than 65 years in age who have undergone donor screening and testing in accordance with US Food and Drug Administration (US FDA) regulations and American Association of Tissue Banks® (AATB) standards in order to assure safety and quality. Each ready-to-use vial of PMVT contains a sterilised, lyophilised disk with at least 500 000 microvascular tissue fragments, and is stable at room temperature for up to 5 years. In accordance with US FDA human cellular and tissue-based product (HCT/P) regulations, the PMVT allograft is indicated for the repair, reconstruction, replacement, or supplementation of microvascular tissue.
2.2 Treatment and application
All subjects were seen on a weekly basis and underwent cleansing of the index DFU with either sterile saline or water and sharp debridement of the index ulcer with a scalpel and/or curette when indicated. All subjects received the same secondary three-layer dressing with felt padding. The only difference between the two groups was the primary dressing: a collagen calcium alginate dressing was applied weekly to the index ulcer in the control group versus weekly PMVT allograft covered with a non-adherent dressing (Adaptic Touch™, Acelity, St. Paul, MN). Offloading, which can be one of the biggest confounding variables in the outcome of clinical trials in DFUs, was standardised across all study sites and subjects, and was achieved using a DARCO diabetic cam boot with a tri-laminar insert (DARCO International, Inc., Huntington, WV) or equivalent. The quantity of PMVT allograft applied to the ulcer was based on the wound surface area, with application of one-half disk of PMVT for every 1.5 cm2 of wound area.
2.3 Assessments
Weekly assessments for all subjects included wound and local peripheral neuropathy assessment. Wound measurement assessment was performed using the eKare InSight® System (eKare Inc., Fairfax, VA), a 3D digital infrared imaging technology connected to an iPad tablet to scan the wound topography and accurately capture wound images. Calibrated software then translates the wound image into an accurate measurement of the wound area. In addition, for the purposes of blinded adjudication of the closed wounds, high resolution photographs were taken at each visit using identical Sony 20.1 Megapixel cameras at each site.
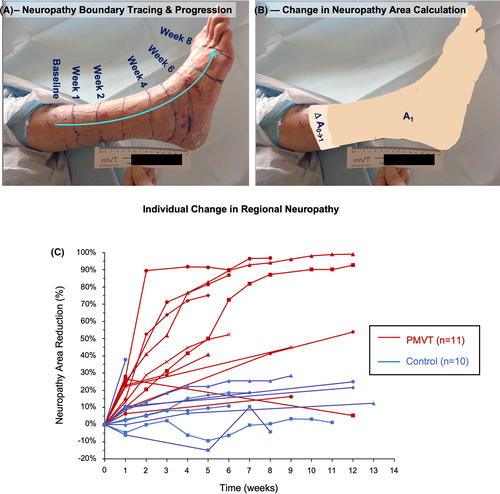
Local peripheral neuropathy was assessed with the standard 10-point Semmes-Weinstein monofilament (SWM) exam using a 5.07 gauge/10 g target force monofilament pressed against the predefined 10 areas of the patient's foot.34, 35 For exploratory evaluation of regional peripheral neuropathy, a technique mapping the stocking glove pattern of neuropathy36 was conducted on the first consecutive21 patients (PMVT = 11; control = 10) at two pre-selected study sites. This technique used the same monofilament to mark the boundary of sensation on the lower extremity at multiple treatment visits. This boundary was outlined with a permanent marking pen and photographs of the lateral leg and plantar foot were taken, and changes to the neuropathy area over time were recorded. Digital contour tracing from the boundary of sensation to the base of the foot allowed the three-dimensional area of neuropathy to be calculated using ImageJ image analysis software. In both SWM and stocking glove assessments, subjects were prevented from visually observing the monofilament being pressed against their foot and lower limb.
For an evaluation of tissue perfusion, the first10 consecutive subjects (PMVT = 5; control = 5) at one study site also underwent microcirculation analysis at the baseline visit, at 1 week, and at wound resolution or end of the study period, whichever occurred first. Microcirculation assessment was performed using indocyanine green fluorescence microangiography (ICGFA) using the LUNA device (Stryker Corp., Kalamazoo, MI), which provides real-time visualisation and objective assessment of tissue perfusion to a specific area of concern.37 Average ingress rate was measured at the centre of the wound bed and in the wound periphery, no more than 1 cm from the wound margin, at the 1, 4, 7, and 10 O'clock positions using the maximum observed ICG intensity as the injection was monitored in real time. Dynamic image capture from dye ingress to egress and measurements taken within this period were performed in the same fashion for each imaging sequence to allow for serial analysis. Blinded evaluation of ICGFA video and images were performed by three independent clinicians from different institutions with expertise using the LUNA system for tissue perfusion in DFUs.
2.4 Study endpoints
The primary endpoint of the study was complete wound closure at 12 weeks. FDA guidance defines complete wound closure as a wound that has reepithelialised without drainage or dressing requirements confirmed at two consecutive study visits 2 weeks apart. We expanded upon the US FDA guidance38 by using a recently proposed standard definition that includes four criteria for complete closure: (a) 100% epithelialisation, (b) normal coloration with no marginal recurrence, (c) complete absence of exudate, and (d) no clinical signs of infection.39 The investigator and a physician blinded to the subject's care made the initial determination of wound closure, followed by adjudication and confirmation of closure by a panel of three independent blinded plastic surgeons with >10 years of experience in wound care. In order to avoid the introduction of bias, the adjudicators only saw blinded images of the “closed” wound and evaluated them based on the four criteria. If an image was unclear, adjudicators could request additional images because several were taken at each visit. Wound closure was documented 2 weeks after adjudication at the healing confirmation visit. Secondary endpoints included the percent wound area reduction (PAR) at 4, 6, 8, and 12 weeks, time to healing, and local neuropathy. Exploratory endpoints were changes in wound perfusion and regional peripheral neuropathy.
2.5 Statistical analysis
Continuous data were described at baseline and follow-up using descriptive statistics (mean, SD, median, minimum, and maximum), while categorical variables were characterised as counts and proportions or percentages. The intent-to-treat (ITT) population included all subjects who attended at least one treatment visit. All analyses were performed on the ITT population only.40 Subjects who were lost to follow-up were included in the ITT analysis of primary and secondary endpoints using last observation carried forward (LOCF) principles to impute missing data.41 The study was designed to detect a difference of 0.3 between control and PMVT (proportions healed 0.4 and 0.7, respectively) with 50 subjects in each group and a statistical power of 88% (Pass 13 software). A chi-square test was performed to determine if there was a statistically significant difference in the proportion of wounds healed at 12 weeks between control and PMVT (unadjusted analysis). Logistic regression was used to adjust for available variables likely to affect wound healing. Kaplan-Meier analysis was used to determine the time to healing for both groups within 12 weeks. PAR between treatment groups at 4, 6, 8, and 12 weeks was statistically tested using general linear mixed modelling (GLMM), which is a repeated measures (PAR value at each week) version of general linear modelling. A P value of .05 was considered statistically significant. Statistical analysis was performed using PASW 27. The Hochberg step-up procedure was used for simultaneous adjustment of all secondary endpoints in regard to multiplicity of statistical testing.
3 RESULTS
3.1 Study demographics
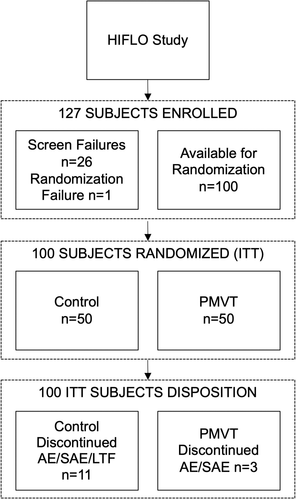
A total of 127 subjects were enrolled at six study sites. Twenty-seven subjects failed to meet inclusion and exclusion criteria, leaving 100 subjects for randomisation (n = 50, control arm; n = 50 PMVT arm). Of these 100 subjects, 14 subjects discontinued the study early because of adverse or serious adverse events (PMVT = 3; control = 11) and one subject (control) was lost to follow-up at week 12 (Figure 1). These 15 subjects were all counted as failed treatments in the final data analysis in accordance with LOCF principles. Baseline demographics between the control and PMVT groups were similar apart from there being deeper wounds in the control group and more wounds located on the toes in the PMVT group (Table 2). Approximately half of the subjects in both cohorts were current or past smokers, and the average BMI of both groups was above 30. While there were no statistically significant differences between the control and treatment cohorts for either demographic parameter (P = .91 and P = .54, respectively), these data attest to the high risk of impaired healing for the subjects enrolled in the HIFLO study.
| Subject demographics | |||
|---|---|---|---|
| Variable | PMVT (n = 50) | Control (n = 50) | P value |
| Age (y) | 59.4 ± 13.22 | 61.2 ± 9.79 | .43 |
| Race n (%) | |||
| Caucasian | 44 (88) | 46 (92) | .74 |
| African American | 6 (12) | 4 (8) | |
| Sex n (%) | |||
| Male | 32 (64) | 34 (68) | .67 |
| Female | 18 (36) | 16 (32) | |
| BMI | 34.3 ± 8.79 | 32.5 ± 6.65 | .54 |
| Smoking status n (%) | |||
| Never | 26 (52) | 24 (48) | .91 |
| Former | 18 (36) | 19 (38) | |
| Current | 6 (12) | 7 (14) | |
| Hypertension | 39 (78) | 37 (7) | .64 |
| HbA1c | |||
| Screening | 8.1 ± 1.65 | 7.4 ± 1.50 | .047 |
| End of studya | 8.0 ± 1.88 | 7.0 ± 1.57 | .008 |
| Creatinine | 1.1 ± 0.36 | 1.1 ± 0.39 | .80 |
| DFU History | |||
| Significant deformities n (%) | 12 (24) | 14 (28) | .65 |
| Age first DFU appeared (y) | 54.0 ± 14.3 | 55.7 ± 11.09 | .51 |
| Prior number of DFUs | 3.2 ± 4.35 | 2.7 ± 2.48 | .70 |
| Amputations (study foot) n (%) | 13 (26) | 16 (32) | .51 |
| Amputation (contralateral foot) n (%) | 6 (12) | 11 (22) | .18 |
| Concurrent DFUs (screening) n (%) | 6 (12) | 12 (24) | .12 |
| Wound characteristics | |||
|---|---|---|---|
| Variable | PMVT (n = 50) | Control (n = 50) | P value |
| Wound area (cm2) (randomisation) | 3.1 ± 3.4 | 3.5 ± 2.3 | .081 |
| Initial depth (mm) n (%) | |||
| <1 | 14 (28) | 10 (20) | .024 |
| 1 | 27 (54) | 19 (38) | |
| 2 | 5 (10) | 12 (24) | |
| >2 | 4 (8) | 9 (18) | |
| Wound age (wk) | 15.2 ± 10.4 | 15.6 ± 10.8 | .71 |
| Wagner grade n (%) | |||
| Wagner 1 | 26 (52) | 17 (34) | .07 |
| Wagner 2 | 24 (48) | 33 (66) | |
| Plantar location n (%) | 41 (82) | 35 (70) | .16 |
| Wound position n (%) | |||
| Lateral | 24 (48) | 21 (42) | .55 |
| Medial | 26 (52) | 29 (58) | |
| Wound location n (%) | |||
| Toe | 13 (26) | 7 (14) | .04 |
| Forefoot | 17 (34) | 14 (28) | |
| Midfoot | 10 (20) | 13 (26) | |
| Hindfoot | 1 (2) | 2 (4) | |
| Heel | 8 (16) | 8 (16) | |
| Ankle | 1 (2) | 6 (12) | |
| Mean duration of offloading at screening (wk) | 16.0 ± 14.8 | 14.0 ± 11.4 | .96 |
| Mean percent time wound offloaded during study | 82.1 ± 11.1 | 81.1 ± 9.1 | .88 |
- a Values for one subject in the PMVT group and six subjects in the control group were missing. Bold value show *p < 0.05.
3.2 Primary and secondary wound closure endpoints
Subjects who received PMVT had a statistically significant increase in the percentage of wounds closed at 12 weeks compared with the control arm (74% vs 38%, P = .0003), meeting the study's primary endpoint (Figure 2A). The final statistical power of this endpoint was 96%, exceeding the projected power of 88% and signifying a greater ability to detect if a difference in outcomes existed between the two treatment groups. Logistic regression analysis to determine the effect of baseline wound area, baseline HbA1c level, and treatment group assignment on healing outcomes was also performed to calculate the adjusted results. Linearity of independent variables with log odds ratio (OR) was verified. While the odd ratios for healing decreased with each unit increase in value for baseline wound area (OR = 0.82; P = .023) and HbA1c (OR = 0.57; P = .001), assignment into the PMVT group increased the odds of healing by approximately 9-fold (OR = 9.0; P = .00008) compared with the control.
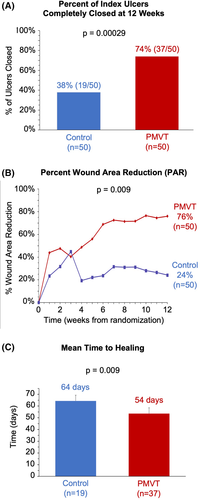
Change in PAR showed a divergence in trajectories with statistical significance seen between the PMVT and control groups beginning at 4 weeks and continuing through 12 weeks of treatment (P = .009). Subjects in the PMVT group had a mean PAR of 76%, over 3-fold more than the mean PAR seen in subjects in the control group (Figure 2B). The mean time to healing was statistically significantly faster for the PMVT group compared with subjects in the control group (54 days (95% CI: 46–61) vs 64 days (95% CI: 57–72); P = .009). This 10-day reduction in time to complete closure is particularly significant as it suggests a change in the wound healing trajectory in the cohort of subjects who achieved wound closure.42 This is further substantiated by Kaplan-Meier analysis showing clear divergent trajectories for time to healing between the PMVT and control groups beginning around 6 weeks, with a 16% faster time to healing seen for subjects in the PMVT group compared with the control group (Figure 2C).
3.3 Exploratory wound perfusion endpoint
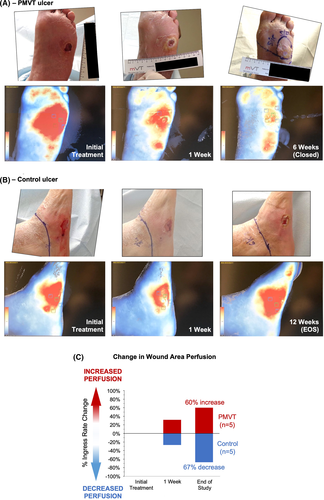
Representative image analyses of the10 consecutive subject subset who underwent wound perfusion assessment via ICGFA to determine ingress rate are shown in Figure 3A,B. ICGFA was analysed for all 10 subjects and timepoints, except for two control subjects who exited the trial prematurely. End study data for these two subjects were imputed using LOCF principles. As a standardised table denoting ingress rates consistent with healing does not exist, change in average ingress rates from baseline to 12 weeks was assessed. A consistent, steady decrease in the mean ingress rate, corresponding to an increase in perfusion of 60% was seen for the PMVT group, whereas the control group a showed a consistent increase in the mean ingress rate corresponding to a significant decrease in perfusion (67%) (Figure 3C).
3.4 Secondary local neuropathy endpoint and exploratory regional neuropathy endpoint
The 10-point SWM exam conducted on all 100 study subjects showed those treated with PMVT had a statistically significant improvement in peripheral neuropathy at end of treatment compared with those in the control group (118% vs 11%; P = .028) (Figure 4). Improved peripheral neuropathy was seen within the first 2 to 4 weeks of the study period.
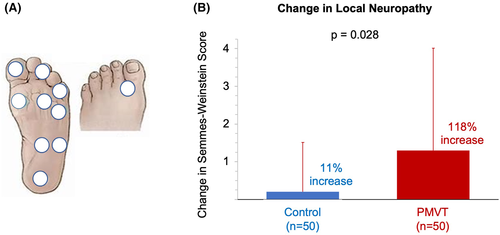
Mean percent reduction in the area of regional peripheral neuropathy assessed with the stocking glove technique was also greater in the PMVT group (62 ± 31%) compared with patients from the control group (16 ± 12%). An example of boundary of sensation marking over time and corresponding change in neuropathy area using image analysis is shown in Figure 5A,B. Although this assessment was only performed on the subset of 21 subjects, the change in sensation over time showed a trend of a rapid reduction of neuropathy area in PMVT treated subjects (Figure 5C).
3.5 Safety
No adverse events (AEs) or serious adverse events (SAEs) related to the study treatment or the procedure were reported. A total of three AEs/SAEs in the PMVT arm and eight in the control arm related to the study wound were recorded (Table 3). Although the total AEs/SAEs in the control arm exceed that of the PMVT arm, all are typical of patients with DFUs, and none were deemed by the investigators to be a direct result of the topical treatment. Other AEs/SAEs unrelated to the study wound were typical of this patient population. In total, one PMVT subject and seven control subjects were hospitalised during the treatment phase of the study, although with the exception of one control subject hospitalised as a result of an infection of the index ulcer that led to amputation, all hospitalisations were because of non-study wound issues.
| Type of event | Control (n = 50) | PMVT (n = 50) |
|---|---|---|
| Adverse event (AE) | Infection (1) Cellulitis (1) Bursitis (1) Dehiscence (1) |
Infection (1) Swelling (1) |
| Serious adverse event (SAE) | Infection (2) Osteomyelitis (1) Amputation (1) |
Dermatitis (1) |
| Total AE + SAE | 8 | 3 |
4 DISCUSSION
Diabetic foot ulceration, caused by peripheral neuropathy and an impaired microcirculation, remains a significant complication of diabetes. Lower extremity amputation is the end result of untreated DFU that become infected, leading to gangrene and osteomyelitis. Standard conservative approaches to DFU will resolve approximately 70% of DFUs after 4 months of treatment, but the remaining 30% of DFUs will become chronic,43 placing the patient at risk for cellulitis, osteomyelitis, gangrene, and lower extremity amputation.44 Advanced wound care techniques such as skin grafts, recombinant growth factors, hyperbaric oxygen, and bioengineered skin can be effective for therapy, but none directly address the defects of the microcirculation or neuropathy present in diabetic wounds. We here report that microvascular therapy can be effective to healing chronic DFU, achieving complete wound closure, with evidence of improved perfusion and improvement in neuropathy.
HIFLO is a randomised, prospective, single-blinded, multi-centre clinical trial designed to validate use of a microvascular tissue allograft (PMVT) in a diabetic population with non-healing Wagner Grade 1 and 2 DFU. The results demonstrated a statistically significant increase in complete wound closure at 12 weeks, reduced time to healing, increased PAR, and improved lower extremity peripheral neuropathy. In addition, the odds of healing with PMVT treatment were nine times greater than a control group in which standard wound care was applied along with a collagen alginate dressing. One of the challenges for clinicians in comparing available DFU treatment options is that there is an inconsistent definition of closure within the field. HIFLO therefore incorporated a stringent definition of complete wound closure with adjudication verification, use of a robust and consistent standard of care treatment, and the final power (96%) of the statistical analysis performed. Confirmation of wound closure was determined by the investigator, a separate blinded physician at the study site who had not been treating the wound, and a panel of three independent blinded adjudicators with expertise in wound care, and then reconfirmed 2 weeks after adjudication at the healing confirmation visit.
Improving the quality of healing is a critical goal for DFU, given the variety of advanced wound care technologies that offer improved wound closure. In the HIFLO Trial, numerous primary, secondary, and exploratory endpoints were assessed, including the use of functional imaging to evaluate perfusion and examination of changes in regional peripheral neuropathy in subsets of subjects. These exploratory endpoints suggest mechanisms by which PMVT expedites DFU healing.
While the cause of diabetic neuropathy is not fully understood, the compromise of both vascular and neural components, which are innately interconnected, are recognised as important elements in its pathophysiology. Vascular insufficiency, ischaemia, hypoxia, and inflammation have all been implicated in the development and progression of diabetic neuropathy.45-47 Neuronal dysfunction correlates closely with the development of blood vessel abnormalities, such as capillary basement membrane thickening and endothelial hyperplasia, which contribute to diminished oxygen tension and hypoxia. Neuronal ischaemia is a well-established characteristic of diabetic neuropathy.
The ability of PMVT to improve local neuropathy is an important observation in this study. Diabetic neuropathy is an underlying factor for skin injury that progresses to ulceration. The insensate feet contribute to decreased quality of life.48 While the monofilament technique is known to have some variability, the large number of subjects analysed in the HIFLO Trial combined with the standardised training across all study sites enabled consistency in technique and the demonstration of statistically significant improvement in local neuropathy with PMVT. The improved sensation documented in this study is likely because of improved angiogenesis, although the mechanisms of action are not known and warrant further investigation. An intervention that improves diabetic neuropathy would address a primary risk factor for DFU formation, and has the potential to reduce wound recidivism.18
The increase in local tissue perfusion documented by fluorescence microangiography addresses another key risk factor for DFU, because microvascular pathology and reduced tissue oxygenation are observed in diabetes.49 The restoration of the microcirculation allows increased oxygen and nutrient delivery to the wound, which promotes granulation and wound epithelialisation.50 The finding that there appears to be an inflection point in the rate of wound closure between 2 and 4 weeks, where the control group's rate of closure falls off relative to the treatment group (Figure 2B), may suggest that PMVT promotes a transition towards a more normal wound healing process that enables closure of the wound.
The structure and composition of PMVT may provide insight into possible mechanisms that resulted in improved perfusion and wound healing. PMVT is microvascular tissue, which not only includes microvessels but also extracellular matrix (ECM). PMVT's microvessel fragments and ECM provide physical scaffolding, mechanical stability, and biochemical cues necessary for angiogenesis, tissue formation, and maintenance of stability in the microenvironment.30, 31, 51 The ECM modulates a whole host of processes including cell migration, attachment, differentiation, and repair, and serves as a reservoir and binding site for growth factors.52 Integrins bind to the extracellular matrix, which in turn triggers endothelial cell adhesion and migration, early indicators of the process of angiogenesis.53, 54 PMVT preclinical data and the scientific literature are supportive of these mechanisms for the positive results seen with PMVT in this clinical study, and are deserving of future exploration.
4.1 Limitations
One limitation of this study is the small subset size of subjects assessed for exploratory evaluation of changes in perfusion. Wound perfusion assessment is a relatively new technology, and ICGFA has not been validated in wound healing. The images can be influenced by various environmental and patient-related factors, including room temperature and patient activity prior to imaging. However, the fluorescence patterns observed for wounds that progressed to healing in this study followed the same trajectory of a chronic wound transitioning from stagnation in a dysfunctional inflammatory phase to the proliferative phase of healing as that reported in several other peer-reviewed publications.37, 55, 56 In addition, although the trial was powered to study local peripheral neuropathy as a secondary endpoint, the assessment in neuropathy area by the stocking glove technique was exploratory. The significant reduction in local and regional neuropathy seen upon PMVT treatment is an important novel observation, but this outcome may be because of factors unrelated to PMVT, and it is possible that successful healing is accompanied by improved neuropathy that has not been measured in other controlled studies of DFU wound healing.
5 CONCLUSION
Weekly application of PMVT, a microvascular tissue allograft, resulted in significantly greater wound closure and improved local neuropathy. These findings support the utility of microvascular therapy as a new approach to treating chronic DFUs. Exploratory results suggest that the increased wound site perfusion and reduction of regional neuropathy accompany wound resolution accelerated by PMVT. Therefore, the restoration of a functional microcirculation is a new approach to diabetic wound healing. By addressing the vascular deficiencies and impaired sensation underlying diabetes, PMVT treatment may also reduce wound recidivism, which would lower medical complications, improve quality of life, and lower associated healthcare costs because of wound recurrence. Further clinical and translational studies of microvascular therapy using PMVT will help to validate the outcomes of the HIFLO Trial.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Nathan Young (Foot & Ankle Associates of Southwest Virginia), Allen Jacobs (SSM Health), and Rachel Hoyal (Sutter Health) for participation as clinical study investigators; Tracey Ikerd (Riverview Health), Jonathan Arnold (Mercy Healing Center), and John Lindberg (Emanate Health) for fluorescence angiography analysis and expertise; Travis Smith and Emmanuel Wilson (eKare, Inc.) for wound measurement system support; Morgan Stepanek and Kim Young (PERI, Inc.) for clinical study support; Douglas M. Arm (MicroVascular Tissues, Inc.) for assistance with study design, clinical study support, data analysis, and manuscript review/editing; and Valerie L. Marmolejo (Scriptum Medica) for assistance with manuscript preparation. L.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The HIFLO Trial was sponsored by MicroVascular Tissues, Inc. (San Diego, CA).
CONFLICT OF INTEREST
C.Z. is an employee of Professional Educational Research Institute, the CRO that conducted this trial. W.L. is a consultant to MicroVascular Tissues, Inc.
AUTHOR CONTRIBUTIONS
Lisa Gould was the principal investigator for the study, reviewed the data, contributed to the discussion, and reviewed/edited the manuscript. Dennis P. Orgill served as an adjudicator for the study, contributed to the discussion, and reviewed/edited the manuscript. David G. Armstrong contributed to the discussion and reviewed/edited the manuscript. Robert D. Galiano served as an adjudicator for the study, contributed to the discussion, and reviewed/edited the manuscript. Paul M. Glat served as an adjudicator for the study, contributed to the discussion, and reviewed/edited the manuscript. Charles M. Zelen was a clinical investigator for the study, contributed to the study design, reviewed the data, and reviewed/edited the manuscript. Lawrence A. DiDomenico was a clinical investigator for the study, contributed to the discussion, and reviewed/edited the manuscript. Marissa J. Carter contributed to the development of the statistical plan, analysed data, and reviewed/edited the manuscript. William W. Li contributed to the study design, reviewed the data, and reviewed/edited the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are the property of the study sponsor, MicroVascular Tissues, Inc., and therefore are not publicly available. IRB informed consent requirements limits disclosure of subject data to their health care provider, study sponsor and their representatives, regulatory agencies and the IRB.




