Diagnosis and initial management of children presenting with premature loss of primary teeth associated with a systemic condition: A scoping review and development of clinical aid
Abstract
Background
Premature loss of primary teeth (PLPT) can be a rare presentation of systemic medical conditions. Premature loss of primary teeth may present a diagnostic dilemma to paediatric dentists.
Aims
To identify systemic conditions associated with PLPT and develop a clinical aid.
Design
OVID Medline, Embase and Web of Science were searched up to March 2023. Citation searching of review publications occurred. Exclusion occurred for conference abstracts, absence of PLPT and absence of English-language full text.
Results
Seven hundred and ninety-one publications were identified via databases and 476 by citation searching of review articles. Removal of 390 duplicates occurred. Following the exclusion of 466 records on abstract review, 411 publications were sought for retrieval, of which 142 met inclusion criteria. Thirty-one systemic conditions were identified. For 19 conditions, only one publication was identified. The majority of publications, 91% (n = 129), were case reports or series. Most publications, 44% (n = 62), were related to hypophosphatasia, and 25% (n = 35) were related to Papillon–Lefèvre. Diagnostic features were synthesised, and a clinical aid was produced by an iterative consensus approach.
Conclusions
A diverse range of systemic diseases are associated with PLPT. Evidence quality, however, is low, with most diseases having a low number of supporting cases. This clinical aid supports paediatric dentists in differential diagnosis and onward referral.
Why this paper is important to paediatric dentists
- Children with premature loss of primary teeth are often referred for specialist paediatric dentistry input, in which they present a diagnostic dilemma.
- This article provides the first scoping review of systemic conditions associated with premature loss of primary teeth in primary case reports.
- It also provides an accessible clinical decision aid to support differential diagnosis and onward referral of these rare cases.
1 INTRODUCTION
Exfoliation of primary teeth as part of dental development usually begins with the primary incisors from the age of 5.5 to 6 years.1, 2 Physiological exfoliation tends to be bilaterally symmetrical, with loss of mandibular primary teeth occurring before their maxillary counterparts, with the exception of second primary molars.1, 3 Girls tend to exfoliate their teeth at an earlier age than boys, but the sequence of tooth loss remains consistent in both groups.2, 3
Premature loss of primary teeth (PLPT) most commonly occurs due to dental caries or traumatic dental injury. Loss of the primary incisor teeth prior to the age of 5 years, in the absence of caries or trauma, can be considered premature loss. The loss of primary incisors prior to the age of 3 years is less likely to be a variation in normal physiological exfoliation and presents a particular diagnostic concern. Such incidences of premature loss of primary teeth can be a rare presentation of a systemic medical condition, which may be of metabolic, inflammatory, neoplastic or immunological origin. These children will often be referred for specialist paediatric dentistry input for management in secondary or tertiary settings, in which they present a diagnostic dilemma to paediatric dentists and may require further clinical advice and support from our paediatric medical colleagues.2
The literature surrounding this topic has not been previously systematically explored; narrative reviews and book chapters have been previously published,2, 4 and these secondary texts may be referred to, but primary cases are often not cited. Additionally, there is conflation of generalised or molar-incisal pattern periodontitis with premature loss of primary teeth in many texts.2 As a result, paediatric dentists may face uncertainty on how to manage and refer these children for subsequent medical investigation for potentially serious underlying medical conditions. This scoping review was therefore conducted to synthesise the primary cases in this area, and to support the development of a clinical aid to serve as a practical, diagnostic tool for paediatric dentists.
2 AIM
To evaluate and synthesise the primary literature relating to PLPT as a manifestation of systemic disease.
3 OBJECTIVES
- To identify systemic conditions associated with PLPT through a scoping review of the literature.
- To develop a clinical aid to support the diagnosis and immediate management of children presenting with PLPT.
4 MATERIALS AND METHODS
4.1 Objective 1: Scoping review
OVID Medline, Embase and Web of Science were searched up to March 2023 with the search strategy (“early exfoliation” OR “premature exfoliation” OR “attachment loss” OR “early loss” OR “premature loss” OR “advanced dental development” OR “early shedding”) AND (“primary teeth” OR “baby teeth” OR “milk teeth” OR “primary dentition” OR “deciduous dentition” OR “deciduous teeth”). Search term was developed iteratively, omitting terms relating to systemic disease to avoid producing too narrow of a search. Initial search occurred in August 2021, with repeat search completed in March 2023 to the review literature published in the interim analysis period. No restrictions were placed on publication date. Secondary and tertiary texts identified in the database searches underwent citation searching.
Duplicate records were removed and abstracts were screened by two researchers (CH and HA), with disagreement resolved by the third researcher (RB). Data were extracted directly to Microsoft Excel® (Microsoft®, Washington). Exclusion occurred for conference abstracts, where excessive mobility or premature loss was not reported first-hand, and where no English-language full text was available. Cases with reported excessive mobility were included with acknowledgement that this may progress to unobserved premature loss.
Included publications underwent full-text review in which the following data were extracted: type of publication, systemic condition, dental features, age at presentation (to determine whether exfoliation was truly premature), initial medical investigations and results, onward medical referral, secondary investigations and results and oral management. A descriptive statistical analysis was carried out.
Results were synthesised by grouping systemic diseases by prevalence and quality of evidence. Further literature review of medical features, investigations and treatment of systemic conditions was completed to support data synthesis and clinical aid development.
5 RESULTS
Identification of 791 records occurred through database searches. Seven review papers were identified:2, 4-9 two relating to premature loss of primary teeth in general, four relating to hypophosphatasia and one relating to Papillon–Lefèvre syndrome. Citation searching was completed, resulting in the identification of 476 further unique records.
After removal of duplicates (n = 390), the screening of abstracts resulted in a further 466 exclusions. The remaining 411 publications were sought for retrieval; records could not be retrieved for 78 publications due to lack of availability of full text (n = 46) or lack of an English-language full text (n = 22). Following full-text screening, exclusion occurred for: 46 conference abstracts and 145 publications in which PLPT was not reported first-hand. This included the following: literature reviews without primary cases (where citation search was subsequently completed), cases in which exfoliation was reported after age 5 years, or clinical attachment loss without mobility or loss of teeth was reported. A total of 142 primary texts therefore underwent full data extraction (Figure 1).
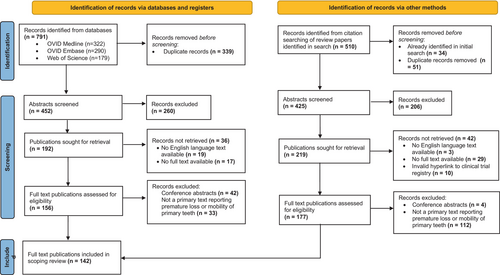
Thirty-one distinct systemic conditions were identified, but for 19 of these conditions, only one case report was identified in the scoping review (Table 1). Publications consisted predominantly of case reports and case series (91%; n = 129), followed by laboratory studies, for example of exfoliated teeth or genetic analysis (6%; n = 8). One publication related to a clinical trial of hypophosphatasia treatment asfotase alfa, and two cross-sectional studies and two cohort studies were included.
| Condition and number of publications identified in scoping review (n=) | Medical features | Intra-oral features | Medical investigations | Referral | Medical management |
|---|---|---|---|---|---|
| Relatively more common systemic conditions associated with premature loss of primary teeth | |||||
|
Hypophosphatasia |
Mutation in the ALPL gene causing non-function of tissue nonspecific alkaline phosphatase, a key regulator of hard tissue metabolism. Varied phenotype from fatal perinatal to mild adult onset.85
|
Multiple included cases with patient-reported history of premature loss,39, 40, 61, 68 or mobility of primary teeth (not reported premature loss).60, 62 | Metabolic bone | Asfotase alfa for childhood-onset hypophosphatasia.86 | |
|
Odontohypophosphatasia |
Included on the spectrum of hypophosphatasia; premature loss of primary teeth without characteristic bone problems in other forms of hypophosphatasia. Children may develop bone symptoms with age and be re-diagnosed with a more severe form.57 |
One included a case of mobility of primary teeth (not reported premature loss)88
|
Metabolic bone | Monitor for the development of hypophosphatasia | |
|
Papillon–Lefèvre |
Autosomal recessive mutation to CTSC gene (Capthesin C).120 Variable severity of palmoplantar keratosis.121 Capthesin C has immune system activating role—susceptibility to cutaneous and systemic infections due to T cell, B cell and neutrophil dysfunction.121 |
Multiple included cases with patient-reported history of premature loss,95-97, 105-107, 109, 113, 115, 116 or mobility of primary teeth (not reported premature loss)93, 112 |
|
Dermatology |
Oral hygiene instruction and non-surgical periodontal therapy.121 |
|
Ehlers Danlos (n = 1)123 |
Collective group of disorders of different genetic defects in collagen. Internationally classified into subgroups based on genetic cause and phenotype, including periodontal type (pEDS).124
|
Periodontal type includes disease of periodontal tissues and premature tooth loss.125
|
|
Paediatrician or Rheumatology |
|
|
Childhood haematological cancers (n = 1)126 |
|
Uncertain association with premature loss of primary teeth, one case series identified from 1983 with premature loss in children with acute lymphoblastic leukaemia and non-Hodgkin's lymphoma.126
|
Oncology |
|
|
|
Severe congenital neutropenia (n=1)16 |
Group of disorders in which absolute neutrophil count is <500 with maturation arrest of myeloid precursors.130 Most commonly due to mutation in ELANE gene most common (70–80% of cases).131
|
|
Severe neutropenia.
|
Haematology or Immunology |
|
|
Cyclic neutropenia |
Inherited autosomal dominant disorder. Most commonly due to mutations in ELA-2 or ELANE.132 Severe neutropenia occurring in typically 21-day cycles, with recovery of neutrophil counts in intervening period.136
|
One included case was premature loss of primary molar at age 7.5 years134
|
|
Haematology or Immunology |
|
|
Autoimmune neutropenia (n = 1)18 |
Usually occurs within the first 2 years of life and may be associated with development of neutrophil specific auto-antibodies.133
|
Included case reports mobility of primary teeth (rather than premature loss)18
|
|
Haematology or immunology |
Up to 90% of cases will spontaneously resolve.133
|
|
Chronic idiopathic neutropenia |
Chronic neutropenia in the absence of identification of neutrophil specific auto-antibodies.133 |
|
Haematology or Immunology |
||
|
Idiopathic immune deficiency (n = 1)139 |
|
|
|
Haematology or Immunology | |
| Rare systemic conditions associated with premature loss of primary teeth | |||||
|
Coffin–Lowry syndrome |
X linked dominant mutation in RPS6KA3 gene.144, 30
|
|
|
Paediatricians Neurology |
|
|
Langerhans cell histiocytosis |
Inflammatory myeloid neoplasia with mutations in MAPKinase (MAPK) pathway.149 Single or multiple locations therefore varied presentations.149 In children, the skull is most affected.150
Skin involvement.149 |
Two included cases of mobility of primary teeth (rather than reported premature loss)147, 148 |
Haematology and Oncology |
|
|
|
Cherubism |
Childhood-onset inflammatory bone disease characterised by bilateral, symmetrical proliferative fibro-osseus lesions limited to the mandible and maxilla.154 Mutation in SH3BP2 gene, usually inherited in autosomal dominant manner.154, 155
|
|
Oral and maxillofacial surgery |
|
|
|
Leukocyte adhesion deficiency (n = 1)157 |
Autosomal recessive group of rare disorders (types I-III) affecting leukocyte transport.
Impaired platelet function in type III:
|
Included case reports mobility of primary teeth (rather than reported premature loss).157 Type I:
Type II:
Type III:
In addition:
|
|
Immunology | |
|
Wiedemann–Steiner syndrome |
Autosomal dominant mutation in KMT2A gene.164 |
|
|
Paediatrician | |
|
Chediak–Higashi syndrome (n = 1)166 |
Autosomal recessive CHS1 gene mutation a.k.a LYST gene137, 167, 168 Rare Lysosomal storage disorder.169
|
|
|
Haematology or Immunology |
|
|
No reported diagnosis |
Possibly generalised periodontal disease without systemic cause or undiagnosed systemic conditions. |
||||
| Rare systemic conditions with limited and uncertain evidence of association with premature loss of primary teeth | |||||
|
Chronic graft vs host disease (n = 1)171 |
Haematopoietic stem cell transplant recipients. Donor T cells respond to histo-incompatible antigens on host tissues.172 Skin, gastroinstestinal tract and liver most common target organs.172 |
|
Predominantly a clinical diagnosis
|
Will already be under care of oncology and transplant teams. |
Prevention:
Treatment:
|
|
Congenital adrenal hyperplasia (n = 1)174 |
Group of autosomal recessive disorders affecting cortisol biosynthesis. Results in low cortisol and/or aldosterone deficiency and/or excess androgens.175 Excessive androgens:
Aldosterone deficiency
Low cortisol
|
Included case reports mobility of primary teeth (rather than reported premature loss)174
|
Endocrinology |
|
|
|
Congenital syphilis (n = 1)176 |
Mother-to-child transmission of Treponema pallidum. Early syphilis (<2 years old)
Late syphilis (>2 years old)
|
|
GP or Paediatrician |
|
|
|
Erythromelalgia (n = 1)180 |
Mutations in SCN9A gene resulting in altered sodium channels and neuron excitability. Neuropathy resulting in triad of redness, burning pain and warmth in extremities relieved by cooling and elevation. Episodic but of continuous nature.181 In episodes, triad of symptoms in extremities:
|
|
|
Paediatrician or Neurology | Symptomatic relief
|
|
Familial (Infantile) malignant osteopetrosis (n = 1)182 |
Dysregulated osteoclasts resulting in osteosclerosis. Excessive bone deposition around cranial foramina resulting in nerve compression. Excess bone interferes with medullary haematopoiesis resulting in bone marrow suppression.183 |
|
|
Metabolic bone team or haematology |
|
|
Fanconi syndrome (n = 1).185 |
Congenital or acquired inadequate reabsorption in the proximal renal tubules of small molecules, for example, glucose, amino acids, uric acid, phosphate, bicarbonate.186 Loss of phosphate affects bone development.
|
|
|
Renal team |
|
|
Hereditary sensory and autonomic neuropathy type VIII (n = 1).187 |
Autosomal recessive mutation in PRDM12 gene.188
|
Premature loss in included case believed to be due to auto-extraction.189
|
|
Neurology | |
|
Metaphyseal dysplasia—Braun–Tinschert type (n = 1)190 |
Metaphyseal widening and under-modelling of bone of extremities (tubular bones). Unusually severe varus deformity of the radii and flat exostoses of the long bones. Skull is unaffected.191 |
Premature eruption permanent successors in reported case.190 | Paediatrician | ||
|
Microcephalic osteodysplastic primordial dwarfism or Seckel syndrome Type II |
Autosomal recessive mutation in PCNT gene.194 May have chronic kidney disease, anaemia, thrombocytosis, hypertension, insulin resistance and Moyamoya194
|
|
|
Paediatrician | |
|
Weary–Kindler syndrome (n = 1)195 |
Associated with FERMT1 pathogenic variants.196
|
Case report with patient-reported history of premature loss of primary teeth
|
|
Dermatology |
|
|
Acatalasemia (n = 1)197 |
Genetic enzyme defect resulting in catalase deficiency.198 Catalase deficiency results in an inability to break down hydrogen peroxide.199
|
Included case is of patient-reported history of mobility of primary teeth (not reported premature loss)197
|
|
Paediatrician |
|
|
Familial renal hypophosphataemia with intracerebral calcifications (n = 1)200 |
Reporting of a novel case series of siblings with:
|
|
Paediatrician |
|
|
|
Mucocutaneous dyskeratosis with premature tooth loss |
Two cases with similar presentations of:
|
One included case reports primary teeth mobility (rather than reported premature loss)201 | Paediatrician |
|
|
|
Short-bowel syndrome (n = 1)203 |
Short-bowel syndrome can be either congenital or acquired following the surgerical resection of the small intestine.203 Case series of two children with other complex co-morbidities including recurrent infections
|
Two cases presented, with premature loss occurring in one patient.203
|
Paediatrician |
|
|
| Considered to be associated with premature loss of primary teeth in the seminal literature but no evidence identified in scoping review | |||||
|
Haim–Munk syndrome (n = 0) |
Autosomal recessive mutation of CTSC as in Papillon–Lefèvre.121
Features to differentiate from Papillon–Lefèvre:
|
|
|
Dermatology |
|
|
Glycogen storage disease (n = 0) |
Enzyme deficiency affecting glucose storage and glycogen breakdown. Results in neutrophil dysfunction. 23 distinct diseases, classified by the enzyme deficiency involved. |
Type Ib most associated with oral features.137, 206
|
|
Metabolic medicine, endocrinology, and immunology |
|
|
Scurvy (n = 0) |
Severe vitamin C deficiency resulting in reduced antioxidant immune defences to oxidative stress. Defective collagen synthesis resulting in weakened capillary vessels and bleeding tendency.207
|
|
|
Paediatrician |
|
|
Wiskott–Aldrich syndrome (n = 0) |
Severe X linked mutation of WASp,209 characterised by thrombocytopenia, immune deficiency and eczema.137, 210 Additionally, increased risk of lymphoma.210
|
|
|
Paediatrician or haematology |
|
Most publications, 44% (n = 62), were related to hypophosphatasia. A further 25% (n = 35) were related to Papillon–Lefèvre syndrome and 2% (n = 3) were related to odontohypophosphatasia, and Coffin–Lowry and Wiedemann–Steiner syndromes. Seven publications (5%) were related to neutropenias or immune deficiencies. No diagnosis of systemic disease was found in four cases (3%). No publications were related to glycogen storage disease, scurvy or Haim–Munk syndrome, which are referred to in previous summaries.2
5.1 Objective 2: clinical aid development
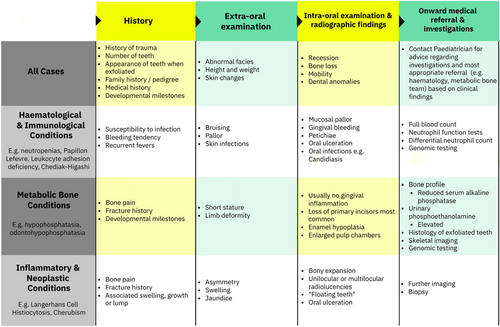
Scoping review findings were synthesised with a further literature review of medical features, investigations and treatment of each systemic condition to support clinical aid development (Table 1). Through this process, four distinct categories were identified as distinguishing features: haematological features, skeletal features (including craniofacial), neurological features and skin features. An iterative consensus approach was applied to streamline findings into an accessible clinical aid (Figure 2); this summarises pertinent diagnostic features to support specialists in paediatric dentistry in assessing, diagnosing and referring children with PLPT.
6 DISCUSSION
This evidence synthesis provides a broad overview of systemic features of conditions associated with PLPT. This paper is novel in its comprehension, and in condensing this information into a practical clinical diagnostic aid.
Clinicians should take a systematic approach to history and examination for these children. Although many conditions will already have been identified, PLPT can aid or confirm a systemic diagnosis. In conditions such as delayed development, recurrent infection or skeletal problems, although these may already be under investigation, PLPT may support definitive diagnosis.10 In some cases, premature loss, however, may be the first sign of a systemic condition. This was evident in the scoping review in multiple published hypophosphatasia,11-15 neutropenia16-18 and Papillon–Lefèvre19-22 cases in particular.
The aim of this study was to identify systemic conditions associated with PLPT. As such, the inclusion criteria of the scoping review were for cases in which loss of teeth or excessive mobility had occurred, although attachment loss was included in the search strategy for completeness. Search strategies with specific terms relating to systemic disease were trialled but were found to produce more narrow results, with publications utilising more specific disease terms and diagnoses omitted. This is noted in a recent narrative review of PLPT, which yielded roughly a third of the results when compared to this scoping review in a more specific search strategy.4 A search strategy omitting reference to systemic disease and inclusion of citation searching of review papers was more time-consuming, with manual exclusion of cases of PLPT relating to caries and traumatic dental injury occurring. However, it supported the completion of a comprehensive scoping review. The number of included cases of hypophosphatasia and Papillon–Lefèvre identified in the scoping review was impacted by citation searching of five review articles specific to these individual diseases.5-9 Two review papers more broadly relating to PLPT, however, also underwent citation searching for inclusion.2, 4
There were a small number of cases included in the scoping review in which a definitive systemic diagnosis was not found at the time of publication, but premature tooth loss had occurred and a diagnosis of periodontitis was made.11, 23-25 Similarly, conditions previously associated with PLPT were not identified in the scoping review (e.g., Haim–Munk syndrome, glycogen storage disease and scurvy).
It is beyond the scope of this article to provide the detailed oral and general management of each condition, however, a few general principles apply. Clearly, by definition, management needs to be interdisciplinary with close liaison with the paediatric team. In some cases, it is likely that if the systemic condition can be addressed, then PLPT can be delayed or arrested.26 The management of the early loss may therefore be intrinsically connected to the management of the systemic condition. Clinicians should be aware that in most cases, the premature loss will extend to the permanent dentition should intervention not be possible or undertaken.
The maintenance of optimal oral hygiene is a good principle to follow. Poor oral hygiene can exacerbate the periodontal health and accelerate premature loss.27 Families should have access to regular, high-quality, preventative services. Although children may not be at high risk of dental caries, they should be managed as being high risk due to the implication of developing dental caries on their reduced dentition. Children should be considered high risk for periodontal disease and managed appropriately. Consideration should be made as to whether the systemic condition is likely to have implications for the treatment of any oral disease that does develop.
Children should remain under specialist paediatric dental care. Parents and carers should be kept fully informed of the consequences of the disease process, particularly its implications for the permanent dentition. The management decisions will be complex, and clinicians should engage in shared decision-making whenever possible.
As this literature review focused on primarily reported cases, it is acknowledged that there may be other oral features not identified in the evidence synthesis. Clinicians are encouraged to take a pragmatic approach so as not to exclude a differential diagnosis from this diagnostic aid due to a novel finding.
Premature loss of primary teeth secondary to systemic conditions is a rare but important presentation. This scoping review has demonstrated that it can be caused by a diverse range of medical conditions, many of which may be unfamiliar to the clinician. Furthermore, there are a number of conditions reported to be associated with premature loss but for which no, or limited, primary evidence has been found. The clinical aid developed from the scoping review provides an evidenced-based tool to which clinicians can refer when presented with such cases. This clinical aid acts as an aide memoir to remind clinicians of relevant information to gather in the history and enables clinicians to link specific features to broad aetiological categories.
AUTHOR CONTRIBUTIONS
R.B. conceived the idea. C.H. and H.A. collected and collated data. C.H., H.A., and R.B. analysed data. C.H. led the writing. All authors provided input to clinical aid development and have approved the manuscript. The BSPD QIRC provided peer review and methodological support.
ACKNOWLEDGEMENTS
In collaboration with the British Society of Paediatric Dentistry Quality Improvement and Research Committee.
FUNDING INFORMATION
No funding was received.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest were identified by any of the authors.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




