Prime–boost bacillus Calmette–Guérin vaccination with lentivirus-vectored and DNA-based vaccines expressing antigens Ag85B and Rv3425 improves protective efficacy against Mycobacterium tuberculosis in mice
Summary
To prevent the global spread of tuberculosis (TB), more effective vaccines and vaccination strategies are urgently needed. As a result of the success of bacillus Calmette–Guérin (BCG) in protecting children against miliary and meningeal TB, the majority of individuals will have been vaccinated with BCG; hence, boosting BCG-primed immunity will probably be a key component of future vaccine strategies. In this study, we compared the ability of DNA-, protein- and lentiviral vector-based vaccines that express the antigens Ag85B and Rv3425 to boost the effects of BCG in the context of immunity and protection against Mycobacterium tuberculosis in C57BL/6 mice. Our results demonstrated that prime–boost BCG vaccination with a lentiviral vector expressing the antigens Ag85B and Rv3425 significantly enhanced immune responses, including T helper type 1 and CD8+ cytotoxic T lymphocyte responses, compared with DNA- and protein-based vaccines. However, lentivirus-vectored and DNA-based vaccines greatly improved the protective efficacy of BCG against M. tuberculosis, as indicated by a lack of weight loss and significantly reduced bacterial loads and histological damage in the lung. Our study suggests that the use of lentiviral or DNA vaccines containing the antigens Ag85B and Rv3425 to boost BCG is a good choice for the rational design of an efficient vaccination strategy against TB.
Abbreviations
-
- BCG
-
- bacillus Calmette–Guérin
-
- CFU
-
- colony-forming unit
-
- CTL
-
- cytotoxic T lymphocyte
-
- DAR
-
- DNA vaccine expressing Ag85B and Rv3425
-
- IFN-γ
-
- interferon-γ
-
- IL-2
-
- interleukin-2
-
- LAR
-
- lentivirus-vectored vaccine expressing Ag85B and Rv3425
-
- PAR
-
- subunit protein vaccine expressing Ag85B and Rv3425
-
- PPD
-
- purified protein derivative
-
- OADC
-
- oleic acid-albumin-dextrose-catalase
-
- TB
-
- tuberculosis
-
- Th1
-
- T helper type 1
-
- TNF-α
-
- tumour necrosis factor-α
Introduction
Tuberculosis (TB) remains a global health threat, and an estimated 8·6 million new cases of active disease and 1·3 million deaths occurred worldwide in 2012.1 Co-infection with HIV, the increased occurrence of drug resistance, and inadequate treatment and preventive measures worsen this situation.2 Mycobacterium bovis bacillus Calmette–Guérin (BCG) is the only TB vaccine available for human use, but this vaccine fails to protect against the most prevalent form of this disease and against pulmonary TB in adults.2, 3 Therefore, new vaccines and vaccination strategies that can replace BCG or boost immunity against TB in BCG-vaccinated individuals are urgently needed.
Due to the success of BCG in protecting children against miliary and meningeal TB, as well as the proven 80-year safety record of this vaccine and the typically excellent tolerance of this vaccine among healthy individuals,4 different approaches for generating new and more effective vaccines have focused on improving the efficacy of BCG. These approaches include Mycobacterium tuberculosis mutants, recombinant BCG, and subunit and virus-vectored vaccines that can be used as a boost for BCG.5 However, because the majority of individuals will have been previously vaccinated with BCG, heterologous boosting of BCG-primed immunity will probably be a key component of future multicomponent vaccine strategies. A booster vaccine may be administered in infancy or adolescence, when the effects of BCG may begin to wane.4, 6 Purified antigens in the presence of appropriate adjuvants, DNA vaccines or virus-encoded mycobacterial antigens are at the forefront of the approaches currently being tested for the capacity to strengthen or prolong BCG-primed immunity against TB.
To date, several subunit vaccines (i.e. Hybrid1–IC31, AERAS 404–IC31 and M72) and recombinant viral vectors (i.e. MVA85A, AdAg85A and Ad35) have been under clinical investigation for their efficacy in boosting BCG-primed immunity.2 Different forms of booster vaccines have their own merits and confer various capacities for boosting BCG-primed immunity. Purified antigens are safe and appropriate adjuvants that can enhance humoral responses and cellular responses.7 DNA vaccines and viral vector-based vaccines offer the advantage of endogenous antigen production within antigen-presenting cells, leading to CD8+ and CD4+ T-cell stimulation.5 Therefore, the best format for boosting BCG must be chosen for each antigen.
Ag85B, which is encoded by fbpB, is one of the most immunodominant antigens of M. tuberculosis8 and is the major component in many vaccines that entered into phase I and II clinical trials. Rv3425, which is a member of the PPE protein family, elicits strong humoral and cellular immune responses in mice after immunization.9 Our previous study also demonstrated that recombinant BCG co-expressing Ag85B and Rv3425 induced strong immune responses after immunization and provided better protective efficacy against M. tuberculosis challenge than BCG.10
To compare the boosting effect of different Ag85B and Rv3425 fusion antigens on BCG-primed immunity, we constructed three different forms of vaccines expressing Ag85B and Rv3425: a subunit protein vaccine (i.e. PAR), a DNA vaccine (i.e. DAR) and a lentivirus vector vaccine (i.e. LAR). We evaluated the efficacy of these vaccines for boosting BCG-induced immunity and protection against M. tuberculosis in mice.
Materials and methods
Bacterial strains and cell lines
A Danish strain of M. bovis BCG (ATCC no. 35733) and the H37Rv strain of M. tuberculosis were grown in Middlebrook 7H9 broth (Difco, Franklin Lakes, NJ) supplemented with 10% oleic acid–albumin–dextrose–catalase, 0·2% glycerol and 0·05% Tween-80. The 293T cell line was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution.
Preparation of vaccines for boosting immunization
For production of the lentiviral vaccine recombining Ag85B and Rv3425 (i.e. LAR), the coding sequences for the antigens Ag85B and Rv3425 were amplified from M. tuberculosis H37Rv genomic DNA via PCR using specific primers. All primer sequences are presented in the Supporting information, Table S1. The PCR products were subsequently cloned into pLenti6.3 (Invitrogen, Carlsbad, CA), generating pLenti-Ag85B-Rv3425. The DNA construct was verified by sequencing. The recombinant lentiviral vaccine was generated in 293T cells as described previously.11 Expression levels of recombinant viruses were analysed by Western blot.
To construct the DNA vaccine harbouring Ag85B and Rv3425 (i.e. DAR), the coding regions of Ag85B and Rv3425 were PCR-amplified from LAR and sub-cloned into the eukaryotic expression vector pVax (Invitrogen). The DNA construct was verified by sequencing. 293T cells were transfected with the expression vectors using Lipofectamine™ 2000 (Invitrogen) according to the manufacturer's instructions. After 48 hr, the cells were rinsed twice with ice-cold PBS and subsequently harvested and lysed using lysis buffer. The protein concentration of the lysate was determined using the Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA). Protein expression was analysed by Western blot using anti-Ag85B-Rv3425 mouse polyclonal antibody.
To generate subunit protein vaccines expressing Ag85B and Rv3425 (i.e. PAR), PCR products of Ag85B and Rv3425 were subcloned into the pET28a plasmid (Invitrogen). Recombinant Ag85B-Rv3425 protein was purified using the HIS-Select® Nickel Affinity Gel (Sigma-Aldrich, St Louis, MO) according to the manufacturer's instructions.
Mice and immunization
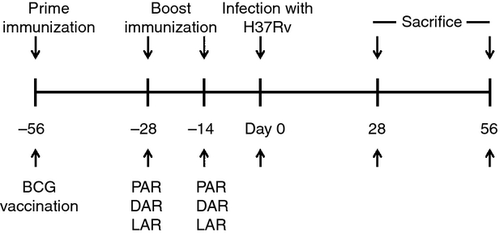
Six- to eight-week-old female C57BL/6 mice were obtained from the Shanghai Laboratory Animal Centre (SLACCAS; Shanghai, China). All mice were maintained under specific pathogen-free conditions and used in accordance with the Guide for the Care and Use of Laboratory Animals from the National Institutes of Health. The experimental protocol was approved by the Animal Care and Use Committee of Wuhan University. The mice were killed by dislocation of the cervical vertebra, which was performed under sodium pentobarbital anaesthesia, and all efforts were made to minimize suffering. A total of six mice per group were first immunized subcutaneously with 5 × 106 colony-forming units (CFU) of BCG in 100 μl of PBS and subsequently boosted twice at 2-week intervals with PAR, DAR or LAR.12 Meanwhile, the mice were boosted with the controls for these three types of vaccines, which were PNC (i.e. monophosphoryl lipid (MPL) + trehalose dicorynomycolate (TDM), DNC (i.e. pVax) and LNC (i.e. pLenti6.3). For DAR immunization, 50 μg of the DAR DNA vaccine in 100 μl of sterile PBS was administered to the mice intramuscularly in the right thigh. For LAR immunization, the mice were immunized with 5 × 106 plaque-forming units of LAR in the foot pad. For PAR immunization, the mice were immunized subcutaneously with 50 μg of PAR formulated with the adjuvants MPL and TDM (Sigma). The vaccination schedule is presented in Fig. 1.
ELISPOT analysis
Two weeks after the last vaccination, the mice were killed and their spleens were removed aseptically. Lymphocytes were isolated from spleen cells using Lympholyte-M density-gradient centrifugation (Cedar Lane Lab, Burlington, NC) and diluted in RPMI-1640 medium containing 10% fetal calf serum. The lymphocytes were plated for 36 hr at a final concentration of 5 × 105 per well with an appropriate stimulus [i.e. 10 μg/ml of purified protein derivative (PPD) or Ag85B-Rv3425]. A mouse interferon-γ (IFN-γ) ELISPOT kit and a mouse tumour necrosis factor-α (TNF-α) ELISPOT kit (U-Cytech Biosciences, Utrecht, the Netherlands) were used according to the manufacturers' instructions to determine the relative number of IFN-γ- and TNF-α-expressing cells, respectively, in the single-cell spleen suspensions. Finally, the spots were counted microscopically.
Cytokine analysis using cytometric bead array
The lymphocytes were plated at a concentration of 2 × 106 cells/well in 24-well plates in RPMI-1640 medium containing 10% fetal calf serum. The cells were stimulated with purified recombinant Ag85B-Rv3425 protein (10 μg/ml) or PPD (10 μg/ml) for 36 hr at 37° After stimulation, the supernatants were harvested and interleukin-2 (IL-2), IL-4, IL-6, IL-10, TNF-α, IFN-γ and IL-17A were analysed using the BD Cytometric Bead Array Mouse T helper type 1 (Th1)/Th2/Th17 Cytokine Kit (BD, Biosciences, San Jose, CA) according to the manufacturer's instructions.
Intracellular staining and flow cytometry
To analyse intracellular cytokine production by lymphocytes, the cells were cultured in the presence of 0·6 μl/ml of BD GolgiStop for 4 hr and surface-stained for CD8a or CD4 expression using anti-CD3- phycoerythrin (PE)-Cy7, anti-CD8a-FITC, anti-CD4-FITC antibodies (BD Pharmingen, San Diego, CA). The samples were incubated at 4° for 30 min, washed and resuspended in PBS. Then, the cells were permeabilized using the BD Cytofix/Cytoperm™ Fixation/Permeabilization Kit according to the manufacturer's instructions and stained with anti-IFN-γ-PE or anti-Perforin-PE (eBioscience, San Diego, CA). CD3+ CD4+ IFN-γ+ and CD3+ CD8+ Perforin+ cells were analysed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA).
Infection of C57BL/6 mice with M. tuberculosis
All mice were maintained under specific pathogen-free conditions in an Animal Biosafety Level 3 Facility at Wuhan University. Groups of 12 C57BL/6 mice were vaccinated using the prime–boost strategy described in Fig. 1. A total of 2 weeks after the last vaccination, all mice were challenged intravenously via the lateral tail vein with 1·2 × 106 CFU of M. tuberculosis H37Rv.13, 14 The mice were monitored every week for weight changes.
Determination of CFU
The mice were killed 4 or 8 weeks after challenge. The organs were separated into two parts for bacterial viable counts and histopathology. The bacterial burdens in the lungs and spleen were determined by plating serial dilutions of tissue homogenates on Middlebrook 7H11 agar. Colony-forming units were counted after incubation at 37° for 3 weeks.
Histopathology and lesion analysis
The lungs and spleens were collected and fixed in 10% neutral-buffered formalin and paraffin for histological analysis. Sections with a thickness of 4–6 mm were then cut and stained with haematoxylin and eosin using standard methods. All histopathological diagnoses were made by two board-certified pathologists, who were both blinded to the experimental groups, and were evaluated at least twice to verify the reproducibility of the observations.
Statistical analysis
The results were tabulated using Excel 2007 and graphpad prism 5.0. The bars represent the mean ± standard deviation (SD). Statistical significance was determined using one-way analysis of variance, followed by the Tukey post-test with a 5% significance level, with *P ≤ 0·05, **P ≤ 0·01.
Results
Construction of vaccines for boosting BCG immunization
To obtain three different types of vaccines expressing Ag85B and Rv3425 (i.e. PAR, DAR and LAR), the two genes were inserted into pET28a, pVax and pLenti6.3, respectively. DAR and PAR were transfected into 293T cells and generated 55 000 molecular weight products in the lysates of the cells; these products were recognized by anti-Ag85B-Rv3425 mouse polyclonal anti-sera. PAR was purified using the HIS-Select® Nickel Affinity Gel, and the molecular weight of this product was slightly greater because of the His tag (Fig. 2).

Cellular immune responses in mice induced by BCG priming and PAR, DAR or LAR boosting immunization
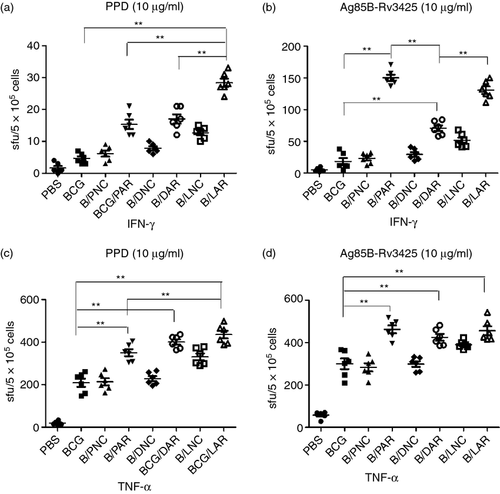
To assess the cellular immune responses elicited by the vaccines, IFN-γ and TNF-α ELISPOT assays were conducted to detect cellular immune responses induced in the spleens of mice by BCG priming and the PAR, DAR or LAR boost vaccination strategy (i.e. BCG/PAR, BCG/DAR or BCG/LAR, respectively). As shown in Fig. 3, IFN-γ and TNF-α levels, which were produced in response to either PPD or to Ag85B-Rv3425, were dramatically increased in BCG/PAR, BCG/DAR and BCG/LAR mice compared with mice vaccinated with BCG only (P < 0·01). Importantly, the spleens of LAR-boosted mice exhibited the most robust IFN-γ and TNF-α immune responses to PPD when compared with those of PAR-boosted and DAR-boosted mice (P < 0·01) (Fig. 3a, c). In contrast, BCG/PAR vaccination elicited more robust IFN-γ immune responses to Ag85B-Rv3425 in the spleens than BCG/DAR or BCG/LAR immunization (Fig. 3b). However, no significant differences in TNF-α responses were observed among BCG/PAR-, BCG/DAR- and BCG/LAR-vaccinated mice (Fig. 3d). In addition, secreted levels of IFN-γ and TNF-α were increased in BCG/PAR-, BCG/DAR- and BCG/LAR-vaccinated mice compared with the corresponding controls. Hence, our results suggested that BCG priming and the LAR boost vaccination strategy could increase PPD-specific IFN-γ and TNF-α levels and enhance cellular immune responses in mouse spleen.
Th1/Th2/Th17 cytokine profiles
The production of Th1 cytokines (i.e. IL-2, IL-6, TNF-α and IFN-γ), Th2 cytokines (i.e. IL-4 and IL-10) and Th17 cytokines (i.e. IL-17) by splenocytes was assayed using the Cytometric Bead Array to evaluate the effects of the vaccines on Th1/Th2/Th17 immune responses (Table 1). Mice immunized with BCG/LAR and stimulated with PPD produced nearly eightfold more IL-2 than mice immunized with BCG/PAR and BCG/DAR. After PPD stimulation, IFN-γ, IL-17A and IL-6 levels were twofold higher in BCG/LAR-vaccinated mice than in the BCG/PAR- and BCG/DAR-immunized groups. The remaining cytokines (i.e. TNF-α, IL-4 and IL-10) were marginally higher in BCG/LAR-vaccinated mice than in BCG/PAR- and BCG/DAR-immunized mice. The levels of these cytokines produced in response to Ag85B-Rv3425 in the groups that were primed with BCG and boosted with PAR were higher than those in the groups that were boosted with LAR or DAR (P < 0·05). Further, the observed concentrations of TNF-α and IFN-γ were consistent with the data presented in Fig. 3.
| Cytokine | PPD | Ag85B-Rv3425 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PBS | BCG | B/PAR | B/DAR | B/LAR | PBS | BCG | B/PAR | B/DAR | B/LAR | |
| IFN-γ | – | 2·46 ± 0·85 | 14·68 ± 1·39 | 15·46 ± 1·09 | 37·81 ± 7·14 a | – | 19·55 ± 3·99 | 1886·0 ± 188·82 a | 317·13 ± 35·67 | 449·75 ± 42·63 |
| IL-10 | 3·14 ± 0·72 | 5·15 ± 1·02 | 8·97 ± 1·49 | 10·69 ± 2·82 | 19·70 ± 5·88 | – | 23·86 ± 11·10 | 95·24 ± 13·38 a | 48·31 ± 17·17 | 84·87 ± 12·38 a |
| IL-17A | – | – | 1·67 ± 0·97 | 1·29 ± 0·48 | 3·57 ± 1·62 a | – | – | 50·14 ± 9·23a | 1·82 ± 0·54 | 2·05 ± 0·84 |
| IL-2 | – | 2·09 ± 0·24 | 2·96 ± 1·23 | 2·71 ± 0·86 | 16·70 ± 1·56 a | – | 2·21 ± 0·28 | 68·65 ± 16·06 a | 21·6 ± 4·13 | 26·58 ± 3·55 |
| IL-4 | – | – | 1·72 ± 1·02 | 1·34 ± 0·48 | 2·83 ± 0·89 | – | – | 5·32 ± 1·24 | 1·60 ± 0·45 | 3·82 ± 0·85 |
| IL-6 | 12·53 ± 0·89 | 30·15 ± 9·35 | 28·60 ± 6·57 | 29·38 ± 8·50 | 72·21 ± 16·82 a | 26·66 ± 4·89 | 71·36 ± 5·39 | 294·76 ± 23·28 a | 99·00 ± 10·52 | 172·13 ± 16·82 a |
| TNF-α | 48·9 ± 5·69 | 164·88 ± 25·90 | 222·97 ± 62·33 | 232·45 ± 32·01 | 254·25 ± 26·25 | 84·21 ± 10·58 | 219·9 ± 38·28 | 778·03 ± 80·16 a | 346·68 ± 69·14 | 336·26 ± 50·86 |
- Abbreviations: BCG, bacillus Calmette–Guérin; DAR, DNA-based vaccine; IFN-γ, interferon-γ; IL, interleukin; LAR, lentiviral-based vaccine; PAR, protein-based vaccine; PPD, purified protein derivative; TNF-α, tumour necrosis factor-α.
- Unit: pg/ml. The results represent the mean and standard deviation of six animals per group.
- (Cytokine not tested).
- a Constant variance P value <0·05 for B/LAR versus B/PAR, B/LAR versus B/DAR or B/PAR versus B/DAR; highlighted in bold.
IFN-γ-positive CD4+ T-cell analysis and perforin-producing CD8+ T-cell analysis
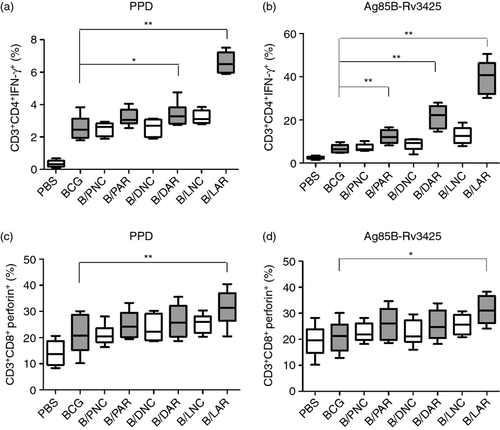
To determine the percentage of CD4+ T cells that were capable of producing IFN-γ and the percentage of CD8+ T cells that expressed perforin, we used an intracellular staining method with different combinations of monoclonal antibodies. As shown in Fig. 4(a), the average percentage of CD3+ CD4+ IFN-γ+ cells (6·49%) among the total CD4+ T-cell population after PPD stimulation in mice boosted with LAR was twofold to threefold higher than that observed in mice boosted with PAR and DAR (P < 0·01). Similarly, the highest overall frequency of CD3+ CD4+ IFN-γ+ cells after Ag85B-Rv3425 stimulation was also observed in mice boosted with LAR (Fig. 4b). Further, the frequency of CD3+ CD8+ perforin+ T cells was increased approximately 1·2- to 1·3-fold in mice boosted with LAR compared with the PAR- and DAR-boosted groups, when stimulated with either PPD or Ag85B-Rv3425 (Fig. 4c, d). Moreover, the results demonstrated that the PAR-, DAR- and LAR-boosted groups exhibited enhanced percentages of CD3+ CD4+ IFN-γ+ T cells and CD3+ CD8+ perforin+ T cells compared with their corresponding control groups. These results demonstrated that BCG priming and the LAR boost vaccination strategy could improve the ability of CD4+ T cells to secrete IFN-γ and CD8+ T cells to produce perforin.
Protective efficacy
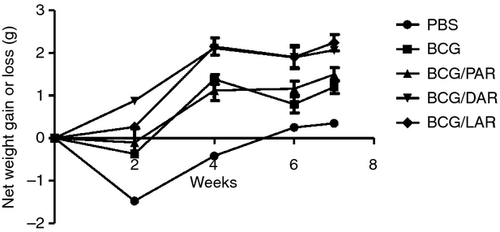
The protective efficacy of immunization with BCG only, BCG/PAR, BCG/DAR or BCG/LAR was then studied after challenge with live M. tuberculosis H37Rv. The total body weight was measured as a clinical evaluation of the critical status of the different groups (Fig. 5). Animals immunized with BCG/DAR or BCG/LAR gained weight from the 2nd week after challenge, and by the end of the experiment, an 11–13% weight gain was observed in these groups. In contrast, animals immunized with PBS, BCG or BCG-PAR lost weight during the 2 weeks after challenge and regained only 2–8% of the total body weight by the 7th week. Therefore, immunization with BCG/DAR or BCG/LAR protected challenged animals from clinical signs, as indicated by protection against weight loss.
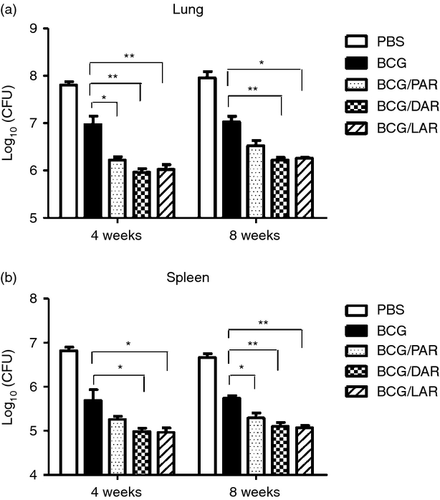
The number of CFUs of M. tuberculosis was determined to assess the bacterial load in the lungs and spleens of mice after challenge. All non-PBS-immunized mice exhibited a statistically significant reduction in viable M. tuberculosis in the lungs and spleens compared with PBS controls (Fig. 6). In the lungs (Fig. 6a), a significant reduction in bacterial load was observed 4 weeks after challenge in mice vaccinated with BCG/PAR, BCG/DAR and BCG/LAR compared with mice immunized with BCG only. However, a significant reduction in bacterial load was observed only 8 weeks after challenge in the BCG/DAR- and BCG/LAR-immunized groups compared with the BCG-immunized groups. We found that the bacterial load in mice vaccinated with BCG/DAR and BCG/LAR was reduced by 1 log10 compared with the BCG-immunized group (P < 0·01). Interestingly, the numbers of CFUs in the BCG/DAR-immunized groups were slightly lower than those of the BCG/LAR groups; however, this difference was not statistically significant. Moreover, a reduction of approximately 0·5 log10 in the burden of M. tuberculosis was observed in BCG/PAR-vaccinated animals compared with the BCG-immunized group. In the spleens (Fig. 6b), BCG/DAR and BCG/LAR immunization significantly reduced bacterial load compared with BCG immunization only at 4 weeks after challenge. Similarly, greater protection and significantly fewer CFUs were also observed in BCG/DAR- and BCG/LAR-immunized mice compared with mice immunized with BCG only at 8 weeks after challenge (P < 0·01). These results demonstrated that vaccination of C57BL/6 mice with BCG/DAR and BCG/LAR promoted improved control of bacterial replication in the lungs and spleens compared with vaccination with BCG and BCG/PAR.
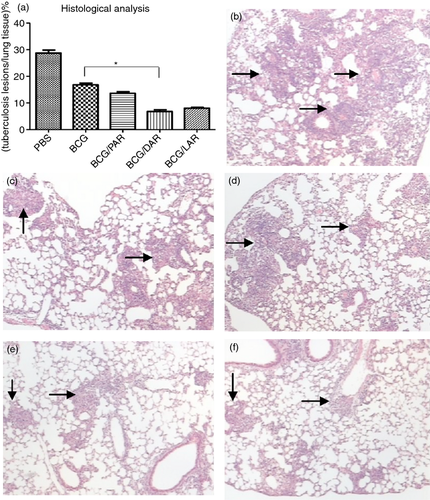
Histological analyses were performed 4 weeks and 8 weeks after M. tuberculosis H37Rv challenge. With respect to the percentage of TB lesions in the lung tissues (Fig. 7), 28% of the lung tissue of PBS-immunized mice developed pulmonary lesions 4 weeks after challenge. In sharp contrast, the percentages of pulmonary lesions in BCG-, BCG/PAR-, BCG/DAR- and BCG/LAR-vaccinated mice were significantly lower, at 16%, 13%, 6% and 8%, respectively. In addition, the area of the pulmonary lesions was significantly lower in mice immunized with BCG/DAR than in mice immunized with BCG alone; this finding is in accordance with the markedly reduced bacterial loads in the lungs of these animals. The lung histology of different groups of mice is illustrated in Fig. 7(b–f). After TB challenge, the PBS control mice exhibited severe tissue damage, with more tubercles and significant lamellar coalesced foci. In contrast, BCG- and BCG/PAR-immunized mice developed only mild tubercles and alveolar macrophage proliferation (Fig. 7c, d). In addition, very little alveolar macrophage proliferation and mild inflammation were observed in mice vaccinated with BCG/DAR and BCG/LAR (Fig. 7e, f). These results indicate that BCG/DAR and BCG/LAR significantly protected mice from severe lung damage caused by TB challenge.
Discussion
Effective TB vaccines with the ability to replace or boost BCG are urgently needed to limit the global spread of TB. Because the majority of the human population in the developing world is already BCG-vaccinated, an attractive strategy would be to boost these existing immune responses.15 However, homologous boosting with BCG failed to increase or lengthen protection against TB in humans.16 Heterologous prime–boost strategies rely on antigens that are present in BCG and capable of priming protective immunity. Three major types of heterologous boost vaccine candidates exist: protein-based, plasmid DNA-based and virus-based.17 Our previous studies revealed that a fusion antigen containing Ag85B and Rv3425 was immunogenic and could induce robust immune responses in mice after vaccination. In this study, we compared the ability of three formulations of this fusion antigen to enhance the immunity and protective efficacy of BCG priming in mice.
Mycobacterium tuberculosis is a typical intracellular pathogen; hence, T-cell-mediated immune responses that include CD4+ T cells, CD8+ T cells or both of these T-cell subsets are important for controlling infection and preventing or delaying the onset of disease.18 The ability of CD4+ T cells to produce IFN-γ, which activates phagocytes to engulf the intracellular pathogen, is central to protection.19 CD8+ T cells may modulate phagocyte activity or produce molecules such as perforin, which mediates the killing target cells and reduces bacterial survival.19 Our results demonstrated that an increased proportion of CD8+ T cells and a highly coordinated expression of the antimicrobial effector molecule perforin occurred in response to either PPD or to Ag85B-Rv3425 stimulation in BCG prime–boost-vaccinated mice compared with mice immunized with BCG only, particularly in BCG/LAR-vaccinated mice. Likewise, we also found that BCG/LAR induced a significant increase in the CD3+ CD4+ IFN-γ+ population compared with the other groups. To control M. tuberculosis growth, CD4+ T-cell responses are required throughout the infection; in contrast, when a chronic infection is established, the role of the CD8 T-cell response becomes more important.20, 21 Therefore, our results suggested that BCG/LAR could elicit M. tuberculosis-specific CD4+ T-cell responses, potentially increasing protective efficacy, and enhance CD8+ cytotoxic T lymphocyte (CTL) responses, potentially reducing bacterial loads during chronic infection.
Naive CD4+ T cells can differentiate into specialized effector T cells (i.e. Th1, Th2 or Th17 cells), which are identified based on cytokine profiles.22 Th1 cells produce IFN-γ, TNF-α and IL-2, which promote the proliferation, maturation and activation of macrophages and granulocytes. Th2 cells generate IL-4, IL-5, IL-6 and IL-10, which promote antibody production and suppress Th1 immune responses.23 Th17 cells secrete the IL-17 family of proteins, which mediate the recruitment of protective Th1 cells to the lung after M. tuberculosis challenge.24 In our study, a Th1/Th2/Th17 cytokine profile analysis indicated that different levels of Th1/Th2/Th17cytokines occur in BCG prime–boost-vaccinated mice compared with the group immunized with BCG only. The observed rise in Th1/Th17 cytokines was higher than the observed rise in Th2 cytokines. Our results suggested that effector immune responses and suppressive immune responses occurred simultaneously in BCG prime–boost-vaccinated mice but that effector immune responses were predominant. In this respect, BCG/LAR induced more IFN-γ, TNF-α, IL-2 and IL-17 secretion in response to PPD than BCG/PAR and BCG/DAR. It is worth mentioning that the secretion of IL-2 was enhanced eightfold in BCG/LAR mice compared with the BCG group. Interleukin-2 amplifies effector T-cell responses and is crucial for programming CD8+ T cells for improved memory capacity and effector function.25, 26 Consequently, BCG/LAR immunization could increase Th1/Th17 responses and promote the expansion of CD4+ and CD8+ T cells.
Moreover, cytokine release at the single cell level using the ELISPOT assay also demonstrated that BCG/LAR immunization resulted in higher levels of IFN-γ and TNF-α secretion after stimulation with PPD than BCG/PAR and BCG/DAR. Previous studies provided convincing evidence that for M. tuberculosis infection, Th1 responses and the protective cytokines IFN-γ, IL-17 and TNF-α are necessary and may be sufficient for vaccine-elicited protection.27 In this work, we also used the antigen-specific IgG2b/IgG1 ratio (data not shown) as an indicator of whether a predominantly Th1 or Th2 response was induced by vaccination. Our results demonstrated that vaccination with BCG/LAR and BCG/DAR resulted in the production of an antigen-specific antibody response biased toward IgG2b, which is a Th1-type antibody isotype.
The loss of body weight, which is a vital indicator of TB severity in experimental animals and humans, was used to monitor TB progression.28 Our results clearly demonstrated that no weight loss occurred in the BCG/DAR- and BCG/LAR-immunized groups after M. tuberculosis challenge. Considering the relevance of weight loss to survival and disease progression, we conclude that DAR and LAR could significantly enhance the ability of BCG to prevent TB progression and severity in the context of our prime-boost strategy. Histological analysis further confirmed that BCG/DAR- and BCG/LAR-vaccinated mice exhibit less severe histopathology than BCG- and BCG/PAR-vaccinated mice after M. tuberculosis H37Rv challenge.
In the intravenous model, both spleen and lung bacterial load counts (i.e. CFUs) are typically used to determine whether a candidate vaccine exerts a protective effect.29 Previous studies demonstrated that if the bacterial load is significantly reduced (i.e. by a minimum of 0·7–0·8 log10), then the vaccine can be considered effective.30 In our study, BCG/DAR and BCG/LAR immunization induced a 1·0 log10 reduction in CFUs in the lung 4 weeks after challenge compared with BCG vaccination. The CFU data from the lung indicates the level of circulating T cells capable of entering inflammatory sites in the tissues;30 hence, BCG/DAR and BCG/LAR recruited more circulating T cells to the site of inflammation in the lung than BCG. Likewise, in the spleen, BCG/DAR and BCG/LAR yielded significantly fewer CFUs than BCG 8 weeks after challenge, indicating that in the BCG/DAR- and BCG/LAR-immunized groups, higher levels of sensitized T cells were generated in the spleen. Higher numbers of these cells also entered the circulation via the thoracic duct from lymph nodes draining the sites of vaccine inoculation.30 Therefore, the T cells that are recruited and sensitized by BCG/DAR and BCG/LAR vaccination would be able to inhibit or delay TB replication and activation in the lung and consequently improve the protective effect of BCG against M. tuberculosis.
Prime-boost immunization can significantly augment the breadth of the induced immune responses, possibly because the divergent cell-targeting and antigen-processing routes complement one another and because the diversity of epitopes is greater than with either agent alone.31 In our study, we found that PAR, DAR and LAR could increase the immunogenicity of BCG to different degrees. Several comparative studies demonstrated that lentiviral vectors are more potent for eliciting cellular protective immunity than other viral vectors or other vaccine strategies, such as DNA vaccines and peptide-pulsed vaccines.32-34 In this study, we also demonstrated that BCG/LAR induced significantly stronger immune responses, including Th1 and CD8+ CTL responses, than BCG/PAR and BCG/DAR. However, BCG/DAR provided similar or better protective efficacy against M. tuberculosis challenge than BCG/LAR. DNA vaccination can alter the hierarchy of immunodominance in T-cell responses,35 possibly through more efficient MHC loading after synthesis in antigen-presenting cells.31 Therefore, for the Ag85B and Rv3425 fusion antigen, lentiviral-based vaccines and DNA vaccines represented the best heterologous boosting strategies for BCG priming. In addition, this study supported the view that the immune responses that are currently considered to be crucial for protection against TB are not sufficient for protection and do not represent usable correlates of risk or protection in the context of vaccine trials.19, 36, 37
In conclusion, in this study, we provide evidence that lentiviral vaccines containing coding sequences for Ag85B and Rv3425 induce significantly stronger Th1 and CD8+ CTL immune responses than protein- and DNA-based vaccines when used for booster immunization after BCG vaccination. BCG priming and a LAR or DAR vaccination boost strategy could improve the protective efficacy of BCG vaccination against M. tuberculosis, as indicated by a lack of weight loss and significantly reduced bacterial loads and histological damage in the lung. Our study suggests that for the Ag85B and Rv3425 fusion antigen, the use of lentiviral and DNA-based vaccines to boost BCG priming is a good choice for the rational design of an efficient vaccine against TB.
Acknowledgements
We thank Zhijiao Tang and Rong Bao for technical support in the mouse challenge experiment. This work was supported by grants from National Major Special Projects (2012ZX10003008), the NSF of China (31100660) and the NSF of Shanghai Sci. Tech. Committee (11ZR1401600).
Disclosures
The authors have declared that no competing interest exists.




