Muscle disorders: the latest investigations
Abstract
Patients with muscle disorders can present a diagnostic challenge to physicians because of the different ways they can present and the large number of different underlying causes. Recognition of the ‘myopathic phenotype’ coupled with investigations usually including electrodiagnostic and histological investigations have been essential for diagnosing the underlying cause of a myopathy. Despite these standard investigations, some patients can remain undiagnosed. New tests including more specific antibody tests for immune-mediated myopathies and the introduction of next-generation sequencing promise to revolutionise diagnostic approaches for immune and inherited myopathies, but clinical expertise remains essential to choose the most appropriate tests and interpret the results. The aim of this review is to provide an overview of the different presentations to the neuromuscular clinic and the latest investigations that can be helpful in the diagnosis of muscle disorders.
Abbreviations
-
- ANA
-
- antinuclear antibody assay
-
- CK
-
- creatine kinase
-
- EM
-
- electron microscopy
-
- EMG
-
- electromyography
-
- FSHD
-
- facioscapulohumeral muscular dystrophy
-
- HMGCR
-
- 3-hydroxy-3-methylglutaryl-coenzyme A reductase
-
- MHC
-
- major histocompatibility class
-
- MRI
-
- magnetic resonance imaging
-
- NAM
-
- necrotising autoimmune myositis
-
- NGS
-
- next-generation sequencing
-
- sIBM
-
- sporadic inclusion body myositis.
Introduction
Muscle disorders comprise a heterogenous group of diseases that are either inherited or acquired. These can present in a wide variety of ways, but the most common symptom attributable to primary muscle disease is weakness.1 There has been a tremendous increase in the number of diagnostic tests available for investigation of muscle disorders in recent years, particularly for genetic myopathies. Gene sequencing is now available for many more disease genes, and the advent of new genetic techniques such as high-throughput sequencing of specialised gene panels, and even whole exome and whole genome screening is leading to higher success rates for identifying causative mutations compared with standard candidate gene sequencing. However, prior to embarking on an exhaustive and often costly list of investigations, a thorough history and examination are important to aid in the selection of the investigations that are most likely to yield results and to identify conditions for which specific treatment is available.
Overview of the clinical presentations of muscle disorders
When a patient presents with muscle weakness, ascertaining the segment of the neuroaxis that is primarily compromised is the first step, then investigations are directed towards finding the underlying pathology. Muscles can become weak for many reasons, but history and examination usually can differentiate between peripheral neuropathy, neuromuscular junction and myopathy. These disorders can overlap clinically, and further investigations including nerve conduction studies and electromyography (EMG) are often required. Once the pathology has been localised to the muscle, the clinician must then establish if it is a primary disorder of muscle, or whether the muscle symptoms are part of a more generalised disorder. Systemic disorders that involve muscle are summarised in Table 1. In addition, the clinician must establish whether the disorder is likely to be inherited or acquired, as this can change the order in which investigations are performed.
| Endocrine causes |
Diabetes mellitus Vitamin D deficiency Hypothyroidism and hyperthyroidism Adrenal dysfunction (e.g. Cushing's syndrome) Parathyroid (both hyperparathyroidism and hypoparathyroidism) |
| Drugs and toxins (common) |
Corticosteroids Alcohol Statins Colchicine Drugs of abuse (heroin, amphetamines, cocaine, phencyclidine) |
| Connective tissue disorders |
Rheumatoid arthritis Systemic lupus erythematosus Mixed connective tissue disease |
| Tumours | Tumours causing a paraneoplastic myopathy/NAM |
| Infections | Viruses (e.g. influenza, HIV) |
| Inflammatory | Idiopathic inflammatory myositis |
- HIV, human immunodeficiency virus; NAM, necrotising autoimmune myositis.
Skeletal muscle has only a limited number of responses to an insult, and therefore, patients present with only a limited number of symptoms and signs. Positive symptoms include myalgias, cramps, contractures, myoglobinuria and muscle stiffness. Negative symptoms are weakness, exercise intolerance, fatigue and muscle atrophy. Myalgias, cramps and fatigue are non-specific symptoms2 that are common among patients referred to the neuromuscular clinic and do not necessarily indicate a primary muscle disease, especially in the setting of a normal physical examination. Some constellations of symptoms in the history and signs on examination can be suggestive for a particular muscle disorder.
Clues to specific diseases with particular symptoms and signs
A history of muscle ‘stiffness’ in the presence of myotonia (such as grip myotonia) with distal weakness on examination points to myotonic dystrophy type 1. Myotonia that is worse after rest such as difficulty standing up from sitting or starting to walk, often in association with muscle hypertrophy, is suggestive of myotonia congenita. Myotonia exacerbated by physical activity, particularly in the cold and most severe in the face and forearms, points to paramyotonia congenita.
The presence of contractures on clinical examination when striking and present early in the disease course can be suggestive of Emery–Dreifuss muscular dystrophy (with early elbow contractures) or a collagen VI disorder such as Bethlem myopathy. Prominent spinal rigidity and/or scoliosis because of paraspinal muscle contractures occur in collagen VI, and SEPN1- and MYH7-myopathies. Contractures are due to fibrosis and muscle shortening with weakness of antagonistic muscles.
Cramping is often present in neurogenic disorders associated with motor nerve hyperactivity and less commonly with primary muscle disorders. Neuromyotonia and stiff-man syndrome can present with muscle cramps. Cramping on physical exertion can also occur with some of the metabolic myopathies and occasionally muscular dystrophies such as rippling muscle syndrome (because of mutations in CAV3, which encodes caveolin 3).
Myoglobinuria results from the release of myoglobin from muscle during periods of muscle destruction (rhabdomyolysis). If severe, this can result in renal failure from acute tubular necrosis. Recurrent myoglobinuria often points to an underlying myopathy such as a metabolic myopathy, muscular dystrophy or RYR1-myopathy (Table 2).3, 4 Recessive mutations in LPIN1 are associated with life-threatening episodes of rhabdomyolysis in children during intercurrent illnesses with normal muscle function between episodes.
| Single | Recurrent |
|---|---|
| Physical factors (exercise, surgery) | Severe childhood: LPIN1 |
| Infections | Glycogen metabolism disorders |
| Drugs | Carnitine palmitoyltransferase II (CPT2) |
| Malignant hyperthermia | Mitochondrial |
| Malignant hyperthermia loci (MH) | |
| Acyl-CoA dehydrogenase (VLCAD) |
The presence of muscle pain in isolation without other clinical signs is a common complaint and one in which investigation is often unrewarding. It may be a symptom of a systemic disorder such as chronic fatigue syndrome, fibromyalgia, drug use (including amiodarone, statins, fibrates, steroid withdrawal, alcohol or heroin), infection or inflammation (including eosinophilia-myalgia syndrome, sarcoidosis or myositis). However, exercise-induced myalgia can be suggestive of a glycogen-storage disorder, a lipid storage disorder, a muscular dystrophy or ischaemia (claudication). Muscle fatigue can also be non-specific, but abnormal fatigability after exercise occurs in certain metabolic and mitochondrial myopathies in addition to neuromuscular junction disorders (Table 3).3, 5
|
Disorders of glycogenoses (enzyme deficiency)
Disorders of lipid metabolism (enzyme deficiency)
Disorders of mitochondrial metabolism (enzyme deficiency)
|
Clues on examination from the pattern of muscle involvement
Clinical signs, in particular the pattern of muscle weakness, provide important clues to the diagnosis because some patterns of weakness are pathognomonic of particular muscle conditions. For example, sporadic inclusion body myositis (sIBM) is characterised by severe weakness in the knee extensors and long forearm flexors. Facioscapulohumeral muscular dystrophy (FSHD) is characterised by asymmetrical involvement of the biceps, humeral head of the pectoralis major, tibialis anterior, serratus anterior and facial muscles. Scapulospinal myopathy, resulting in head drop or camptocormia, occurs typically in scleroderma. Involvement of the extraocular muscles occurs in myasthenia gravis, myotonic dystrophy, thyroid disorders, mitochondrial myopathies, oculopharyngeal muscular dystrophies and some congenital myopathies. The presence of a selective pattern of muscle involvement often points to an inherited muscle condition, in contrast with the acquired myopathies that are generally non-selective, and affect the large proximal girdle muscles (with the notable exception of sIBM).
In addition, the presence of muscle hypertrophy or pseudohypertrophy can occur in a number of muscle disorders such as muscular dystrophies (particularly the dystrophinopathies and some limb girdle muscular dystrophies), myotonia congenita and neuromyotonia.
Other organ involvement
Ascertaining the involvement of other organs such as respiratory, cardiac and central nervous system involvement can aid the diagnostic process. For example, muscle disorders associated with cardiac involvement include myotonic dystrophy, dystrophinopathies, mitochondrial myopathies, dermatomyositis, lipid storage disorders and some muscular dystrophies. Moreover, some muscle disorders involve the respiratory muscles early in the disease course (most notably Pompe disease). Typically, respiratory dysfunction begins with nocturnal hypoventilation, which may present with excessive daytime somnolence or morning headache. Mitochondrial myopathies can involve multiple systems and can present with a multitude of problems including deafness, stroke-like episodes, encephalopathy, seizures, diabetes, retinal pigmentary changes, gastrointestinal problems, peripheral neuropathy, multiple lipomas and renal failure, in addition to myopathy. These may be unrecognised until clinical examination or investigations are undertaken. Electrocardiogram, holter monitor, echocardiogram, respiratory function tests (erect and supine forced vital capacity, maximal inspiratory and expiratory pressures, and nasal pressures) and occasionally formal sleep studies are indicated to find cardiorespiratory involvement.
Importance of family history
A thorough family history is essential, and a three-generation pedigree should be constructed. Sometimes, this reveals strong evidence for a particular inheritance pattern that provides an important diagnostic clue. However, many patients with a genetic neuromuscular disorder have no family history because of recessive inheritance, the presence of a new dominant mutation (20–30% of probands with FSHD and dystrophinopathies), or because disease is mild or subclinical in previous generations, which is a characteristic of trinucleotide repeat disorders because of anticipation.
When the typical clinical presentation for a genetic muscle disorder is found on history or examination (as often is the case in FSHD or myotonic dystrophy), it is now standard to go directly to gene testing. However, when the answer is not clear after the initial evaluation, further investigations are required.
Initial investigations
Blood tests
A stepwise approach to narrowing down the diagnosis of patients presenting with muscle disorders can be helpful in selecting the investigations that are likely to be of highest yield (Fig. 1).
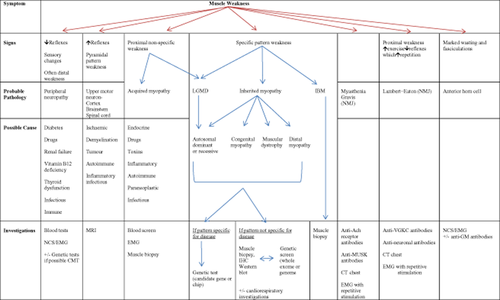
History, examination and investigation of muscle weakness. CT, computed tomography; EMG, electromyography; GM, antiganglioside antibody (subtype); IBM, inclusion body myositis; IHC, immunohistochemistry; LGMD, limb girdle muscular dystrophy; MUSK, muscle-specific kinase; NCS, nerve conduction study; NMJ, neuromuscular junction; VGKC, voltage-gated potassium channel.
Initial investigations should screen for reversible causes of a myopathy. This should include serum electrolytes (potassium, calcium, phosphate and magnesium), glucose, as well as a thyroid-stimulating hormone assay and vitamin D to screen for endocrine myopathies. Vitamin D deficiency can cause osteomalacia,6 predispose to the development of statin myopathy and exacerbate other underlying myopathies.7 More specific assays can be performed if clinical suspicion for an endocrine myopathy is high.2 A blood spot for Pompe disease is a useful screen in patients with a limb girdle pattern of weakness or prominent respiratory muscle weakness.
Creatine kinase (CK) level should also be checked. A persistent, markedly raised CK level usually indicates myositis, a muscular dystrophy or recurrent rhabdomyolysis. Although CK is an important test that should be performed routinely, it is not specific for myopathy as it can be moderately elevated in some neuropathies and systemic diseases such as hypothyroidism and hypokalaemia,8 and normal in some myopathies. In addition, it can be slightly elevated in some families without an obvious underlying cause, often referred to as familial hyperCKaemia, which has a benign prognosis.9, 10 It is also important to remember that baseline CK levels can also be influenced by physiological conditions such as muscle mass, ethnicity or recent physical activity.11
The next step is to differentiate between inflammatory, metabolic and primary genetic myopathies, including muscular dystrophies. Blood tests should include an autoimmune screen, C-reactive protein, an erythrocyte sedimentation rate, rheumatoid factor, anticyclic citrullinated peptide antibodies, antinuclear antibody assay (ANA) and consider myositis specific antibodies that are helpful in suspected immune-mediated myopathies.
Many patients with myositis have auto-antibodies that bind to components of the nucleus or cytoplasm of cells, and can be detected with standard immunofluorescence assays for ANA. Some are specific for myositis, while others are associated with multisystem autoimmune diseases that may include myositis (Table 4).12-14 Notable among the myositis-specific antibodies are those that bind to the transfer RNA synthetases. These are markers of the ‘antisynthetase syndrome’ that includes myositis, interstitial lung disease, polyarthritis, fever, Raynaud's phenomenon and calloused hands.
| Myositis-specific antibodies | Disease association | Myositis-associated antibodies | Disease association |
|---|---|---|---|
|
Anti-tRNA Synthetases: Anti-Jo-1, Anti PL-7, Anti-PL-12, Anti-OJ, Anti-EJ |
Antisynthetase syndrome (in PM or DM) | Anti-PM/SCL | PM, DM, systemic sclerosis |
| Anti-SRP | NAM | Anti-U1RNP | Overlap syndrome (SLE/systemic sclerosis/MCTD) |
| Anti-Mi-2 | DM (classical DM with skin lesions and muscle involvement, often more favourable response to treatment) | Anti-RNA polymerase | Overlap syndrome (SLE/systemic sclerosis) |
| Anti-MDA5 | DM (dermatopulmonary syndrome with absent or mild muscle disease, high risk interstitial lung disease, characteristic mucocutaneous disease, arthralgia) | Anti-TH/TO | Overlap Syndrome (systemic sclerosis) |
| Anti-TIF1(γ) (Anti-155/140) | DM with higher risk of malignancy | ||
| Anti-NXP-2 | Juvenile DM (associated with severe muscle weakness, polyarthritis, joint contractures, higher incidence of calcinosis and interstitial vasculitis) | ||
| Anti-HMG-CoA Reductase | Statin-associated NAM | ||
| Anti-cytosolic 5′-nucleotidase 1A (NT5C1A) | Sporadic IBM |
- DM, dermatomyositis; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-CoA; IBM, inclusion body myositis; NAM, necrotising autoimmune myositis; PM, polymyositis; RNA, ribonucleic acid; RNP, ribonucleoprotein; SCL, scleroderma; SRP, signal recognition particle.
Another important marker of a severe myositis, now called necrotising autoimmune myositis (NAM), is anti-signal recognition particle.15-17 This entity has also been associated with tumours, viral infections (human immunodeficiency virus and hepatitis C) and statin use.18, 19 Statin-related NAM can present after years of statin use and mimics polymyositis, which continues or even progresses despite cessation of statins. Antibodies against the 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) protein were found to be highly associated with previous statin use. Testing for HMGCR antibodies can now be performed and are highly specific for statin-associated NAM.20 The sensitivity of this test is still being determined.
Recently, myositis-specific autoantibodies known as cytosolic 5′-nucleotidase IA were found in sIBM patients.21, 22 These antibodies were detected in the sera of 33% of sIBM patients compared with <5% in dermatomysositis and polymysositis patients. This represents the first serological biomarker for sIBM. It may facilitate the diagnosis of this type of myositis and help differentiate it from polymyositis in the early stages where the biopsy can look as inflammatory.14, 22 In addition, some have been discovered to predict a higher risk of malignancy in patients with dermatomyositis.13
Myositis-specific autoantibodies can be useful to confirm autoimmune myositis, but their main role is to subclassify and thereby to assist with prognosis and choice of treatment.
Aerobic forearm exercise testing
Forearm exercise testing can also be useful as part of the investigation for a suspected glycogen storage disorder. The test should be performed aerobically without the use of a tourniquet to reduce the risk of compartment syndrome. The patient is asked to use a handgrip dynamometer and perform isometric contractions for 4 s and then releasing for 1 s over a 1-min period at 80% of maximum grip strength with maximal effort.23 Venous oxygen, lactate and ammonia are collected at baseline and at 1, 2, 5 and 10 min. A blunted rise in lactate is an indication of acid maltase deficiency (Pompe disease), while myophosphorylase or a phosphofructokinase deficiency will not result in a change in the lactate level. A normal rise in lactate with minimal or no rise in ammonia level is an indication of myoadenylate deaminase deficiency.23
Role of EMG
EMG is most helpful differentiating myopathies from motor neuropathies, anterior horn cell and neuromuscular junction disorders. EMG assesses several components of muscle electrical activity: the muscle's spontaneous activity, its response to the insertion of a probe, the character of the muscle's individual motor unit action potentials and the rapidity with which additional motor units are recruited in response to an electrical signal. For example, in an inflammatory myopathy, there is increased spontaneous activity with presence of fibrillations, complex repetitive discharges and positive sharp waves.24, 25 EMG can also be helpful in identifying myotonic disorders insertion of the electrode and may trigger an extended series of repetitive discharges lasting up to 30 s.26
More specialised investigations
Muscle ultrasound
Ultrasound muscle imaging has been mainly used as a non-invasive tool in the paediatric population for assessment of skeletal muscle disorders.27-29 Specific neuromuscular disorders can differ in the pattern of muscles involved, which is reflected in the ultrasonographic appearance of various muscles.28, 29 For example, with many muscular dystrophies, there is a homogeneously increased echo intensity in the biceps and quadriceps, while in spinal muscular atrophy, muscle changes are typically inhomogeneous with both atrophic and hypertrophic regions present.28
Muscle magnetic resonance imaging
Magnetic resonance imaging (MRI) can be a useful tool in the evaluation of deep muscles not readily accessible to EMG and can identify subclinical muscle involvement aiding the diagnostic process. Several previous studies have reported distinct patterns of muscle involvement (fatty infiltration and muscle atrophy) of thighs and lower legs in various forms of inherited myopathy.30, 31 Some myopathies, such sIBM (Fig. 2) and those because of mutations in RYR1 and DNM2 have distinctive patterns that can direct genetic testing to those genes. It provides a multiplanar and non-invasive examination of the muscles without exposure to radiation in adults and children over 5 who can tolerate the procedure without a general anaesthetic.30, 31 T2-weighted fat-suppressed and T2-weighted images can be used for the detection of oedema and thus inflammation (most helpful in myositis). T1-weighted images are useful for detecting fatty infiltration that often accompanies dystrophic processes.32 A secondary role for muscle MRI is to provide information about the best site for a muscle biopsy by showing which muscles are involved in the myopathic process.
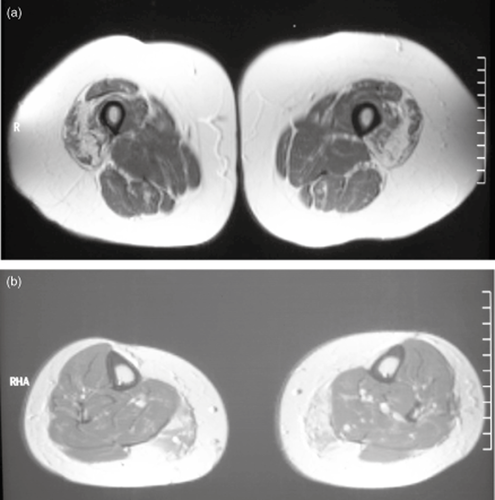
(a) Magnetic resonance imaging (MRI) of cross-sections through the thighs demonstrating selective atrophy of the vastus muscles with relative sparing of the rectus femoris and hamstring muscles. (b) MRI of cross-sections through the lower leg demonstrating fatty atrophy of the medial head of the gastrocnemius muscles. (Courtesy of Professor Frank Mastaglia.)
Muscle biopsy
The decision to undertake a muscle biopsy is usually the next step in patients when the diagnosis remains inconclusive after non-invasive investigations have been completed. The best muscles to biopsy are those moderately affected by the disease process but not diseased to the point of severe atrophy. Previous sites of injections, EMG examination or trauma should be avoided. The most common biopsy sites are the deltoid, biceps and vastus lateralis. If a susceptibility to malignant hyperthermia is suspected, then an in vitro contracture test can be performed at the same time as the muscle biopsy, usually by an anaesthetist.33
The clinical history should be available to the pathologist when analysing the biopsy, as it influences which specialised stains are to be performed and whether electron microscopy (EM) is required. While the presence of ‘dystrophic features’ is helpful to narrow the diagnosis to a muscular dystrophy, the features are rarely specific for a particular subtype, and further testing is usually required. Immunostaining and Western blotting that use specific antibodies to detect the presence and levels of various proteins in frozen muscle biopsies can sometimes provide presumptive or definitive diagnoses (e.g. merosin-deficient congenital muscular dystrophy, dystrophinopathies, sarcoglycanopathies).34, 35 Other more specific findings that can be demonstrated include glycogen accumulation (glycogen storage diseases), lipid accumulation (lipid storage myopathy), ragged red fibres (mitochondrial myopathies) or amyloid deposits (in amyloidosis or sIBM).2 In inflammatory myopathies, immunostaining for major histocompatibility classes I and II (MHC-I/II) are upregulated in myofibres, whereas MHC-I immunostaining alone is non-specific.36, 37 It should be noted that some muscular dystrophies can be very inflammatory, and occasionally, this causes confusion.
Other non-standard histochemical stains have specific roles in diagnosing particular myopathies (Table 5), which is one reason that communication between the clinician and pathologist is important.
| Stain | What it demonstrates |
|---|---|
| Hematoxylin and eosin (H&E) | Basic cellular and tissue structure |
| Fibre type staining (ATPase; fast/slow myosin) | Differentiates type I, IIA and IIB fibres |
| NADH | Mitochondrial disorders |
| Core myopathies | |
| Succinate dehydrogenase | Mitochondrial disorders |
| Cytochrome oxidase | Mitochondrial disorders |
| Modified Gomori trichome | Mitochondrial disorders |
| Nemaline myopathy | |
| Vacuoles | |
| Phosphorylase | Phosphorylase deficiency |
| Phosphofructokinase | Phosphofructokinase deficiency |
| Acid phosphatase | Activated macrophages |
| Periodic acid-Schiff (PAS) | Glycogen |
| Myoadenylate deaminase | Myoadenylate deaminase deficiency |
| Sudan black, oil-red-o | Disorders causing lipid accumulation |
| Congo rod | Amyloid |
- NADH, nicotinamide adenine dinucleotide plus hydrogen.
EM
EM allows visualisation of the ultrastructural components of muscle fibres and can show abnormalities that are not apparent on light microscopy stains. It is used in suspected mitochondrial myopathies to identify paracrystalline inclusions in suspected sIBM to identify the tubulofilamentous inclusions and characterise accumulations of abnormal material such as in amyloid and myofibrillar myopathies. Not all biopsies require evaluation with EM.
Exome sequencing
Until now researchers have relied on candidate gene sequencing or linkage analysis in large families to attempt and identify the genes that cause hereditary myopathies. With the advent of improved computing power combined with genetic techniques next-generation sequencing (NGS), we now have the potential to identify genetic variations at basepair resolution throughout the human genome in a single experiment. NGS methods included modalities such as microarray or ‘chip’ technology, the sequencing of custom gene panels, whole exome sequencing and whole genome sequencing.
Sequencing custom gene panels usually involves targeted ‘capture’ or amplification of a specific list of genes, for example all genes known to cause neuromuscular disease. New gene sequencing techniques often make it cheaper to sequence 200 genes in a custom panel than standard (Sanger) sequencing of a single large gene.38 Custom panels for various combinations of neuromuscular genes are available overseas, and Australian panels are being established.
Whole exome sequencing targets the proportion of DNA that codes for proteins (the Exome, which accounts for ∼2% of the human genome), ‘capture’ and sequencing. In whole genome sequencing, the ‘capture’ step is omitted, and the entire DNA code (both the coding and non-coding) is sequenced generating a wealth of information that requires sophisticated filtering and analysis. The new sequencing techniques are revolutionising our ability to check the sequence of multiple genes at once at minimal cost but pose new challenges for laboratoriesand clinicians. A current challenge is to differentiate between DNA sequence changes that cause disease from the thousands of small variations in genetic code between individuals that do not lead to disease (polymorphisms).39 Over time, our understanding of the range of variations in DNA in healthy individuals will improve, which will help laboratories and researchers distinguish these from disease-causing DNA changes. These new ‘genome-wide’ techniques have greatly improved our ability to identify new disease genes, even in rare conditions in which only a handful of families has been identified in the world.40
As whole-genome sequencing becomes more commonly used, the data can be applied to study complex traits and multigenetic disorders.
The major challenge in the next few years will be to analyse and manage the large amounts of sequencing data generated. Bioinformatic approaches are required to filter out potential candidate pathogenic variants/genes. Currently, the variations detected by exome sequencing require validation by Sanger sequencing.
There are also several limitations of these new technologies that need to be addressed. One limitation relates to the sensitivity and specificity of the detection of some classes of mutations. For example, guanine-cytosine-rich sequence stretches can be difficult to capture and sequence as found by Hoischen et al. and so many genes may not be 100% sequenced by the new technologies.41 The current methods have a limited ability to detect large deletions and duplications that are an important class of mutation in many neuromuscular diseases. Common examples include Duchenne muscular dystrophy and Charcot–Marie–Tooth disease type 1A. Expansions or contractions in repetitive sequences are particularly difficult to detect by current high-throughput sequencing methods. For this reason, different methods are needed to diagnose FSHD and myotonic dystrophy type 1, the two most common adult-onset muscular dystrophies.
The new genetic technologies bring with them a range of new ethical issues to consider. For example, when hundreds or thousands of genes are analysed at once, it is possible to uncover genetic information about health risks quite separate to the neuromuscular condition being investigated. What type of information should be given to patients before these new tests are undertaken, what type of consent should be sought, and how should ‘incidental’ genetic findings be handled? The volume of health-related information that is potentially extractable from whole genome sequencing is enormous. How should the health system help patients to understand and use this information in an informed and positive way?42, 43 Laboratories and clinics are addressing these issues as new tests make their way into routine clinical care. All medical staff, and to some extent patients as well, will need to become familiar with many of these issues so the exciting opportunities to understand our genetic sides translates into better lives rather than anxiety or impractical demands on our health system.
Conclusion
Patients with muscle disorders can present a diagnostic challenge. Narrowing down the differential diagnoses requires a stepwise approach to minimise the number of investigations required to establish a diagnosis. Although traditional investigations such as muscle biopsies remain useful, NGS methods will enable non-invasive diagnosis of inherited muscle diseases. Establishing the correct diagnosis is a vital first step in the future treatment of muscle disorders. However, using these genetic tools for diagnosis will also present multiple challenges in terms of interpretation of the generated data and also raise issues regarding consent. Conveying this information to the patient and their family will often require the involvement of clinical geneticists and counsellors. Finally, as this technology becomes more established in clinical practice, it will be important to consider how such testing is best coordinated to ensure optimal patient care.
Acknowledgement
We gratefully acknowledge Professor Frank Mastaglia's helpful comments on the manuscript and the addition of Figure 2 from his archives.




