CC chemokine receptor 6 (CCR6) in the pathogenesis of systemic lupus erythematosus
Abstract
The CC chemokine receptor 6 (CCR6) and its sole chemokine ligand, CCL20, are an intriguing pair that have been implicated in a growing number of inflammatory, autoimmune and malignant disease processes. Recent observations have also highlighted this chemokine axis in the regulation of humoral immune responses. Through this review article, we explore the emerging links of CCR6–CCL20 with an archetypal autoimmune disease of humoral dysregulation: systemic lupus erythematosus (SLE). CCR6 is expressed prominently on several immune cells involved in the pathogenesis of SLE, such as dendritic cells and T-helper 17 cells. CCR6's expression is correlated with disease activity and serological markers of disease severity, suggesting a possible role in disease pathogenesis. However, there are numerous holes in our understanding of the functions of CCR6 and CCL20, and future studies are required to determine if there are any diagnostic, prognostic or monitoring roles for these important molecules.
Introduction
Systemic lupus erythematosus (SLE) is an intriguing and archetypal autoimmune disease that has kept researchers and clinicians pondering about its etiology and pathogenesis for decades. SLE entails a large spectrum of clinical manifestations from serologically active, clinically quiescent disease to florid multisystem, end-organ dysfunction. Immunologically, it is characterized by autoantibodies against self-antigens, hypocomplementemia and cytopenias, and is thought to be, in part, a B-cell-driven disease leading to pathogenic immune complex formation.1 Current treatments comprise immunomodulators and targeted biologic agents that focus on minimizing end-organ damage, alleviating symptoms and improving quality of life.
Our limited understanding of the immunological basis of SLE requires more research in order to better understand the underlying mechanisms that drive the disease and to develop new therapeutic targets. Furthermore, current biomarkers such as the hallmark anti-double stranded DNA (anti-dsDNA) autoantibody and serum acute phase reactants have limitations in their sensitivity, specificity and prognostic and monitoring capabilities.2
One possible candidate for therapeutic research is the CC chemokine receptor 6 (CCR6), a G-protein-coupled receptor that is found prominently on interleukin (IL)-17-positive T-helper (TH17) cells, regulatory T (Treg) cells, memory T cells, dendritic cells (DCs) and most B-cell subsets.3, 4 CCR6’s only chemokine ligand is CCL20, although human β-defensins are known to bind and activate the receptor.5 Functionally, it is involved in cell chemotaxis, mucosal immunity and wound healing.6
CCL20 is largely an inflammatory mediator that is found in significant quantities at mucosal sites and skin. In secondary lymphoid organs such as lymph nodes, CCL20 can be detected, whereas in the spleen, its presence is below the detection threshold in bulk analysis of the entire organ.7 At a cellular level, it is expressed by T cells (including TH17 cells), macrophages, neutrophils and endothelial cells.8, 9 Together with CCR6, the CCR6–CCL20 axis has been implicated in a wide range of autoimmune diseases, cancers and viral infections such as HIV infections.10-12
More recently, however, CCR6 has been implicated in coordinating humoral immune responses.13 In a gene-deficient model, Ccr6-negative mice have shown susceptibility to an increased tendency to form T–B-cell conjugates and germinal centers (GCs), and to undergo isotype switching.13 It is not clear, at present, which CCR6-deficient cells or mechanisms drive these observations. This suggests that CCR6, as a whole, plays an important role in coordinating the humoral immune response and may act as a “checkpoint” to dampen humoral immune responses.
CCR6 and Autoimmunity
Over the last few decades, attention has turned to examining the role of CCR6 and CCL20 in the immunopathogenesis of numerous autoimmune disorders. The primary focus of studies has been on the role that the chemokine axis has in recruiting pathogenic immune cells, such as TH17 cells, to cause pathology at sites of inflammation. At homeostasis, the chemokine pair is thought to maintain a delicate balance of CCR6+ TH17 and Treg cells at the gastrointestinal mucosa.14 In inflammatory bowel disease, for instance, both CCR6 and CCL20 messenger RNAs (mRNAs) are increased in colonic tissue of inflammatory bowel disease patients which corresponded to an influx in CCR6+ and CCL20+ cells in active disease.15 Similarly, in multiple sclerosis, there is a delicate balance between TH17 and Treg cells which controls inflammation in the disease,16 and CCR6 appears to be important in helping to mediate this balance. In the murine model for multiple sclerosis, experimental autoimmune encephalitis, TH17 and Treg cells that lack CCR6 have aberrant migration into inflamed tissue and had an attenuated disease course compared with wild-type mice.9 Serum CCL20 is also significantly higher in multiple sclerosis patients than healthy controls (HCs), reinforcing the role for the CCR6–CCL20 axis in this disease.17 Finally, in psoriasis, keratinocytes secrete CCL20 which recruits CCR6+ pathogenic TH17 cells to inflamed skin.18 This has paved the way for novel therapeutics such as anti-IL-17 therapy for this condition, which has shown efficacy in randomized placebo-controlled clinical trials.19
Given the pleotropic effects of the CCR6–CCL20 chemokine axis and its role in autoimmunity, we examined its putative role in the classical systemic autoimmune disease, SLE. We established that most work thus far has been restricted to the analysis of CCR6 in the context of T cells—specifically, TH17 cells—however, there are emerging implications for the receptor and its ligand in the greater repertoire of immune cells, and thus, its usefulness as a potential target for therapy. A variety of effector and Treg cells are CCR6+ and are correlated with disease activity and various organ manifestations of the disease. It is possible that CCR6 helps direct effector cells to sites of inflammation, perhaps up a CCL20 gradient. We will, therefore, review the putative role that the CCR6–CCL20 axis may have in contributing to the pathogenesis of SLE.
Dendritic cells
Myeloid (classical) DCs are typically CCR6+ and migrate in response to its chemokine ligand CCL20.20 In SLE patients, circulating classical and plasmacytoid DCs are increased, and monocyte-derived DCs in SLE patients are unable to induce TH17 cell polarization and show a reduced ability to induce Treg cells.21 It is unclear if CCR6 expression across all DC subtypes is affected in SLE, but one study did note increased CCR6 expression on classical DCs in patients with discoid lupus erythematosus which correlated with disease activity (Revised Cutaneous Lupus Erythematosus Disease Area and Severity Index scores).22 It is possible that this CCR6 expression reflects an increased activation state in the DCs because CCR6 expression is upregulated upon immune cell activation.13
T cells
T-helper subsets
The bulk of studies examining the CCR6–CCL20 axis in SLE has focused on T cells—in particular, the involvement of the CCR6+ TH17 and Treg cell axis in the pathogenesis of SLE. Compared with HCs, a lower percentage of peripheral blood T cells express CCR6 in inactive SLE patients but not in active [Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) ≥ 6] SLE patients.23 This may reflect chronic binding of CCR6 to its cognate ligands in an inflammatory environment as this leads to downregulation of CCR6 surface expression.24
However, amongst SLE patients, those that are positive for anti-dsDNA antibodies have a higher proportion of peripheral blood T cells which are CCR6+. In particular, these cells include TH22, TH17 and TH17/TH1 (TH17 cells that mimic TH1 cells and produce interferon-γ) cells.25, 26 Interestingly, the same authors found that the percentage of CCR6+ T cells positively correlated with SLEDAI, urinary protein and erythrocyte sedimentation rate (ESR).25, 26 Given the function of CCR6 in the chemotaxis of inflammatory and T cells, it is conceivable that CCR6 is a surrogate marker of activated T cells that perpetuates the cycle of helper T-cell recruitment. However, it is also possible that the authors’ observations reflect an immunological epiphenomenon associated with more severe disease. TH17 cells will be covered in more detail in a separate section later.
Recently, CD4+CCR6+IL-7R+ nonfollicular helper T (TFH) non-TH17 cells were identified in the blood and lymph nodes of SLE patients which were able to secrete the immunomodulatory cytokine IL-10.27 These cells have been previously identified as being autoreactive and proliferate in the presence of autologous DCs.28 These CCR6+IL-7R+ cells positively correlate with higher SLEDAI and anti-dsDNA, and indeed were able to induce spontaneous autoantibody secretion from autologous cocultured B cells.27 It is not clear what role, if any, the CCR6 positivity confers on these cells, which were found near GCs and at T–B cell borders27; however, it is possible that these could act as TFH-like cells, which express high amounts of CCL20,8 and may assist with positioning of these unique T cells.
Follicular helper T cells and subsets
TFH cells help coordinate the GC B-cell reaction in lymphoid organs. Recently, a small percentage of TFH cells have been shown to be CCR6+.8 In SLE studies, circulating TFH cells—rather than tissue TFH cells—have been commonly studied owing to ease of acquisition. They are thought to have originated from, and be functionally similar to, their GC compartment counterparts, although have discernible differences in their epigenetic profile.29 In SLE, there are higher percentages of circulating TFH cells which correlate with clinical manifestations, SLEDAI and autoantibodies.30-32 These circulating TFH cells do not correlate with previous organ damage, indicating that they may be a marker of current disease activity.33
Circulating TFH cells are CXCR5¯ to allow them to migrate away from the GC; nevertheless, they likely still play a direct role in providing B cell help because these cells have greater BCL6 protein expression compared with their CXCR3+ and PD-1– counterparts.32 The fact that these nonclassical TFH cells are CCR6+ hints that they are likely the CXCR5– equivalent of TFH17 cells which are also CCR6+.31 Taken together, these studies imply that CCR6 is an important marker for the identification of circulating TFH cells that may play an important role in disease activity and pathogenesis. It is difficult to speculate what role their CCR6 positivity plays and further functional studies are required to complement these observational studies.
Subsets of circulating TFH cells (TFH1, TFH2 and TFH17 cells) do not appear to be more numerous in SLE patients relative to HCs31; however, when they were stratified for CXCR5 positivity, only TFH17 cells (CXCR3+CCR6+) were elevated in SLE patients.34 TFH17 cells are becoming of interest because they have been increasingly recognized as cells implicated in a wide range of autoimmune disorders.35
TH17 cells
TH17 cells are a class of helper T cells that are reliant on transforming growth factor-β, IL-6 and IL-21 for their differentiation from naïve T cells, and on the master transcription factor, RORγt, for their differentiation and function. In the absence of IL-6 and IL-21, naïve T cells differentiate into the functionally opposing Treg cells. TH17 cells secrete a number of cytokines including IL-17A, IL-21, IL-22, IL-23 and CCL20.36 They are classically CCR6+, and have been used as a defining flow cytometric marker of TH17 cells.37
TH17 cells have been implicated in a number of autoimmune conditions. These cells have received marked interest owing to their association with SLE disease activity and pathogenesis. Most studies report increased circulating, peripheral TH17 cells in SLE patients,38-41 whereas other studies find no increase.42, 43 This may be, in part, a result of how TH17 cells were phenotypically defined, as the studies that found increased circulating cells characterized the cells as CD4+IL-17A+, whereas those that found the opposite defined TH17 cells via chemokine receptor expression (CD4+CCR6+). However, at least one study did not find TH17 (CD4+IL-17A+) cells frequency differing between inactive and active SLE.44
Although using CCR6 is an accepted method to define these cells,37 it does not take into account TH9 and TH22 cells which are also CCR6+, thereby limiting its use as specific marker of TH17 cells. To illustrate this point, one study found higher circulating IL-17+ T cells in active and inactive SLE patients over HC but not CD4+CCR6+ T cells.45
In SLE, circulating TH17 cells are correlated with SLEDAI, ESR and anti-dsDNA;26, 39, 40, 46 are increased in vasculitic flares;47 but they do not reliably correlate with organ (renal, neuropsychiatric or hematologic) involvement.41 Yet, in lupus nephritis, IL-17A staining of renal biopsies also correlated with SLEDAI and circulating TH17 cells, and the TH17 cell-promoting cytokine, IL-23, similarly correlated with renal SLEDAI.48 Not surprisingly, TH17 cells and their cytokines have recently been associated with interferon gene expression in peripheral blood mononuclear cells (PBMCs) of SLE patients in one small study42; however, it is not clear to what extent TH17 cells are contributory to the interferon mRNA signature and this study did not explore this aspect. These findings strongly implicate TH17 cells in the pathogenesis of SLE.
Although the classic function of TH17 cells in SLE is to recruit neutrophils and create inflammation,49 TH17 cells can also provide effective B-cell help and induce isotype switching.50 Consequently, IL-17A is correlated with a proliferation-inducing ligand (APRIL).51 In the SLE-prone mouse model, BXD2, TH17 cells reside in the GC and perpetuate autoimmunity, supporting a putative role in mediating humoral immunity.52
TH17 cell-related cytokines, IL-12, IL-17A and IL-23 are generally elevated in the serum of SLE patients.38, 53, 54 As expected, most studies find that these cytokines correlate with SLEDAI and central nervous system involvement.38, 53, 54 The inflammatory and TH17 cell-promoting cytokine, IL-6, was also found in increased concentrations,40, 48 therefore helping to cement the significance of TH17 cells in SLE pathogenesis.
It is of no surprise, therefore, that higher levels of IL17 mRNA from isolated PBMCs are seen in SLE patients compared with HCs, and correlated with SLEDAI, proteinuria and lupus nephritis.38, 55 Other TH17 cell-related genes have, curiously, been downregulated including IL23R, IL17RE and RORC in untreated SLE patients.56 However, it is conceivable that looking for TH17 cell-related genes in a general population of PBMCs may not reflect the active TH17 cell populations which may be sequestered at immunological sites such as secondary lymphoid organs.
There is evidence that TH17 cells may be directly pathogenic in SLE. In a murine model, blockade of TH17 cell differentiation led to an amelioration of experimentally induced nephritis and reduction of infiltrating TH17 cells.57 Correspondingly, there was a decrease in Il17a, Ccr6 and Ccl20 mRNA expression in the kidneys of these mice relative to control mice.57 Similarly, Yang et al.58 demonstrated that when a TH17 cell-inhibiting compound derived from a herb (Baicalin) was administered to lupus-prone mice, the mice had a marked reduction in glomerulonephritis as assessed by histology and urine protein. The treated mice also had a reduction in infiltrating TH17 cells and a corresponding reduction in Ccl20 in kidneys compared with vehicle control-administered mice. In MRL/lpr Fli-1+/– transgenic mice protected from autoimmune glomerulonephritis, the mice had a reduction of infiltrating CCL20+ monocytes and TH17 cells in the kidneys compared with wild-type mice, suggesting that a possible means of TH17 cell reduction is via a disrupted CCR6–CCL20 axis.59
These preliminary findings do provide some evidence for the pathogenic role for TH17 cells in SLE/lupus nephritis and may be a possible target for treatment. However, it is not clear from these studies to what extent blockade of the CCR6–CCL20 axis could inhibit TH17 cells and hence ameliorate pathology. As a multisystemic and heterogenous disease, further functional studies are required to assess the effect that TH17 cells and CCR6–CCL20 axis have on other disease compartments (such as cutaneous manifestations) in SLE.
Regulatory T cells
Treg cells may antagonize the actions of TH17 cells and in a murine model for SLE, they downregulated TH17 cells and prevented end-organ damage.60 These cells are CCR6+ and the CCR6–CCL20 axis functions to regulate migration of these cells to sites of inflammation.9 CCL20 has been observed to phosphorylate important T-cell signaling molecules (such as mammalian target of rapamycin (mTOR) and signal transducer and activator of transcription 3 (STAT3)) and bias the differentiation pathway from Treg to TH17 cells in in vitro experiments.61
In active SLE patients, there is a higher percentage of Treg cells that are CCR6+ compared with inactive SLE patients,62 which may represent a role for CCR6 in directing migration of these cells to sites of inflammation. Indeed, in a murine model for glomerulonephritis, CCR6 was responsible for recruiting CCR6+ Treg cells to kidneys and attenuating TH1 cell-induced inflammation.63
However, there is some inconsistency with the frequency of circulating Treg cells in SLE patients compared with HCs. Some studies find a reduction in circulating Treg cells,41 no difference39, 64 or even higher frequencies.38, 62 Although this may be, in part, related to how Treg cells were flow cytometrically defined in these studies, Treg cells are affected by the heterogenous treatments that patients are taking which may alter the circulating frequencies.65
Owing to the mixed observations of circulating Treg cells, these studies currently are limited in their contribution to our understanding of how Treg cells may contribute to pathogenesis or tolerance in SLE. As a counterbalance to TH17 cells and custodian of inflammation, further study of how the CCR6–CCL20 pair may modulate the activity of these cells and affect migration to sites of inflammation is required.
B cells
Dynamic expression of CCR6 is seen as B cells progress through various maturation phases.3 Curiously, despite prominent expression of CCR6 on B cells, only few studies have assessed the chemokine receptor as it relates to B cells. Although the major B-cell subsets were found not to differ between SLE patients and HCs, CCR6 was found to be more prominently expressed on B cells of SLE patients compared with HCs.66 This is in contrast to a previous study that did not establish any difference between CCR6 across B cells and their subsets.67
Diagnostic utility of CCR6–CCL20 in SLE
The search for biomarkers for diagnosis, prognosis and monitoring treatment remains a significant part of medical research in diseases. To that end, the use of CCR6 remains a possibility. As explored earlier (see the “T cells” section), the expression of CCR6 on circulating T cells is higher in SLE patients and correlates with SLEDAI and clinical and biochemical parameters of disease activity.25, 26, 57 A study found higher expression of CCR6 protein on PBMCs from SLE patients which correlated with SLEDAI, but not with vasculitic, myositis or hematological manifestations.68 However, the same study did not differentiate which cells displayed CCR6 and found other chemokine receptors were also elevated on these PBMCs and correlating with SLEDAI (e.g. CCR5).
These studies indicate that CCR6 expression on circulating cells may be a suitable monitoring marker of SLE disease activity. Yet, surface CCR6 expression nonspecifically increases with inflammation,13 and further work is required to elucidate the value of CCR6 as a monitoring biomarker over other established clinical biomarkers, for example, anti-dsDNA. No studies thus far have examined the prognostic potential for CCR6 expression on circulating leukocytes, nor its role as a diagnostic biomarker. Similarly, raised CCR6 expression is unlikely specific enough to be of value for SLE diagnosis.
Studies of CCL20 in the blood of SLE patients are limited and conflicting. Pan et al.56 found no difference in CCL20 mRNA in circulating leukocytes between SLE patients and HCs, whereas AlFadhli et al.69 found a marked reduction in CCL20 in whole blood of SLE patients. This reduction seen in AlFadhli et al.’s study may be the result of treatment the patients received or the fact that there may be a degree of dilution in the starting cDNA with plasma and other nonleukocytes. Moreover, active SLE patients may be leukopenic, thereby diluting available nucleic acid material even further. In fact, a more recent study found higher concentrations of serum CCL20 (via ELISA) in SLE patients over HCs,70 although serum CCL20 may be reflective of other nonleukocyte sources of this chemokine such as lymph nodes.71
Further studies are required to dissect the clinical utility of CCL20 detection in patient blood, and clarify these conflicts. Nevertheless, it is possible that CCR6 and/or CCL20 will be components of multiplex, transcriptomic or proteomic assays in the future to provide prognostic, monitoring and disease risk information for the patient and clinician.
Targeting CCR6 and CCL20 in SLE
Chemoattractants and their receptors have become the focus of manipulation and inhibition for a myriad of diseases. Owing to the prominent nature of TH17 cells and related cytokines in the pathogenesis of SLE, it remains to be seen if blockade of the TH17 cell axis directly or via the CCR6–CCL20 axis will be clinically beneficial in humans. Promise has already been demonstrated in the blockade of the TH17 cell axis in other autoimmune diseases such as psoriasis.19, 72 Disruption of CCR6 would also have the possible result of disruption of the various pathogenic CCR6+ effector T cells which, preliminarily, appear to be associated with serologically more active SLE. However, the precise role and function of CCR6 on these cells have not be dissected. A possible theory is that CCL20 allows these cells to migrate to effector sites and cause more end-organ damage. Another possibility is that CCR6 upregulation is simply a by-product of an immunologically more active cell.
It is puzzling that CCR6 may act as a negative regulator of T–B cell interactions and GC formation.13 How, then, can blockade potentially lead to less autoimmunity? Although this has not been elucidated completely, the answer may lie in the relative contributions of CCR6 to the immune system considered as a whole. A major limitation for most studies on the CCR6–CCL20 axis is the difficulty in capturing the net impact that blockade of the chemokine axis has on the immune system. For example, although blocking CCR6 appears to worsen murine models of colitis in some studies,14 how this may impact on mucosal immunity elsewhere is not well understood. Furthermore, blocking CCR6 through knockout mice may have unwarranted immunological epiphenomena during their development and confound some of the results seen in murine studies. Nevertheless, it is likely that because of the pleiotropic effects of the CCR6–CCL20 axis on various immune cells, the overall outcome will depend on the fine balance between proinflammatory and anti-inflammatory processes.
Modes to target the chemokine axis include monoclonal antibodies against CCR6 and small-molecule inhibitors. For example, in a murine model for psoriasis, a modified CCL20 ligand, which acts as a CCR6 partial agonist, inhibited CCR6+ T-cell migration and was protective for severe dermatitis.73 If therapeutic utilization of this chemokine axis is going to be considered, then careful attention needs to be paid to establishing the clinical phenotype associated with the pair. Because SLE is not one distinct disease—rather, a spectrum of sometimes distinct organ and serological phenotypes—it makes sense that a targeted treatment should be offered where there is an immunological basis. It is plausible, given the known pathologies that CCR6–CCL20 is associated with at present, that it may have an important role to play in gastrointestinal, dermatological and central nervous system SLE.
Discussion
The complex and heterogeneous immunological perturbations that occur in SLE demand detailed studies to unravel the immunopathogenesis. Although the CCR6–CCL20 chemokine axis is involved in a wide variety of autoimmune diseases, its role in SLE has only been fleetingly explored. Frustratingly, from what little research exists, there are numerous apparent contradictions likely related to the heterogeneity of the disease and definitions of cellular subsets. The most studied aspect of this chemokine axis is via the TH17 cells which are CCR6+, home to effector sites via CCL20 and are significantly implicated in the pathogenesis of the disease. However, to what extent the CCR6–CCL20 axis coordinates TH17 cell distribution, and migration of other CCR6+ cells remains largely unexplored. We summarize the key CCR6+ cells thought to be involved in the immunopathogenesis of SLE in Figure 1.
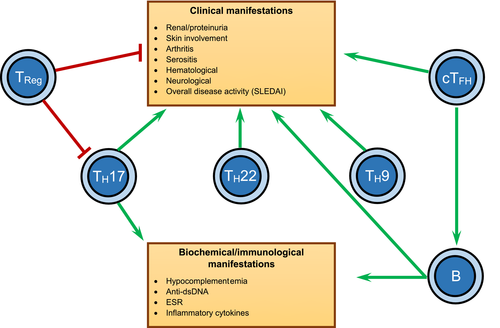
Indeed, a relatively poorly understood area of CCR6–CCL20 biology is how it may act as a negative regulator for the GC reaction and T–B cell interactions,13 having important implications for SLE. CCR6 and CCL20 may assist with positioning TFH and B cells at the T–B cell border and contributing to the “quality” of the T–B cell synapse.8 The role of the possible raised CCR6 on B-cell subsets in SLE patients66 remains a mystery at this stage. However, Elgueta et al.75 have shown that CCR6 expression on memory B cells remains important for proper localization and function of these cells in lymphoid organs, and CCR6 has been identified as a marker of B cells undergoing memory B-cell differentiation.76 As such, it is likely that SLE B-cell CCR6 expression contributes to a pathological immune response. It is quite surprising that given the importance of B cells in SLE pathogenesis and the prominence of CCR6 on B cells,3 there is a paucity of studies looking at the CCR6–CCL20 axis in relation to this cell type.
A considerable challenge for SLE research is tissue samples in human patients which are largely confined to the peripheral blood compartment. This is used as a surrogate measure of the other compartments of the body such as the less accessible lymphoid organs. Of course, this may be far from what is actually happening at key immunological sites, as cells may be sequestered away by chemokines and other molecules. As a result, studies on CCL20 and CCR6 distribution across tissue compartments (including serum) are severely lacking. Furthermore, heterogeneous murine models for SLE as reviewed here are limited in that other clinical effects such as neurological, psychiatric or cutaneous manifestations cannot be readily assessed.
A further challenge of the SLE literature is the fact that many studies presented here have, at best, been correlative studies which have their limitations in helping us understand the intricacies of the pathogenesis of SLE. Furthermore, care should be exercised when interpreting these studies in concluding the direct role of the CCR6–CCL20 chemokine axis in SLE pathogenesis. Nevertheless, they provide helpful clues as to the pathways and effector cells involved in wayward immune responses (Figure 1), and even provide potential diagnostic, prognostic and monitoring biomarkers for clinicians.
In summary, although there are a considerable number of studies looking at CCR6 as an emerging biomarker in SLE, our overall understanding is superficial at best and more work is urgently needed. Its partnering ligand CCL20 remains essentially unexplored. Dissection of the involvement of the two molecules in orchestrating immunological processes in SLE will be instrumental in the development of appropriate diagnostic and therapeutic interventions.
Acknowledgments
The authors thank Associate Professor Bruce Lyons (University of Tasmania) for critically reviewing this manuscript.
Author Contribution
Adrian Lee: Conceptualization; Writing-original draft; Writing-review & editing. Heinrich Korner: Supervision; Writing-review & editing.
Conflicts of Interest
None declared.





