Significance of androgen-deprivation therapy for intermediate- and high-risk prostate cancer treated with high-dose radiotherapy: A literature review
This document has been checked by a professional medical editor, who is a native speaker of English.
Abstract
The real-world benefits of adding androgen-deprivation therapy (ADT) and its optimal duration when combined with current standard high-dose radiation therapy (RT) remain unknown. We aimed to assess the efficacy of and toxicities associated with ADT in the setting of combination with high-dose RT for intermediate-risk (IR) and high-risk (HR) prostate cancer (PCa). This article is a modified and detailed version of the commentary on Clinical Question 8 described in the Japanese Clinical Practice Guidelines for Prostate Cancer (ver. 2023). A qualitative systematic review was performed according to the Minds Guide. All relevant published studies between September 2010 and August 2020, which assessed the outcomes of IR or HR PCa treated with high-dose RT, were screened using two databases (PubMed and ICHUSHI). A total of 41 studies were included in this systematic review, mostly consisting of retrospective studies (N = 34). The evidence basically supports the benefit of adding ADT to high-dose RT to improve tumor control. Regarding IR populations, many studies suggested the existence of a subgroup for which adding ADT had no impact on either overall survival or the BF-free duration. On the other hand, regarding HR populations, several studies suggested the positive impact of adding ADT for ≥1 year on overall survival. Adding ADT increases not only the risk of sexual dysfunction but also that of cardiovascular toxicities or bone fracture. Although the benefit of adding ADT was basically suggested for both IR and HR populations, further investigations are warranted to identify subgroups of patients for whom ADT has no benefit, as well as the appropriate duration of ADT for those who do derive benefit.
Abbreviations & Acronyms
-
- 3D-CRT
-
- three-dimensional conformal radiotherapy
-
- A-ADT
-
- adjuvant androgen-deprivation therapy
-
- ADT
-
- androgen-deprivation therapy
-
- BF
-
- biochemical failure
-
- CAB
-
- combined androgen blockade
-
- CI
-
- confidence interval
-
- EBRT
-
- external-beam radiation therapy
-
- GI
-
- gastrointestinal
-
- GS
-
- Gleason score
-
- GU
-
- genitourinary
-
- HDR
-
- high-dose rate
-
- HR
-
- hazard ratio
-
- HR
-
- high-risk
-
- IMRT
-
- intensity-modulated radiation therapy
-
- IPSS
-
- International Prostate Symptom Score
-
- IR
-
- intermediate-risk
-
- LDR
-
- low-dose rate
-
- MVA
-
- multivariable analysis
-
- NA-ADT
-
- neoadjuvant androgen-deprivation therapy
-
- OS
-
- overall survival
-
- PCa
-
- prostate cancer
-
- PSA
-
- prostate-specific antigen
-
- QOL
-
- quality of life
-
- RCT
-
- randomized controlled trial
-
- RT
-
- radiation therapy
-
- UVA
-
- univariate analysis
INTRODUCTION
Prostate cancer (PCa) is globally the second most common cancer in males.1 Definitive radiation therapy (RT), such as external-beam radiation therapy (EBRT) or brachytherapy, is one of the established treatment modalities for localized or locally advanced PCa. Improvement of disease control via local dose escalation has been demonstrated in several randomized controlled trials (RCTs) and meta-analyses.2, 3 Currently, as high-dose RT, several treatment modalities, such as intensity-modulated radiation therapy (IMRT), low-dose-rate (LDR)/high-dose-rate (HDR) brachytherapy, and proton/carbon-ion particle therapy, are used in the Japanese clinical practice.4-6
Improved outcomes on adding androgen-deprivation therapy (ADT) to RT have been reported based on several RCTs or meta-analyses on RT for intermediate-risk (IR) and high-risk (HR) PCa.7, 8 In addition, for HR PCa, the benefit of adding long-term compared with short-term ADT has been demonstrated. In the phase III RTOG 92–02 trial,9 comparing the benefits of long-term (28 months) with short-term (4 months) ADT among patients with T2c–T4 PCa who received definitive EBRT, long-term ADT led to a significant improvement of disease-specific survival compared with short-term use (p = 0.0042). Similarly, in the phase III EORTC 22961 trial, comparing the benefits of long-term administration (3 years) with short-term ADT (6 months), long-term ADT demonstrated significantly lower PCa-specific mortality (p = 0.002).10 Based on these multiple prospective data, the addition of ADT to RT has become a standard approach for IR and HR PCa. Currently, 4–6-month short-term ADT for IR PCa and 2–3-year long-term ADT for HR PCa are recommended in several treatment guidelines. However, this evidence was based on the results of EBRT using 70 Gy or lower via three-dimensional conformal radiotherapy (3D-CRT), which is suboptimal in the current era. The dose of EBRT used in the aforementioned RTOG 92–02 trial was 65–70 Gy in conventional fractions. Similarly, that of the EORTC 22961 trial was 70 Gy. The currently published Japanese phase II study of moderate hypofractionated IMRT (70 Gy in 28 fractions) with image-guided radiotherapy demonstrated excellent disease control among patients with HR PCa only in combination with short-term ADT (4–8 months).11 Specifically, the 5-year biochemical failure (BF) survival rate of HR PCa patients was 93.9%. Therefore, the true benefits of adding ADT and optimal duration of ADT when combined with current standard high-dose RT remain unknown.
In this systematic review, we aimed to assess the efficacy of and toxicities associated with ADT in the setting of combination with high-dose RT for IR and HR PCa. This article is a modified and detailed version of the commentary on Clinical Question 8 described in the Japanese Clinical Practice Guidelines for Prostate Cancer(ver. 2023).12
METHODS
Literature search
A qualitative systematic review was conducted in 2022 based on the Minds guidelines.13 We performed a comprehensive literature search of two databases (PubMed and ICHUSHI) for all relevant published studies between September 2010 and August 2020, using a search formula created based on three key studies selected by four reviewers (RA, HI, SS, and MK) and the following ten keywords: “prostate cancer,” “radiotherapy,” “androgen deprivation therapy,” “high risk,” “intermediate risk,” “IMRT,” “brachytherapy,” “proton therapy,” “carbon-ion radiotherapy,” and “high-dose.” We also performed a search to include relevant papers. Given the marked advances in this area, papers published prior to August 2010 were not included.
Methods of study selection, quality assessment, and data extraction
As primary screening, two pairs of reviewers inspected the titles and abstracts of studies identified by the search. Half of the studies were independently reviewed by one of the pairs, and the other half were similarly reviewed by the other pair. As secondary screening, full papers of the studies selected in primary screening were inspected. Half of the selected studies were independently reviewed by one pair, and the other half were done so by the other pair. In addition, we also performed hand-based searching of relevant manuscripts. Studies meeting the following criteria were selected: (1) main subjects comprised intermediate- and high-risk cases; (2) overall survival (OS), PCa-specific survival, progression-free survival, BF-free survival, toxicities due to ADT, result of quality of life (QOL) analysis, and late toxicities due to RT were included in the results; (3) comparison of these clinical outcomes according to the duration of hormonal therapy were included in the analyses; (4) total doses exceeding 70 Gy in conventional fractions were prescribed for the prostate in EBRT studies. Any disagreements during primary and secondary screening were resolved through discussion and reaching a consensus.
After the second screening, the results of each paper were organized based on information on, “Patients,” “Interventions,” “Comparisons,” “Control,” and “Outcomes” from each paper and evaluated in the qualitative synthesis.
RESULTS
Evidence analysis
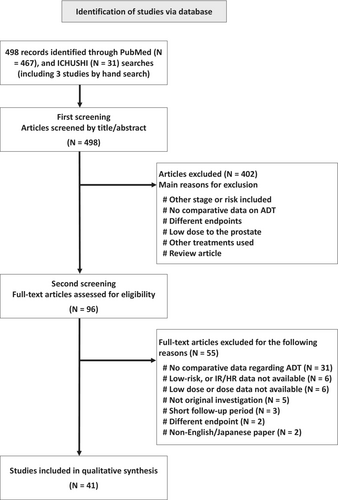
Figure 1 shows a process of literature search. The systematic literature searches yielded 498 references, which included three studies selected by hand search: 467 from PubMed and 31 from ICHUSHI. Among them, 403 papers were excluded based on the title and abstract (the primary screening process). After review of the full text of the remaining 95 papers, 54 were excluded (secondary screening process). Finally, 41 studies were included in the systematic review.14-53 The majority were retrospective cohort studies (N = 34). Regarding RCTs, five papers on four RCTs were included.
Impact on overall survival
The phase III PMH 9907 trial evaluated the effect of adding androgen receptor blocker monotherapy (bicalutamide, 150 mg once daily) for 5 months predominantly among IR PCa patients (94.6% of study population) treated with definitive EBRT (Table 1).17 In this trial, 75.6 to 79.8 Gy in 42 fractions or 78 Gy in 39 fractions were prescribed for the prostate. OS was not significantly different between the two groups: 82% (95% confidence interval [CI]: 75%–90%) in the EBRT plus bicalutamide group versus 86% (95%CI: 80%–94%) in the EBRT-alone group at 9 years, p = 0.37. The other phase III DART01/05 GICOR trial14 compared the outcomes of 4-month ADT (2-month neoadjuvant ADT [NA-ADT] using combined androgen blockade [CAB] followed by 2-month concomitant ADT) (short-term ADT) with those of 28-month ADT (same treatment followed by 24-month adjuvant ADT [A-ADT]) (long-term ADT) in combination with high-dose EBRT (76 Gy in 38 fractions) among patients with clinical stage T1c–T3bN0M0 PCa with IR and HR factors. After a median follow-up of 63 months, OS was significantly better in the long-term ADT group. Specifically, the 5-year OS rates were 95% (95%CI: 93%–97%) in the long-term ADT group vs. 86% (95%CI: 83%–89%) in the short-term ADT group (hazard ratio [HR]: 2.48 [95%CI: 1.31–4.68], p = 0.009).
| Reference | Refs. Number | Study design/Phase | RT method | ADT: experimental arm | Sample size | Outcomes | Comment |
|---|---|---|---|---|---|---|---|
| Intermediate-risk | |||||||
Zapatero et al. DART01/05 GICOR |
13 | Phase 3 | EBRT: median 78 Gy/39 fr | Arm 1: 4 months ADT (4 months Goserelin plus 2 months Bicalutamide) Arm 2: 28 months ADT (28 months Goserelin plus 2 months Bicalutamide) |
81 85 |
5 y-OS: 91% 5 y-OS: 94% p = 0.318 |
|
McPartlin et al. PMH 9907 |
16 | Phase 3 | EBRT: median 78–79.6 Gy/39–42 fr | Arm 1: 5 months Bicalutamide Arm 2: no ADT |
119 122 |
9 y-OS: 82% 9 y-OS: 86% p = 0.37 |
95% of the study subjects were intermediate-risk |
| Hou et al. | 17 | Retrospective | EBRT: median 78 Gy | No ADT ≤6 months >6 months |
27% 35.3% 37.7% |
5 y-OS: 97% 5 y-OS: 93% 5 y-OS: 95% p = 0.651 |
|
| Hervas et al. | 18 | Retrospective | EBRT: median 74 Gy | No ADT ADT (median: 7.1 months) |
184 1310 |
Not significant | |
| Ishiyama et al. | 19 | Retrospective | BT plus EBRT | Neoadjuvant ADT (median: 8.6 months) Neoadjuvant plus adjuvant ADT (median: 36.5 months) |
n/a | p = 0.089 | |
| Dong et al. | 20 | Retrospective | EBRT: 74–80 Gy/1.8–2 Gy/fr, or 70.2 Gy/2.7 Gy /fr | No ADT Neoadjuvant ADT (median: 4 months) |
979 155 |
5 y-OS: 90.7% 5 y-OS: 92.3% p = 0.48 |
|
| Keane et al. | 45 | Retrospective | BT with or without EBRT | No ADT ADT (median: 4 months) |
1677 833 |
p = 0.03 *PCa-specific mortality |
|
| High-risk | |||||||
Zapatero et al. DART01/05 GICOR |
13 | Phase 3 | EBRT: median 78 Gy/39 fr | Arm 1: 4 months ADT (4 months Goserelin plus 2 months Bicalutamide) Arm 2: 28 months ADT (28 months Goserelin plus 2 months Bicalutamide) |
97 92 |
5 y-OS: 82% 5 y-OS: 96% p = 0.015 |
|
| Hou et al. | 17 | Retrospective | EBRT: median 78 Gy | No ADT ≤6 months >6 months |
3.1% 40.8% 56.1% |
5 y-OS: 73% 5 y-OS: 80% 5 y-OS: 89% p = 0.102 |
|
| Ishiyama et al. | 19 | Retrospective | BT plus EBRT | Neoadjuvant ADT (median: 8.6 months) Neoadjuvant plus adjuvant ADT (median: 36.5 months) |
n/a | p = 0.002 (high-risk), and 0.167 (very high-risk) | |
| Stone et al. | 22 | Retrospective | BT plus EBRT | 0–6 months ADT >6 months ADT |
n/a | 10 y-ACS:90.9% 10 y-ACS:82.6% p = 0.004 |
Increased time on ADT was associated with decreased ACS |
| Liss et al. | 23 | Retrospective | BT plus EBRT or EBRT alone (75–79.2 Gy) | No ADT <12 months >12 months |
n/a | Reference p = 0.91 p = 0.066 |
MVA for patients with Gleason Score 8–10 |
| Shilkrut et al. | 24 | Retrospective | BT plus EBRT or EBRT alone (median: 78 Gy) | No ADT <12 months 12–24 months ≥24 months |
n/a | reference p = 0.079 p = 0.11 p = 0.001 *PCa-specific mortality |
|
| Shilkrut et al. | 25 | Retrospective | BT plus EBRT or EBRT alone (median: 77.4 Gy) | No ADT | 28 | Reference | Patients with ≥2 high-risk factors |
<12 months 12–24 months ≥24 months |
46 46 118 |
p = 0.22 p = 0.064 p = 0.004 *PCa-specific mortality |
|||||
| Feng et al. | 26 | Retrospective | EBRT: 75–79.2 Gy | No ADT | 20% | Reference | |
| <12 months | 36% | p = 0.72 | |||||
| ≥12 months | 44% | p < 0.001 | |||||
| Kasuya et al. | 27 | Retrospective | Carbon-ion radiotherapy; 63–66 Gy (RBE)/20 fr | ≥12 vs. <12 months | 295 vs. 29 | 22% vs. 28%, p = 0.23 | |
| ≥24 vs. <24 months | 224 vs. 100 | 21% vs. 26%, p = 0.539 *OAM rate |
|||||
| Guarneri et al. | 28 | Retrospective | EBRT: 78 Gy | ≥6 but <12 months | 49 | Not significant | |
| ≥12 but <24 months | 15 | Not significant | |||||
| ≥24 months | 91 | Not significant | |||||
- Abbreviations: ACS, all-cause survival; ADT, androgen-deprivation therapy; BT, brachytherapy; EBRT, external-beam radiation therapy; n/a, not available; OS, overall survival; PCa, prostate cancer; RBE, relative biological effect; RT, radiation therapy.
The impact of adding ADT on survival outcomes was different between the IR PCa and HR PCa populations. In subgroup analysis of the aforementioned phase 3 RCT,14 no significant difference in OS was observed among IR PCa populations (p = 0.318) while OS was significantly better in the long-term ADT group among HR PCa populations (p = 0.015). According to the results from several retrospective studies, adding ADT consistently failed to improve survival outcomes among IR PCa populations, irrespective of the ADT duration.18-23 On the other hand, its impact on HR PCa populations was not consistent among results from retrospective studies, including this systematic review. According to a Japanese multi-institutional retrospective analysis of HDR brachytherapy combined with EBRT,20 OS was significantly more favorable in patients who received NA-ADT plus A-ADT compared with those who received NA-ADT alone among the HR PCa cohort (HR: 0.46 [95%CI: 0.29–0.75], p = 0.002). However, the reported impact on OS among retrospective studies differed depending on the duration of ADT. When comparing those who received >6-month ADT with those who received no or ≤6-month ADT, the improvement of OS by adding long-term ADT (>6 months) was not observed.18, 23 An increased ADT duration was associated with a lower all-cause or PCa-specific mortality rate (≥12 vs. <12 months, or ≥24 vs. <24 months).24-27 On the other hand, several studies reported that ≥24-month ADT led to no improvement of OS.28, 29
Impact on biochemical progression
Regarding IR PCa, seven studies investigated the impact of adding 4–6-month NA-ADT on high-dose RT.21, 22, 30-34 Among them, one study reported the significant improvement of time to BF by adding NA-ADT31 and one study reported its tendency (p = 0.0937) (Table 2).22 In addition, the benefit of adding NA-ADT was observed in the subgroup aged <70 years old32 and an unfavorable IR population.33 The remaining three studies reported similar BF control between RT alone and short-term (4–6 months) NA-ADT.21, 30, 34 Among these studies, one reported improved BF by adding longer ADT (4–12 months) compared with RT alone.30 No studies reported an improved outcome with RT alone compared with NA-ADT. In addition, according to the phase 3 PMH 9907 trial, which compared EBRT plus androgen receptor blocker monotherapy for 5 months with EBRT alone predominantly among IR PCa patients (94.6% of study population), no significant difference was observed in the BF rate (p = 0.32).17 Regarding the duration of ADT, one RCT14 and two retrospective studies18, 31 reported no improvement of biochemical control by adding >4–6-month ADT while one retrospective study reported a tendency toward improvement of BF-free survival by adding 4–12-month ADT compared with shorter-term ADT (1–3 months) (p = 0.06).30 According to a Japanese multi-institutional retrospective analysis of proton beam therapy,35 although the benefit of adding ADT (with vs. without ADT) to improve BF-free survival was evident (HR: 0.49 [95%CI: 0.26–0.93], p = 0.029), this benefit disappeared when comparing those who received ≤6-month ADT with those administered >6-month ADT (HR: 1.00 [95%CI: 0.37–2.69], p = 0.999). In their subgroup analysis, no benefit was observed among those with a single IR factor (HR: 0.82 [95%CI: 0.33–2.02], p = 0.664) while the benefit of adding ADT was evident among patients with multiple IR factors. According to the phase 3 DART01/05 GICOR trial,14 which compared the outcomes of long-term (28 months) with short-term (4 months) ADT in combination with high-dose EBRT, no benefits of adding long- over short-term ADT were observed among the IR PCa population (HR: 1.82 [95%CI: 0.76–4.33], p = 0.174). Furthermore, according to a retrospective study of high-dose EBRT (75–78 Gy in conventional fractions), which divided the IR population into three risk groups, the benefit of adding short-term ADT (<6 months) over RT alone was observed only among patients with Gleason score (GS) 4 + 3 or T2c disease.33 Another retrospective study reported that the benefit of adding ADT was observed only among patients aged ≤70 years old.32
| Reference | Refs. Number | Study design/Phase | RT method | ADT method | Sample size | Outcomes | Comment |
|---|---|---|---|---|---|---|---|
| Intermediate-risk | |||||||
Zapatero et al. DART01/05 GICOR |
13 | Phase 3 | EBRT: median 78 Gy/39 fr | Arm 1: 4 months ADT (4 months Goserelin plus 2 months Bicalutamide) Arm 2: 28 months ADT (28 months Goserelin plus 2 months Bicalutamide) |
81 85 |
5 y-bDFS: 88% 5 y-bDFS: 92% p = 0.174 |
|
McPartlin et al. PMH 9907 |
16 | Phase 3 | EBRT: median 78–79.6 Gy/39–42 fr | Arm 1: 5 months Bicalutamide Arm 2: no ADT |
119 122 |
9 y-BF rate: 40% 9 y-BF rate: 47% p = 0.32 |
95% of the study subjects were intermediate-risk |
| Hou et al. | 17 | Retrospective | EBRT: median 78 Gy | No ADT ≤6 months >6 months |
27% 35.3% 37.7% |
5 y-BCFFS: 91% 5 y-BCFFS: 97% 5 y-BCFFS: 100% p = 0.24 |
|
| Hervas et al. | 18 | Retrospective | EBRT: median 74 Gy | No ADT ADT (median 7.1 months) |
184 1310 |
p = 0.035 | |
| Dong et al. | 20 | Retrospective | EBRT: 74–80 Gy in 1.8–2 Gy/fr, or 70.2 Gy in 2.7 Gy /fr | No ADT Neoadjuvant ADT (median: 4 months) |
944 153 |
5 y-FFBF: 87.3% 5 y-FFBF: 84.0% p = 0.83 |
|
| Boladeras et al. | 21 | Retrospective | EBRT: median 75 Gy | No ADT ADT (median: 21 months for entire cohort) |
183 163 |
5 y- and 10 y-bRFS: 82.9% and 65.8% 5 y- and 10 y-bRFS: 89.9% and 74.5% p = 0.0937 |
|
| Tabata et al. | 29 | Retrospective | BT with or without EBRT | No ADT 1–3 months 4–12 months |
142 70 80 |
5 y- and 7 y-bPFS: 89.8% and 83.5% 5 y- and 7 y-bPFS: 89.7% and 89.7% 5 y- and 7 y-bPFS: 97.7% and 97.5% p = 0.02 |
|
| Ludwig et al. | 30 | Retrospective | EBRT: median >70 Gy | No ADT with ADT |
797 421 |
p = 0.04 | |
| Amit et al. | 31 | Retrospective | EBRT: 78–82 Gy in 2 Gy /fr, or 73.6 Gy in 2.3 Gy/fr | No ADT vs. with ADT (≤70 years old) no ADT vs. with ADT (>70 years old) |
56 vs. 67 121 vs. 126 |
6 y-bRFS:82.1% vs 94.2% (p = 0.03) 6 y-bRFS:94.4% vs 94% (p = 0.878) |
|
| Castle, et al | 32 | Retrospective | EBRT: 78 Gy/39 Gy /fr, or 75.6 Gy/42 fr | No ADT with ADT |
327 218 |
Clinical or biochemical-failure p = 0.016 |
|
| Schiffmann et al. | 33 | Retrospective | BT plus EBRT | No ADT 3–6 months |
97 84 |
BCR-free survival p = 0.6 |
|
| Murakami et al. | 34 | Retrospective | Proton therapy: 70–80 GyE/35–40 fr or 63–66 GyE /21–22 fr |
no ADT vs. with ADT 1–6 months vs. >6 months |
276 vs. 220 118 vs. 102 |
p = 0.029 p = 0.999 bFRS |
|
| High-risk | |||||||
Zapatero et al. DART01/05 GICOR |
13 | Phase 3 | EBRT: median 78 Gy/39 fr | Arm 1: 4 months ADT (4 months Goserelin plus 2 months Bicalutamide) Arm 2: 28 months ADT (28 months Goserelin plus 2 months Bicalutamide) |
97 92 |
5 y-bDFS: 76% 5 y-bDFS: 88% p = 0.054 |
|
| Ito et al. | 15 | Phase 3 | EBRT: 72 Gy/36 fr | Arm 1: long-term ADT (60 months) Arm 2: intermittent ADT (14 months plus re-start at > PSA 5.0 ng/mL) |
136 144 |
5 y- and 10 y-mBRFS*: 84.8% and 63.7% 5 y- and 10 y-mBRFS*: 82.8% and 57.8% p = 0.5619 |
PSA relapse in this study meat progression to non-metastatic CRPC |
| Hou et al. | 17 | Retrospective | EBRT: median 78 Gy | No ADT ≤6 months >6 months |
3.1% 40.8% 56.1% |
5 y-BCFFS: 67% 5 y-BCFFS: 88% 5 y-BCFFS: 82% p = 0.001 |
|
| Boladeras et al. | 21 | Retrospective | EBRT: median 75 Gy | No ADT ADT (median: 21 months for entire cohort) |
33 303 |
5 y- and 10 y-bRFS: 68.6% and 58.8% 5 y- and 10 y-bRFS: 86.9% and 72.7% p = 0.0319 |
|
| Liss et al. | 23 | Retrospective | BT plus EBRT or EBRT alone (75–79.2 Gy) | No ADT <12 months >12 months |
n/a | Reference p = 0.44 p = 0.02 |
MVA for patients with Gleason Score 8–10 |
| Shilkrut et al. | 24 | Retrospective | BT plus EBRT or EBRT alone (median: 78 Gy) | No ADT <12 months 12–24 months ≥24 months |
n/a | Reference p = 0.019 p = 0.002 p < 0.001 |
|
| Shilkrut et al. | 25 | Retrospective | BT plus EBRT or EBRT alone (median: 77.4 Gy) | No ADT <12 months 12–24 months ≥24 months |
28 46 46 118 |
Reference p = 0.66 p = 0.045 p = 0.012 |
Patients with ≥2 high-risk factors |
| Feng et al. | 26 | Retrospective | EBRT: 75–79.2 Gy | No ADT <12 months (median: 6.1 months) ≥12 months (median: 25.6 months) |
20% 36% 44% |
Reference p = 0.18 p = 0.03 |
|
| Guarneri et al. | 28 | Retrospective | EBRT: 78 Gy | ≥6 but <12 months ≥12 but <24 months ≥24 months |
49 15 91 |
Not significant Not significant Not significant |
|
| Schiffmann et al. | 33 | Retrospective | BT plus EBRT | No ADT 3–6 months |
74 137 |
BCR-free survival p < 0.001 |
|
| Murakami et al. | 34 | Retrospective | Proton therapy: 70–80 GyE/35–40 fr or 63–66 GyE /21–22 fr |
No ADT vs. with ADT 1–6 months vs. >6 months |
66 vs. 448 175 vs. 273 |
p = 0.433 p = 0.018 bRFS |
|
| Chen et al. | 35 | Retrospective | BT with or without EBRT | ≤6 months >6 months |
18 79 |
Biochemical relapse rate: 23.1% Biochemical relapse rate: 0% p = 0.019 |
|
| Cagney et al. | 44 | Retrospective | EBRT: median 74 Gy | ADT (median: 8 months) | 547 | p = 0.017 | ADT duration was evaluated as continuous variable in MVA |
- Abbreviations: ADT, androgen-deprivation therapy; BCFFS, biochemical failure-free survival; BCR, biochemical recurrence; bDFS, biochemical disease-free survival; BF, biochemical failure; bPFS, biochemical progression-free survival; bRFS, biochemical relapse-free survival; BT, brachytherapy; EBRT, external-beam radiation therapy; FFBF, freedom from biochemical failure; GyE, Gy equivalent; n/a, not available; and RT, radiation therapy.
For HR PCa, firstly, among five studies that investigated the benefit of adding <12-month ADT over RT alone,24, 26, 27, 34, 35 one study, which evaluated the benefit of adding ADT to high-dose RT (HDR brachytherapy plus EBRT), reported that improvement of BF-free survival was noted in D'Amico HR patients (p < 0.001).34 Regarding the benefit of adding longer ADT over RT alone, one study, which compared EBRT plus longer ADT (median duration: 21 months) with EBRT alone, reported that BF-free survival was significantly better in the EBRT plus ADT cohort (p = 0.0319).22 Secondly, among studies investigating the impact of the ADT duration on RT, 4–6 months, 1 year, and 2 years were used as cutoff values for the ADT duration. Among the three studies that used 4–6 months as a cutoff,18, 35, 36 two (67%) reported the improvement of BF-free survival by adding longer ADT.35, 36 Among five studies that used 1 year as a cutoff,24-27, 29 four (80%) reported an improvement by adding longer ADT.24-27 Similarly, among four studies that used 2 years as a cutoff,25, 26, 29, 35 two (50%) reported an improvement by adding longer ADT.25, 26 According to a Japanese multi-institutional retrospective analysis,35 the benefits of long-term ADT were observed among patients with multiple HR factors (T3a-4, prostate-specific antigen [PSA] >20 ng/mL, or GS ≥8) when using 21 months as the cutoff for long-term ADT; however, no difference was observed between ≥21 and 6–21 months ADT among patients with single HR factors. Furthermore, in a Japanese phase 3 study that compared medium-term ADT (14 months) under the policy of the early initiation of salvage-ADT (PSA >5.0 ng/mL) and long-term ADT (5 years) among patients with T3-4N0M0 PCa treated with definitive EBRT (72 Gy in 36 fractions), no difference in non-metastatic castration-resistant PCa-free survival was observed (HR: 1.132 [95%CI: 0.744–1.722], p = 0.5619).16 Similarly, in the phase 3 DART01/05 GICOR study, which compared long-term (28 months) vs. short-term (4 months) ADT, although there was the tendency of better BF-free survival in the long-term ADT arm, the difference was not statistically significant (HR: 1.91 [95%CI: 0.97–3.77], p = 0.054).14
Toxicities due to ADT
As ADT-related toxicities, cardiovascular events have been mainly investigated. According to the phase 3 DART01/05 GICOR study,54 which compared 28-month ADT (28 months of goserelin acetate and 2 months of bicalutamide) vs. 4-month ADT (4 months of goserelin acetate and 2 months of bicalutamide), there was no significant difference in the incidence of mortality due to cardiovascular events between the long- and short-term ADT arms (2.8% for long-term ADT group vs. 1.7% for short-term ADT group, p = 0.470) although the cumulative incidence of cardiovascular events was significantly higher in the former compared with latter (17.6 vs. 7.2% at 5 years, respectively, p = 0.014) (Table 3). Similarly, according to a population-based study of cardiovascular mortality among patients who received definitive EBRT with or without ADT, the use of ADT did not increase cardiovascular mortality.37 In addition, according to three studies16, 27, 38 that evaluated the impact of ADT on non-PCa or all-cause mortality, the use of ADT or long-term ADT did not significantly increase the incidence of non-PCa death among IR and HR PCa populations. Of note, among patients with at least a single risk factor of coronary artery disease, the use of ADT was associated with a significantly increased risk of all-cause mortality in men with low-risk PCa (HR: 1.36 [95%CI: 1.07–1.74], p = 0.01) but not in men with IR PCa (HR: 1.13 [95%CI: 0.96–1.35], p = 0.15) or HR PCa (HR: 0.86 [95%CI: 0.66–1.13], p = 0.28).38
| Reference | Refs. Number | Study design/Phase | RT method | ADT method | Sample size | Endpoint | Outcome |
|---|---|---|---|---|---|---|---|
| Ito et al. | 15 | Phase 3 | EBRT: 72 Gy/36 fr | Arm 1: long-term ADT (60 months) Arm 2: intermittent ADT (14 months plus re-start at > PSA 5.0 ng/mL) |
136 144 |
NPCD | 12.5% 15.2% p value: n/a |
McPartlin et al. PMH 9907 |
16 | Phase 3 | EBRT: median 78–79.6 Gy/39–42 fr | Arm 1: 5 months Bicalutamide Arm 2: no ADT |
119 122 |
Gynecomastia | 2.5% in the arm 1 |
Zapatero et al. DART01/05 GICOR |
53 | Phase 3 | EBRT: median 78 Gy/39 fr | Arm 1: 4 months ADT (4 months Goserelin plus 2 months Bicalutamide) Arm 2: 28 months ADT (28 months Goserelin plus 2 months Bicalutamide) |
178 177 |
5 y-cumulative incidence of cardiovascular events | 7.2% 17.6% p = 0.014 |
Zapatero et al. DART01/05 GICOR |
53 | Phase 3 | EBRT: median 78 Gy/39 fr | Arm 1: 4 months ADT (4 months Goserelin plus 2 months Bicalutamide) Arm 2: 28 months ADT (28 months Goserelin plus 2 months Bicalutamide) |
178 177 |
Death due to cardiovascular events | 1.7% 2.8% p = 0.47 |
| Feng et al. | 26 | Retrospective | EBRT: 75–79.2 Gy | No ADT <12 months ≥12 months |
48 84 102 |
NPCD | Reference p = 0.57 p = 0.23 |
| Tabata et al. | 29 | Retrospective | BT with or without EBRT | No ADT With ADT |
142 150 |
Myocardial infarction | 1.4% 2.0% Not significant |
| Kim et al. | 36 | Retrospective | EBRT: median 70 Gy/35 fr | No ADT ≤6 months >6 months |
1933 674 3341 |
Cardiovascular mortality | 2.6% 2.1% 1.4% p = 0.002 |
| Nanda et al. | 37 | Retrospective | BT with or without EBRT | No ADT vs with ADT (intermediate-risk) No ADT vs with ADT (high-risk) |
2225 vs. 2140 353 vs. 1007 |
ACM | p = 0.15 p = 0.28 |
| Elliott et al. | 38 | Retrospective | 3D-CRT | No ADT vs. with ADT | 13 396 vs. 6974 | Hip fracture Distal forearm fracture |
8.4% vs. 8.7%*, p = 0.014 p < 0.002 *cumulative incidence at 10-year |
| Parekh et al. | 42 | Retrospective | n/a | No ADT With ADT |
213 225 |
Complaints of a shortened penis | 0% 2.67% p = 0.016 for UVA, and 0.08 for MVA |
- Abbreviations: 3D-CRT, three-dimensional conformal radiation therapy; ACM, all-cause mortality; ADT, androgen-deprivation therapy; BT, brachytherapy; EBRT, external-beam radiation therapy; MVA, multivariable analysis; n/a, not available; NPCD, non-prostate cancer death; RT, radiation therapy; UVA, univariate analysis.
Regarding toxicities other than cardiovascular diseases, increased risks of hip and distal forearm fracture39 or gynecomastia17 were suggested.
Decrease in quality of life due to ADT
According to a phase 3 study that evaluated the benefit of 5-month bicalutamide among patients with IR and HR PCa who received EBRT, no marked effect of the addition of bicalutamide on the QOL was observed.17 In addition, another prospective study, which compared RT plus ADT for 4–8 months with RT alone, reported that there was no significant difference in the incidence of genitourinary toxicities requiring alpha-blocker medication (Table 4).40 However, the authors reported that the time needed to return to baseline urinary symptoms assessed by the health-related QOL score (UCLA Prostate Cancer Index) was significantly shorter in the RT plus ADT (4–8 months) cohort compared with RT-alone cohort in univariate analysis (UVA) (p = 0.017) although the difference disappeared in multivariable analysis (MVA).40 On the other hand, according to a retrospective study among Japanese patients who received IMRT plus ADT, factors affecting the difference in the International Prostate Symptom Score (IPSS) between the baseline and at 24 months were age, IPSS classification, pretreatment GU medications, and positive biopsy rates (p = 0.023, <0.001, 0.044, and 0.028 in MVA, respectively), while the ADT duration had no impact.41 Similarly, according to a retrospective study that investigated predictive factors for urinary toxicities after LDR brachytherapy with or without ADT, the use of NA-ADT was not associated with the time to IPSS resolution.42
| Reference | Refs. Number | Study design/Phase | RT method | ADT method | Sample size | Endpoint | Outcomes |
|---|---|---|---|---|---|---|---|
McPartlin et al. PMH 9907 |
16 | Phase 3 | EBRT: median 78–79.6 Gy/39–42 fr | Arm 1: 5 months Bicalutamide Arm 2: no ADT |
119 122 |
QOL | Not significant |
| Grant et al. | 39 | Prospective study | EBRT, BT, or BT plus EBRT | No ADT With ADT (4–8 months) |
81 67 |
Time to return to baseline urinary symptoms (health-related QOL) | Shorter in the RT plus ADT group for UVA (p = 0.017), but not significant in MVA |
| Tomita et al. | 40 | Retrospective | IMRT | ADT (median: 29 months) | 216 | IPSS difference between baseline and 24 months | Not significant |
| Eriguchi et al. | 41 | Retrospective | BT with or without EBRT | No neoadjuvant ADT With neoadjuvant ADT |
785 528 |
IPSS resolution | Not significant |
| Parekh et al. | 42 | Retrospective | n/a | No ADT With ADT |
213 225 |
Complaints of a shortened penis | 0% 2.67% p = 0.016 for UVA, and 0.08 for MVA |
| Gay et al. | 43 | Prospective study | EBRT | No ADT With neoadjuvant ADT (>1 y) |
473 100 |
Health-related QOL | Decreased the sexual and hormonal/vitality domain responses in the “with ADT” group |
- Abbreviations: ADT, androgen-deprivation therapy; BT, brachytherapy; EBRT, external-beam radiation therapy; IMRT, intensity-modulated radiation therapy; IPSS, international prostate symptom score; MVA, multivariable analysis; n/a, not available; QOL, quality of life; RT, radiation therapy; UVA, univariate analysis.
Regarding sexual function-related QOL, according to a study using a multicenter observational prospective registry in the United States, the RT plus ADT group made significantly more complaints of reduced penile size than the RT-alone group irrespective of the ADT duration (p = 0.016).43 In addition, according to a multicenter prospective study of QOL involving 1201 patients with non-metastatic PCa treated with EBRT or brachytherapy, in which the health-related QOL was measured using Expanded Prostate Cancer Index Composite-26, among patients who received EBRT, adding neoadjuvant ADT with a duration of ≥1 year significantly decreased the sexual and hormonal/vitality domain responses.44 Specifically, they included questions regarding the ability to achieve an erection, quality of erections, frequency of erections, ability to reach orgasm, ability to function sexually, and lack of energy.
Impact of ADT on late toxicities due to radiotherapy
Among two phase 3 RCTs, one RCT, which evaluated the benefit of 5-month bicalutamide among patients with IR and HR PCa who received EBRT, reported that no significant differences in late genitourinary (GU) or gastrointestinal (GI) toxicities were observed between the RT plus bicalutamide monotherapy arm and RT-alone arm (p = 0.41 for late GU toxicities and p = 0.55 for late GI toxicities) (Table 5).17 Similarly, according to another phase 3 study, which compared 28-month ADT (28 months of goserelin acetate and 2 months of bicalutamide) vs. 4-month ADT (4 months of goserelin acetate and 2 months of bicalutamide), no significant differences in late GU or rectal toxicities were observed between the long- and short-term ADT arms (p = 0.912 for late GU toxicities and p = 0.345 for late rectal toxicities).54 Regarding two retrospective studies, in one analysis, which included 3424 Japanese patients who received HDR brachytherapy plus EBRT, compared with RT alone, the use of neoadjuvant ADT alone was not significantly correlated with the decrease of ≥grade 2 late GU (p = 0.310) or GI (p = 0.560) toxicities, while the use of neoadjuvant plus adjuvant ADT was significantly correlated with decreased ≥grade 2 late GU (p = 0.0) or GI (p = 0.004) toxicities.20 Conversely, in the other retrospective study, which included 1313 Japanese patients who received LDR brachytherapy with or without EBRT, ≥grade 2 GU toxicities were significantly higher in the RT plus neoadjuvant ADT group compared with RT-alone group (p = 0.001).42 The interaction between the prostate volume and use of neoadjuvant ADT was considered one potential explanation for the contradictory results.
| Reference | Refs. Number | Study design/Phase | RT method | ADT method | Sample size | Endpoint | Outcomes |
|---|---|---|---|---|---|---|---|
| GU toxicities | |||||||
McPartlin et al. PMH 9907 |
16 | Phase 3 | EBRT: median 78–79.6 Gy/39–42 fr | Arm 1: 5 months Bicalutamide Arm 2: no ADT |
119 122 |
Grade 2 and 3 late toxicities | 9.6% and 11.4% 5.5% and 11% p = 0.41 |
Zapatero et al. DART01/05 GICOR |
53 | Phase 3 | EBRT: median 78 Gy/39 fr | Arm 1: 4 months ADT (4 months Goserelin plus 2 months Bicalutamide) Arm 2: 28 months ADT (28 months Goserelin plus 2 months Bicalutamide) |
178 177 |
≥Grade 2 late toxicities | 7.6% 11.1% p = 0.345 |
| Ishiyama et al. | 19 | Retrospective | BT plus EBRT | No ADT Neoadjuvant ADT Neoadjuvant plus adjuvant ADT |
n/a | ≥Grade 2 late toxicities | Reference p = 0.31 p = 0.000 |
| Eriguchi et al. | 41 | Retrospective | BT with or without EBRT | No neoadjuvant ADT With neoadjuvant ADT |
785 528 |
≥Grade 2 late toxicities | More common in the “with ADT” group p = 0.001 |
| GI toxicities | |||||||
McPartlin et al. PMH 9907 |
16 | Phase 3 | EBRT: median 78–79.6 Gy/39–42 fr | Arm 1: 5 months Bicalutamide Arm 2: no ADT |
119 122 |
Grade 2 and 3 late toxicities | 3.5% and 0% 4.7% and 0.8% p = 0.55 |
Zapatero et al. DART01/05 GICOR |
53 | Phase 3 | EBRT: median 78 Gy/39 fr | Arm 1: 4 months ADT (4 months Goserelin plus 2 months Bicalutamide) Arm 2: 28 months ADT (28 months Goserelin plus 2 months Bicalutamide) |
81 85 |
≥Grade 2 late toxicities | 7.3% 8.2% p = 0.912 |
| Ishiyama et al. | 19 | Retrospective | BT plus EBRT | No ADT Neoadjuvant ADT Neoadjuvant plus adjuvant ADT |
n/a | ≥Grade 2 late GI toxicities | Reference p = 0.56 p = 0.004 |
- Abbreviations: ADT, androgen-deprivation therapy; BT, brachytherapy; EBRT, external-beam radiation therapy; GI, gastrointestinal; GU, genitourinary; RT, radiation therapy.
CONCLUSIONS AND FUTURE PERSPECTIVE
This systematic review summarized the available evidence regarding the effects of ADT on OS, BF-free survival, and toxicities among patients with IR and HR PCa who received high-dose RT. The evidence supports the benefit of adding ADT to high-dose RT to increase tumor control. However, regarding IR populations who received high-dose RT, a large number of studies suggested the existence of subgroup in which adding ADT had no impact not only on overall survival but also the BF-free duration. On the other hand, regarding HR populations who received high-dose RT, several studies suggested the positive impact of adding ADT for ≥1 year on overall survival, although it was considered difficult to draw a general conclusion regarding its benefit due to the heterogeneity of the ADT duration and backgrounds of patients in HR populations. It is also suggested that adding ADT increases not only the risk of sexual dysfunction but also that of cardiovascular toxicities or bone fracture. Therefore, taking into account QOL after treatment and cost-effectiveness, further investigations are warranted to identify subgroups of patients for whom ADT has no benefit, as well as the appropriate duration of ADT for those who do derive benefit. In addition, evidence is expected to accumulate in this area, based on big data analysis of prospective registry databases, RCTs to verify novel treatment strategies designed based on the results of nomograms to predict the risk of disease progression, and further data accumulation on clinical outcomes following high-dose RT.
ACKNOWLEDGMENTS
The authors would like to express gratitude to committee members of the Clinical Practice Guidelines for Prostate Cancer 2023 for their cooperation.
Committee members:
Akihiro Ito: Department of Urology, Tohoku University Graduate School of Medicine.
Takahiro Inoue: Department of Nephro-Urologic Surgery and Andrology, Mie University Graduate School of Medicine.
Hiroji Uemura: Departments of Urology and Renal Transplantation, Yokohama City University Medical Center.
Osamu Ukimura: Department of Urology, Kyoto Prefectural University of Medicine.
Shiro Saito: Department of Urology, Prostate Cancer Center Ofuna Chuo Hospital.
Hideki Sakai: Nagasaki Rousai Hospital.
Hiroshi Shinmoto: Department of Radiology, National Defense Medical College.
Mikio Sugimoto: Department of Urology, Kagawa University.
Kazuhiro Suzuki: Department of Urology, Gunma University Graduate School of Medicine.
Satoru Takahashi: Department of Urology, Nihon University School of Medicine.
Tsutomu Takeuchi: NPO Prostate Cancer Patients Association.
Atsushi Takenaka: Division of Urology, Department of Surgery, Tottori University Faculty of Medicine.
Tsutomu Tamada: Department of Radiology, Kawasaki Medical School.
Norio Nonomura: Department of Urology, Osaka University Graduate School of Medicine.
Tomonori Habuchi: Department of Urology, Akita University Graduate School of Medicine.
Nobuaki Matsubara: Department of Medical Oncology, National Cancer Center Hospital East.
Hideyasu Matsuyama: Department of Urology, Yamaguchi University Hospital.
Hideaki Miyake: Department of Urology, Hamamatsu University Hospital.
Masahiro Yoshida: Department of EBM and Guidelines, Japan Council for Quality Health Care. Medical information network distribution service (Minds).
AUTHOR CONTRIBUTIONS
Rihito Aizawa: Writing – original draft; writing – review and editing. Hitoshi Ishikawa: Writing – original draft; project administration; conceptualization. Manabu Kato: Writing – original draft; writing – review and editing. Shosei Shimizu: Writing – original draft; writing – review and editing. Takashi Mizowaki: Project administration. Yasuo Kohjimoto: Project administration. Shiro Hinotsu: Project administration. Isao Hara: Project administration.
CONFLICT OF INTEREST STATEMENT
RA reported receiving honoraria for lectures from Janssen Pharmaceutical K.K and Astellas Pharma Inc. TM reported receiving honoraria for lectures from Janssen Pharmaceutical K.K., Takeda Pharmaceuticals and Astellas Pharma Inc. and Bayer Yakuhin, Ltd. IH reported receiving funds for research or education from IQVIA Services Japan K.K., Syneos Health Research, Bristol-Myers Squibb K.K., and Janssen Pharmaceutical K.K., honoraria for lectures from Astellas Pharma Inc., Takeda Pharmaceuticals, and Janssen Pharmaceutical K.K., and donation from Astellas Pharma Inc., ONO PHARMACEUTICAL CO., LTD., Takeda Pharmaceuticals, Novartis Pharma K.K., and Pfizer Japan Inc. YK reported receiving honoraria for lectures from Takeda Pharmaceuticals and Astellas Pharma Inc. HI, MK, SS, and SH report no conflicts of interest related to the subject matter of this study.
Dr. Isao Hara is an Editorial Board member of the International Journal of Urology and a co-author of this article. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication.




