Pneumovesical vesicovaginal fistula repair: Lessons learned from an initial series of 25 patients
Abstract
Objectives
This study aims to share the experiences and outcomes of laparoscopic pneumovesical repair for vesicovaginal fistulas (VVF).
Materials and methods
A retrospective review of medical records from a single institution over 10 years was conducted. The focus was on patients who underwent VVF repair using a pneumovesical approach with three 5 mm laparoscopic ports. The study evaluated perioperative parameters, postoperative outcomes, and complication rates to assess the efficacy and safety of this surgical method. Cumulative sum (CUSUM) analysis was used to determine the learning curve based on operative time.
Results
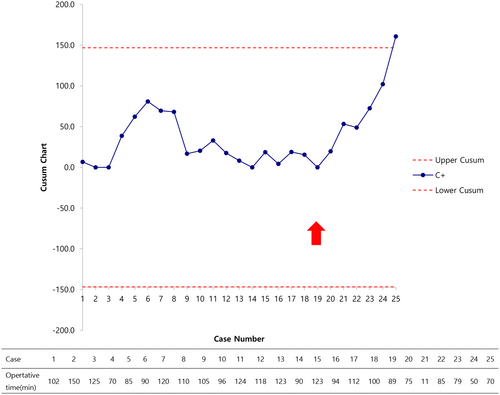
Of the 26 patients with VVF, 23 (88.5%) had successful fistula closure after the first surgery. One patient required open surgery conversion due to challenges in maintaining pneumovesicum, and two experienced recurrences, although successful repairs were achieved in subsequent surgeries. The average patient age was 47.4 years, with a mean operative time of 99.9 min. The postoperative hospital stay averaged 9.1 days, and catheterization lasted about 11 days. The CUSUM chart indicated a learning curve, with fluctuations until the 19th case, followed by a consistent upward pattern.
Conclusion
Laparoscopic pneumovesical VVF repair is an effective and safe technique, especially suitable for fistulas near the ureteral orifice or deep in the vaginal cavity. The method demonstrates favorable outcomes with minimal complications and allows for easy reoperation if necessary.
Abbreviations & Acronyms
-
- CUSUM
-
- cumulative sum
-
- IRB
-
- Institutional Review Board
-
- VVF
-
- vesicovaginal fistula
INTRODUCTION
Vesicovaginal fistulas (VVF) are common genitourinary fistulas with an incidence ranging from 0.3% to 2%, causing considerable distress.1 Although local trauma or prolonged labor can lead to VVF, the primary known cause is hysterectomy, accounting for approximately 85% of cases, occurring in approximately 1 out of 1800 hysterectomies.2, 3 Conservative treatments, such as prolonged bladder drainage or fulguration, may be considered in cases that are small or non-malignant and do not involve radiation therapy.4
However, the majority of VVF cases necessitate surgical intervention. Currently, there is no consensus on the optimal approach for VVF repair that is universally applicable. Generally, VVF repair can be accomplished via abdominal or vaginal route, each having its own merits. The choice of surgical method depends on various factors such as fistula size, location, and surgeon preference. The transvaginal approach is mainly employed for infratrigonal fistulas, offering less invasiveness and cost-effectiveness. Nevertheless, it proves challenging when the vagina is short or when the fistula is situated above the trigone plane or near the ureteral orifice.5, 6 With recent advancements in laparoscopic and robotic surgeries, the preference for the transabdominal approach has gained favor. This technique is commonly employed for supratrigonal fistulas and enables fistula tract removal through layer-by-layer repair.7, 8 However, despite these endeavors, accessing the fistula remains problematic, and bladder closure after fistula removal via bladder incision presents a drawback. Consequently, the surgical procedure is inherently intricate, leading to increased complexity and extended operative time.9
More recently, innovative surgeries utilizing pneumovesicum through CO2 insufflation into the bladder during laparoscopic procedures have been explored for VVF.10-12 This surgical approach offers a minimally invasive means to directly and conveniently access bladder lesions without complications such as postoperative bladder dysfunction, ureteral reimplantation for vesicoureteral reflux, removal of bladder diverticulum, or removal of bladder stones and foreign bodies.13-16 This study aimed to present the outcomes of laparoscopic pneumovesical VVF repair performed at a single institution, with a specific focus on the success rate of the procedure.
MATERIALS AND METHODS
Study population and selection
This retrospective study included 26 patients who had undergone laparoscopic pneumovesical repair for VVF at a single institution, Korea University Ansan Hospital, between July 2013 and July 2023. This study was approved by the Institutional Review Board (IRB) (IRB No. 2018AS0221). Patient data, including age, history of gynecological surgery, intra-operative parameters, and postoperative outcomes, were reviewed and analyzed from medical records. This study included patients who had undergone both primary and secondary repairs. All patients analyzed in this study had a history of gynecological surgery and developed fistulas after surgery; 15 patients had undergone abdominal hysterectomy, five had undergone laparoscopic hysterectomy, and three had undergone robotic hysterectomy. Three patients developed fistulas after cesarean sections. Five patients had a history of radiation therapy for malignancies. The fistula size and location were measured using preoperative cystoscopy and computed tomography urogram. With one exception, all fistulas were located in the supratrigonal region, and one patient had multiple fistulas. Surgical data included operative time, complications during surgery, and postoperative details such as hospitalization period, duration of urinary drainage, and postoperative complications. Following surgery, urinary leakage was confirmed via cystography before removal of the urinary catheter, and surgical success was defined as the absence of urinary leakage observed during follow-up monitoring.
Surgical procedure
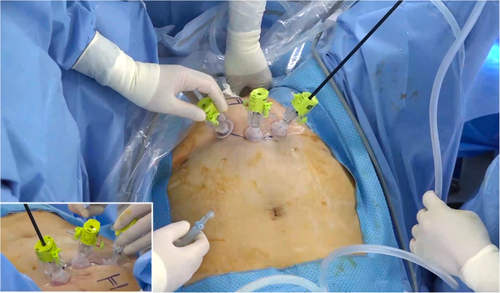
All surgeries were performed by a urologist (J.H.B.) using either the traditional open or pneumovesical approach. The surgeon had previously performed five cases of the pneumovesical approach for Politano-Leadbetter ureteric reimplantation before this surgery. During the period of this study, in addition to VVF repairs, the physician conducted 40 cases of Politano-Leadbetter ureteric reimplantation, and 10 other pneumovesical surgeries including foreign body or stone removal, and bladder diverticulectomy. All surgical procedures were performed under general anesthesia, and the patient was placed in the modified lithotomy position with slight Trendelenburg and straight hands. After the bladder was filled with normal saline, three 5-mm laparoscopic ports were inserted through the bladder wall under cystoscopic guidance. To prevent air leakage, the port was secured internally within the bladder using the Eagle-port laparoscopic port (Dalim Medical, Seoul, Korea), which features a fixation mechanism (Figure 1). After port placement, normal saline was drained while simultaneously filling the bladder with gas (pressure, 8–12 mmHg and flow rate, 2–3 L/min). The vagina was packed with 30 cc balloon 20Fr Foley catheter to prevent gas leakage. After dissecting the peri-fistula tissue, the mucosa surrounding the fistula was circumferentially debrided using endoscissors (Figure 2a). Fresh vaginal tissue was then obtained, and the vaginal layer was repaired in a layered fashion using vicryl 4-0 sutures (Figure 2b,c). Subsequently, the bladder mucosal layer was reconstructed layer-by-layer using vicryl 4-0 sutures (Figure 2d). After confirming bilateral jetting, the bladder was catheterized. The Foley catheter was removed after confirming the absence of leakage using cystography. No ureteral stents were placed.

Statistical analysis
The cumulative sum (CUSUM) series was defined as Sn = ∑(Xi − X0), where Xi represent an individual measurement, and X0 was a predetermined reference level that was set as the mean value of the total operation time for all cases overseen. Sn is plotted against the sequence of operations. The cutoff values were selected according to the points of upward inflection revealed by the plots. Continuous data are expressed as mean ± SD. Statistical analyses were performed using IBM SPSS Statistics (version 21.0; IBM Corp., Armonk, NY, USA).
RESULTS
The patient characteristics and results are outlined in Tables 1 and 2. Of the 26 patients, 23 (88.5%) achieved successful surgical outcomes. However, one patient required conversion to open surgery owing to difficulties related to a small bladder volume resulting from radiation and a relatively large fistula size. These factors led to difficulties in maintaining an airtight space in the bladder. Although leakage was observed on post-surgery cystography in the remaining two patients, both patients were successfully treated through reoperation using the same method. Mean patient age was 47.4 years (range 33–77), and the mean body mass index was 24 kg/m2 (range 18–29.8). The average interval between gynecological surgery and VVF repair was 7 months (range 1–34). The fistula sizes ranged from 3 to 15 mm. The mean operative time was 99.9 min, with no cases necessitating blood transfusions owing to minimal blood loss during surgery. The average hospital stay was 9.1 days, and the mean duration of indwelling urinary catheter insertion was 11 days. During hospitalization, six patients experienced minor hematuria, one experienced vaginal bleeding, and two had urinary tract infections. In the follow-up period, one patient had remnant suture material; however, no case of recurrence occurred. The average follow-up period was 6.96 months.
| Factor | Value |
|---|---|
| The number of patients | 26 |
| Age (years), mean | 47.4 (33–77) |
| BMI (kg/m2) | 24 (18–29.8) |
| Interval between gynecologic injury and VVF repair (months), mean | 7 (1–34) |
| Fistula history, n (%) | |
| Primary | 21 (80.8) |
| Recurrent | 5 (19.2) |
| Previous radiotherapy history | 5 |
| Size (mm) | 6.1 (3–15) |
| Fistula number, n (%) | |
| Single | 25 (96.2) |
| Multiple | 1 (3.8) |
| Fistula location n (%) | |
| Supratrigonal | 26 (96.3) |
| Infratrigonal | 1 (3.7) |
| Etiology of fistula n (%) | |
| Abdominal hysterectomy | 15 (57.7) |
| Laparoscopic hysterectomy | 5 (19.2) |
| Robotic hysterectomy | 3 (11.5) |
| Cesarean | 3 (11.5) |
- Abbreviations: BMI, body mass index; VVF, vesicovaginal fistula.
| Parameters | Value |
|---|---|
| Operative time (min), mean ± SD | 99.9 ± 22.9 |
| Postoperative stay (days), mean ± SD | 9.1 ± 3.1 |
| Duration of Catheterization (days), mean ± SD | 11 ± 4.5 |
| Success rate n (%) | |
| Primary repair | 23/26 (88.5) |
| Secondary repair | 2/2 (100) |
| Postoperative follow-up period (months), mean | 6.96 (1–30) |
| Complications, n (%) | |
| Clavien-Dindo grade I | 8 (30.8) |
| Clavien-Dindo grade II | 2 (7.7) |
| Clavien-Dindo grade III–V | 0 (0) |
Figure 3 displays an upward CUSUM chart illustrating operative time. Initially, a decreasing slope was observed from the first to the third cases, followed by an ascending trend up to the sixth case. Subsequently, fluctuations were noted until the 19th case, beyond which an ascending curve emerged, signifying an acceptable level of performance.
DISCUSSION
VVF primarily results from iatrogenic injuries associated with gynecological surgeries, notably hysterectomies, causing considerable physical and psychological distress to both patients and surgeons.7 Although some cases respond to conservative treatments like prolonged urinary catheterization, most require surgical repair, predominantly via transvaginal or transabdominal approaches. However, the absence of randomized prospective trials has prevented the establishment of a gold standard surgical method for VVF repair, making the choice of technique dependent on various factors including fistula size, location, and the surgeon's expertise and preference.17 Despite different etiologies, the success rate of non-complicated VVF repair in the recent era, whether through a vaginal or abdominal approach, is >90%. For improving the surgical success rate, the study has also reported that preoperative phenazopyridine, physical therapy, and intraoperative antibiotics may be beneficial methods. The transvaginal approach is often used for VVF repair when the fistula is in the lower vagina, given its benefits of shorter surgery times and quicker recovery.18, 19 However, it may not be suitable for deeply located fistulas or in patients with narrow or compromised vaginal tissues due to conditions like radiation therapy.5, 6 For urologists who may not be familiar with the transvaginal approach, the transabdominal approach can be a preferred alternative in cases of high-lying, inaccessible vaginal fistulas, large fistulas, or coexisting intra-abdominal surgical conditions such as synchronous ureteric involvement.20 It allows for extensive mobilization of tissues, complete fistula excision, and effective closure, often with omental interposition for added support collection.21, 22 Laparoscopic or robotic-assisted transabdominal approaches offer enhanced visualization and shorter recovery times but can be challenging due to increased operative time and intraperitoneal adhesions.23, 24 The transvesical approach, while offering improved fistula identification, carries risks such as postoperative bladder dysfunction and bleeding.25, 26
Recently, many studies have reported the use of the pneumovesical approach in various situations such as ureter reimplantation in vesicoureteral reflux or removal of bladder diverticulum, bladder stones, and foreign body.13-16 The pneumovesical approach is even utilized in surgeries related to the prostate, such as surgeries for benign prostatic hyperplasia.27, 28 Compared with traditional laparoscopic surgery, this approach provides quick and straightforward access to the fistula lesion without causing injury to the intra-abdominal organs or unnecessary bladder incision. This approach allows for fast recovery and a satisfactory cure rate and reduces bladder trauma, postoperative hematuria, and detrusor spasm. Our case series demonstrated promising results using the pneumovesical approach for VVF repair in patients with fistulas located in the supratrigonal region, a common site of bladder damage during abdominal hysterectomy.19 This approach is particularly advantageous for fistulas located deep within the vagina or adjacent to ureteral orifices. Furthermore, most patients undergoing VVF repair have a history of abdominal surgery, particularly hysterectomy, which may lead to intra-abdominal adhesions. In cases where patients have coexisting cancer and have undergone radiation therapy, the condition of the vaginal tissues may not be favorable. Considering these factors, accessing the fistula site can be challenging via both the transabdominal and transvaginal approaches. However, the pneumovesical approach offers a distinct advantage, as it allows direct access to the fistula through the bladder, significantly reducing preoperative considerations.
Ultimately, despite two patients requiring reoperation, there was no recurrence of urinary leakage observed in any of the cases included in this study. This is a noteworthy outcome, especially considering the challenging and complicated overall patient condition (20% had undergone previous VVF repair and 16% had undergone radiation therapy). Regarding VVF repair, secondary surgery after the initial procedure often leads to lower success rates.19 However, in our study, regardless of whether the initial surgery was transvaginal or transabdominal, no patient experienced leakage. Notably, if the previous surgical approach was the pneumovesical approach, reoperation can still be performed using the same method.
The timing of the VVF repair in this study was expedited as much as possible upon referral to our department, considering the distress of patients with urinary leakage. However, owing to patient referrals from other hospitals post-surgery, a relatively extended mean interval of 7 months occurred between VVF repair and gynecological surgery. Moreover, in our case series, four cases experienced VVF within 1 month after gynecological surgery. Recent reports have indicated that delaying surgical repair offers no advantage in the absence of an inflammatory reaction, and the success rates of early repairs are comparable with those of the conventional strategy of delayed operation for 3–6 months after fistula formation.29
The upward CUSUM chart for operative time showed a trend of gradual improvement after the first three cases, but a fluctuating pattern was observed up to the 19th case. This is because surgery was initially intuitive and easy to approach, but there have been many trials and errors owing to concerns regarding the surgical method. These challenges include port placement, addressing gas leakage into the peritoneal cavity through Veress needle aspiration, and attempting various vaginal packing techniques. In the initial cases of this study and similar research, vaginal packing involved the use of Betadine or Vaseline gauze.12 However, this method resulted in gas leakage to some extent, necessitating manual intervention by the assistant, which disrupted the surgical procedure. In our subsequent cases, we employed a 30 cc balloon Foley catheter to prevent gas leakage. This was achieved by placing the balloon beyond the fistula site at the vaginal entrance. The restructured surgical approach pointed to a learning curve encompassing approximately 19 cases.
The successful execution of a pneumovesical VVF repair hinges on refined laparoscopic expertise, demanding meticulous dissection, resection, and intracorporeal suturing. Despite technical difficulties, the selected patient pool and the prevailing familiarity among urologists with laparoscopic surgery indicate a formidable yet surmountable learning curve. Recent instances involving robotic systems for pneumovesical VVF repair have emerged. Although there are some limitations, such as the relatively large size of robotic instruments compared with conventional laparoscopic systems and longer operative times, the robotic approach offers clear advantages, including high magnification and a three-dimensional view. Given suturing post-fistula excision is a critical procedure in VVF repair, the use of multi-joint robotic systems presents clear advantages. If tailored robotic systems emerge for pneumovesical surgery, the steep learning curve associated with laparoscopic pneumovesical surgery could be mitigated, rendering it more accessible.
Our study had several limitations. First, its retrospective nature necessitates future research for comparison with conventional surgical methods. Second, the relatively low VVF incidence led to a limited surgical case pool. Third, in scenarios featuring coexisting ureteral injuries or inadequate bladder air pressure retention owing to extensive fistula size or reduced bladder capacity, the pneumovesical approach might not be suitable. Nonetheless, the favorable results in terms of cure and low recurrence rates in our study demonstrate the potential benefits of this approach for VVF repair. Previous studies utilizing the pneumovesical approach for VVF repair predominantly involved cases characterized by modified methods or a relatively limited number of selected patients.10-12 However, our study encompassed a diverse range of cases, regardless of a prior history of VVF repair or radiation therapy, fistula location, or number. As an initial experience, our study demonstrates the effectiveness of the pneumovesical approach, which can be applied to the majority of VVF cases without significant limitations and yields successful outcomes with less bleeding and a short recovery period. The longer hospitalization period observed in our study was influenced by the healthcare insurance policy in Korea, resulting in relatively lower medical costs compared with other countries.
In conclusion, pneumovesical VVF repair emerges as a viable and secure clinical option, boasting a minimal recurrence rate. Owing to the prime benefit of diminished morbidity through the non-peritoneal approach, our pneumovesicoscopic procedure could potentially assume an alternative role in VVF repair, potentially supplanting other surgical methods.
AUTHOR CONTRIBUTIONS
Byeong Jo Jeon: Writing—original draft; conceptualization; methodology; data curation; formal analysis. Bum Sik Tae: Writing—review & editing; investigation; validation; formal analysis; data curation; supervision. Jeong Wan Yoo: Data curation. Ho Young Koo: Data curation. Cheol Young Oh: Resources; data curation. Jae Young Park: Data curation; supervision. Jae Hyun Bae: Writing—review & editing; conceptualization; methodology; project administration; resources; data curation; supervision.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
The study protocol was approved by the Institutional Review Board of Korea University Medicine (IRB No. 2018AS0221) and was conducted in accordance with the Declaration of Helsinki.
INFORMED CONSENT
Not applicable.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
Not applicable.
ANIMAL STUDIES
Not applicable.




