Lower bleeding volume contributes to decreasing surgical site infection in radical cystectomy: A propensity score-matched comparison of open versus robot-assisted radical cystectomy
Abstract
Objectives
To compare the incidence of surgical site infections (SSI) between robot-assisted and open radical cystectomies and investigate the risk factors for SSI after radical cystectomies.
Methods
Consecutive patients who underwent radical cystectomy between July 2008 and December 2022 were retrospectively reviewed. The prevalence and characteristics of SSI after open and robot-assisted radical cystectomies were compared, and the risk factors for SSI were investigated using propensity score matching.
Results
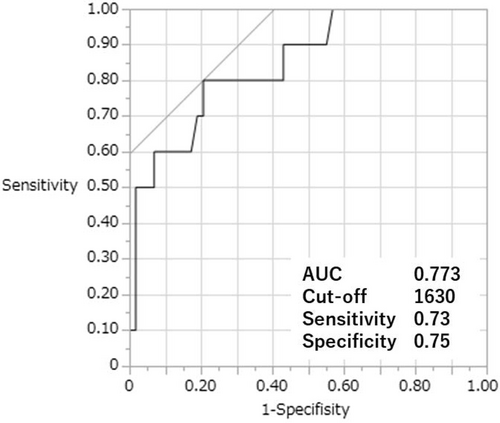
This study enrolled 231 patients (open: 145, robot-assisted: 86). In the robot-assisted group, urinary diversion was performed using an intracorporeal approach. SSI occurred in 34 (open: 28, robot-assisted: 6) patients, and the incidence was significantly lower in the robot-assisted group (19.3% vs. 7.0%, p = 0.007). After propensity score matching cohort (open: 34, robot-assisted: 34), increased bleeding volume, blood transfusion, and delayed postoperative oral feeding were significantly associated with SSI. Only increased bleeding volume remained a significant risk factor in the multivariate regression analysis (odds ratio, 1.13 [per 100 mL increase]; 95% confidence interval: 1.02–1.25; p = 0.001). The cutoff bleeding volume for predicting SSI was 1630 mL with an area under the receiver operating characteristic curve, sensitivity, and specificity of 0.773, 0.73, and 0.75, respectively.
Conclusions
The incidence of SSI after robot-assisted radical cystectomy was significantly lower than that after the open procedure. However, decreased bleeding volume, which was significantly associated with robot-assisted procedures, was an independent and more significant factor for reducing SSI after radical cystectomy than the differences of the surgical procedure even after propensity score matching.
Abbreviations & Acronyms
-
- ASA-PS
-
- American Society of Anesthesiologists Physical Status
-
- AUC
-
- area under the receiver operating characteristic curve
-
- BMI
-
- body mass index
-
- CDC
-
- the Centers for Disease Control and Prevention
-
- ECUD
-
- extracorporeal urinary diversion
-
- ICUD
-
- intracorporeal urinary diversion
-
- OR
-
- odds ratio
-
- ORC
-
- open radical cystectomy
-
- POD
-
- postoperative day
-
- PSM
-
- propensity score matching
-
- RARC
-
- robot-assisted radical cystectomy
-
- ROC
-
- receiver operating characteristic
-
- SSI
-
- surgical site infection
INTRODUCTION
Radical cystectomy with urinary diversion is the standard surgical treatment for muscle-invasive bladder cancer and very-high-risk non-muscle-invasive bladder cancer, for which the previously reported complication rates were relatively high (28–60%).1, 2 Surgical site infections (SSIs) negatively impact the quality of life, prolong hospital stays, and increase healthcare-related costs. They are common complications of radical cystectomy and the incidence rate of SSI after ORC has been reported as 2%–33%.2-6
Robot-assisted radical cystectomy (RARC) has become a standard minimally invasive surgery, expected to reduce perioperative mortality and complications, especially bleeding volume and blood transfusion, with oncological outcomes comparable to ORC.7-10 Minimally invasive surgeries significantly decrease the incidence of SSIs in various surgeries; hence, RARC is also expected to decrease the incidence of SSI compared to ORC.11 However, several previous studies evaluating the perioperative outcomes of RARC did not distinguish between the incidence of SSIs and urinary tract infections when assessing the prevalence of infectious complications or evaluating the prevalence of SSIs according to the criteria defined in the Centers for Disease Control and Prevention (CDC) guidelines.12-14 Furthermore, no studies have evaluated in detail the differences in SSI characteristics between RARC and ORC or the etiology related to the differences in incidence.
Therefore, this study aimed to compare the incidence rates of SSIs after RARC and ORC and investigated the risk factors for SSIs after radical cystectomy, focusing on the difference in the surgical approach using propensity score matching (PSM).
METHODS
Ethical statements and study participants
This study enrolled consecutive patients who underwent radical cystectomy between July 2008 and December 2022 in our institute. ORC was performed from July 2008 to May 2018, while RARC was performed afterward. The clinical characteristics of the patients and surgical, perioperative, and pathological information, including the occurrence of SSI were investigated by reviewing the medical records. This retrospective study was approved by our institutional ethics committee (A22-023). Given the retrospective nature of the study, the requirement for written informed consent was waived.
Radical cystectomy procedure and perioperative management
ORC was performed using a standard intraperitoneal approach with Level IIA lymph node dissection,15 and the uterus and ovaries were concomitantly resected in females. RARC was performed in the 25° Trendelenburg position using the Da Vinci Si Surgical System.16, 17 After completing the cystectomy and extended lymph node dissection, urinary diversion was performed with intracorporeal urinary diversion (ICUD) via the small intestine, with all patients in the 15° Trendelenburg position. Cutaneous ureterostomy was chosen for selected patients instead of urinary diversion using the small intestine. Estimated bleeding volume was calculated by adding the amount of the fluid aspirated during surgery to that absorbed by gauze based on the fact that 4 × 4-inch gauze has an absorption capacity of about 10 mL.
Cisplatin- or carboplatin-based neoadjuvant chemotherapy was administered prior to surgery in selected patients, depending on the surgeon's decision. Patients were basically admitted to the hospital the day before surgery, and pubic hair was either cut in the operating room immediately before surgery or not removed. Prophylactic antibiotics included cephamycin, second-generation cephalosporins, or penicillins and beta-lactamase inhibitors combination, with modifications permitted at the surgeon's discretion according to the patient's history, urine culture, and renal function. SSI was diagnosed by laboratory and clinical findings and defined as infection occurring within 30 postoperative days and involving skin, subcutaneous or deep soft tissues of the incision, or abdominal or perineal spaces according to the criteria in the CDC guidelines.12
Statistical analysis
Multivariable logistic regression analysis was used for PSM to calculate propensity scores, and matching was conducted on the logit of the propensity scores using nearest neighbor matching with a caliper of 0.20. Patient characteristics, clinical variables, and SSI characteristics (classification, location, causative bacteria, and treatment) were compared between the ORC and RARC groups.
The risk factors for SSI after radical cystectomy were statistically evaluated, focusing on the type of surgery (ORC vs. RARC) and other perioperative parameters.
Chi-squared or Kruskal–Wallis tests were performed for each covariate to evaluate the risk factors for SSI. All significant variables in the univariate analysis were considered in the multivariate logistic regression analysis. Receiver operating characteristic (ROC) analysis was performed to obtain the cutoff value of the significant parameters for predicting SSI occurrence. The best cutoff point was determined using the Youden index: sensitivity (1 − specificity). The cumulative incidences of SSI were estimated using the Kaplan–Meier method and compared using the log-rank test. All statistical analyses were performed using JMP 14 software (SAS Institute, Cary, NC, USA). All tests were two-sided, and p-values <0.05 were considered statistically significant.
RESULTS
Analysis before PSM
During the study period, 231 patients underwent radical cystectomy (ORC, n = 145; RARC, n = 86). Patient characteristics and surgical and perioperative outcomes are shown in the left half of Table 1. The RARC group tended to have a higher prevalence of diabetes mellitus, neoadjuvant chemotherapy, and higher ASA-PS scores. The RARC group also showed significantly lower bleeding volume (1880 mL vs. 300 mL, p < 0.001) and shorter periods of antimicrobial prophylaxis (p < 0.001) and fasting (6 days vs. 3 days, p < 0.001) than the ORC group.
| Unmatched cohort | p-value | Matched cohort | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 231) | ORC (n = 145) | RARC (n = 86) | Total (n = 68) | ORC (n = 34) | RARC (n = 34) | |||
| Age, year | 70 (63–75) | 69 (62.5–74) | 70 (65.8–75) | 0.18 | 70 (63–74.5) | 68 (60.8–74.3) | 71.5 (67–75) | 0.13 |
| Male | 178 (77.8%) | 112 (77.2%) | 66 (76.7%) | 0.93 | 36 (52.9%) | 19 (55.9%) | 17 (50.0%) | 0.80 |
| BMI, kg/m2 | 23.1 (21.2–24.8) | 23.0 (21.2–24.8) | 23.1 (20.3–24.9) | 0.96 | 23.4 (21.2–25.0) | 23.2 (21.1–25.3) | 23.6 (21.5–24.5) | 0.75 |
| Smoking history | 0.13 | 0.61 | ||||||
| None or ex-smoker | 219 (94.8%) | 135 (93.1%) | 84 (97.7%) | 64 (94.1%) | 31 (91.2%) | 33 (97.1%) | ||
| Current smoker | 12 (5.2%) | 10 (6.9%) | 2 (2.3%) | 4 (5.9%) | 10 (6.9%) | 2 (2.3%) | ||
| Diabetes mellitus | 52 (22.5%) | 23 (15.9%) | 29 (33.7%) | <0.01 | 14 (20.6%) | 8 (23.5%) | 6 (17.7%) | 0.77 |
| Steroid use | 7 (3.0%) | 4 (2.8%) | 3 (3.5%) | 0.75 | 3 (4.4%) | 1 (2.9%) | 2 (5.9%) | 1.0 |
| ASA-PS | 0.026 | 0.85 | ||||||
| 1 | 24 (10.4%) | 21 (14.5%) | 3 (3.5%) | 7 (10.3%) | 4 (11.8%) | 3 (8.8%) | ||
| 2 | 171 (74.0%) | 101 (69.7%) | 70 (81.4%) | 50 (73.5%) | 24 (70.6%) | 70 (76.5%) | ||
| 3 | 36 (15.6%) | 23 (15.9%) | 13 (15.1%) | 11 (16.2%) | 6 (17.7%) | 13 (14.7%) | ||
| Preoperative hemoglobin, g/dL | 12.0 (11.0–13.0) | 12.1 (11.2–13.2) | 11.7 (10.6–12.7) | 0.019 | 11.9 (10.5–13.2) | 11.9 (10.8–13.5) | 12.0 (10.3–13.0) | 0.50 |
| Preoperative serum albumin, g/dL | 4.0 (3.8–4.3) | 4.1 (3.8–4.3) | 4.0 (3.8–4.2) | 0.2 | 4.0 (3.8–4.2) | 4.0 (3.7–4.3) | 4.0 (3.8–4.2) | 0.60 |
| Preoperative pyuria | 143 (61.9%) | 90 (62.1%) | 53 (61.6%) | 0.97 | 43 (63.2%) | 21 (61.8%) | 22 (64.7%) | 1.00 |
| Preoperative bacteriuria | 48 (25.7%) (n = 187) | 26 (24.3%) (n = 107) | 22 (27.5%) (n = 80) | 0.62 | 21 (37.5%) (n = 56) | 10 (41.7%) (n = 24) | 11 (34.4%) (n = 32) | 0.59 |
| Clinical T stage | 0.08 | 0.41 | ||||||
| Tis, Ta | 36 (15.6%) | 16 (11.0%) | 20 (23.3%) | 17 (25.0%) | 5 (14.7%) | 12 (35.3%) | ||
| T1 | 40 (17.3%) | 29 (20.0%) | 11 (12.8%) | 11 (16.2%) | 6 (17.7%) | 5 (14.7%) | ||
| T2 | 83 (35.9%) | 50 (34.5%) | 33 (38.4%) | 20 (29.4%) | 11 (32.4%) | 9 (26.5%) | ||
| T3 | 61 (26.4%) | 43 (29.7%) | 18 (20.9%) | 15 (22.1%) | 9 (26.5%) | 6 (17.7%) | ||
| T4 | 11 (4.8%) | 7 (4.8%) | 4 (4.7%) | 5 (7.3%) | 3 (8.8%) | 2 (5.9%) | ||
| Neoadjuvant chemotherapy | 136 (58.9%) | 72 (49.7%) | 64 (74.4%) | <0.001 | 32 (47.1%) | 14 (41.2%) | 18 (52.9%) | 0.47 |
| Type of urinary diversion | 0.43 | 0.83 | ||||||
| Ilial conduit | 181 (78.4%) | 115 (79.3%) | 66 (76.7%) | 47 (69.1%) | 24 (70.6%) | 23 (67.7%) | ||
| Neobladder | 4 (1.7%) | 1 (0.7%) | 3 (3.5%) | 2 (2.9%) | 1 (2.9%) | 1 (2.9%) | ||
| Cutaneous ureterostomy | 35 (15.2%) | 22 (15.2%) | 13 (15.1%) | 13 (19.1%) | 6 (17.7%) | 7 (20.6%) | ||
| None | 11 (4.8%) | 7 (4.8%) | 4 (4.7%) | 6 (8.7%) | 3 (8.8%) | 3 (8.8%) | ||
| Simultaneous nephroureterectomy | 27 (11.7%) | 17 (11.7%) | 10 (11.6%) | 0.98 | 12 (17.6%) | 7 (20.6%) | 5 (14.7%) | 0.75 |
| Simultaneous urethrectomy | 164 (71.0%) | 136 (93.8%) | 28 (32.6%) | <0.001 | 50 (73.5%) | 25 (73.5%) | 25 (73.5%) | 1.0 |
| Total operation time, min | 462 (393–518) | 450 (388–510) | 474 (409–533) | 0.17 | 476 (388–529) | 451 (392–506) | 487 (384–547) | 0.34 |
| Bleeding volume, mL | 1145 (400–2300) | 1880 (1185–4606) | 300 (120–473.8) | <0.001 | 790 (262.5–1880) | 1880 (1109–3041) | 265 (118–895) | <0.001 |
| Blood transfusion | 126 (54.1%) | 120 (82.8%) | 6 (7.0%) | <0.001 | 33 (48.5%) | 30 (88.2%) | 3 (8.8%) | <0.001 |
| Length of antimicrobial prophylaxis | <0.001 | <0.001 | ||||||
| 1–2 days | 43 (18.6%) | 10 (6.9%) | 33 (38.4%) | 16 (23.5%) | 3 (8.8%) | 13 (38.2%) | ||
| 3–4 days | 100 (43.4%) | 54 (37.2%) | 46 (53.5%) | 27 (39.7%) | 8 (23.5%) | 19 (55.9%) | ||
| 5 days or longer | 88 (38.1%) | 81 (55.9%) | 7 (8.1%) | 25 (36.8%) | 23 (67.6%) | 2 (5.9%) | ||
| Oral feeding (POD) | 6 (3–7) | 6 (5–7) | 3 (2–6) | <0.001 | 5 (3–7) | 5.5 (3–7) | 4 (2–7) | 0.10 |
| SSI within 30 days | 34 (14.72%) | 28 (19.3%) | 6 (7.0%) | 0.007 | 10 (14.7%) | 8 (23.5%) | 2 (5.9%) | 0.040 |
- Note: Data are presented as number (percentage) or median (interquartile range).
- Abbreviations: ASA-PS, American Society of Anesthesiologists Physical Status; BMI, body mass index; ORC, open radical cystectomy; POD, postoperative day; RARC, Robot-assisted radical cystectomy; SSI, surgical site infection.
SSIs occurred in 34 cases (ORC: 28 and RARC: 6), and their prevalence was significantly lower in the RARC group (19.3% vs. 7.0%, p = 0.007). Table 2 compares SSI characteristics between patients in the ORC and RARC groups. Superficial incisional SSIs were observed in 17 (11.7%) and two (2.3%) patients in the ORC and RARC groups, respectively (p = 0.012). Three of six patients with SSIs in the RARC group had pelvic or abdominal abscesses; two of them required percutaneous drainage. The epididymis was observed in two and one patient(s) in the ORC and RARC groups, respectively. A MRSA strain and an extended-spectrum beta-lactamase-producing E. coli were detected from a case in the RARC group, respectively, and no statistical differences in resistant strains were identified between the two groups.
| ORC (n = 28) | RARC (n = 6) | p-value | |
|---|---|---|---|
| Age, year | 65 (60–73.8) | 73 (66.3–76.3) | 0.14 |
| Days to diagnosis (POD) | 11 (7–13) | 8.5 (6.5–16.5) | 0.87 |
| Classification | 0.34 | ||
| Superficial incisional | 17 | 2 | |
| Deep incisional | 3 | 0 | |
| Organ/space | 6 | 3 | |
| Genital tract | 2 | 1 | |
| Location | 0.59 | ||
| Abdominal incisional scar | 15 | 1 | |
| Perineal incisional scar | 4 | 1 | |
| Stoma site | 1 | 0 | |
| Pelvic abscess | 4 | 2 | |
| Abdominal abscess | 2 | 1 | |
| Epididymis | 2 | 1 | |
| Causative bacteria | 0.14 | ||
| Enterococcus faecalis | 9 | 0 | |
| Enterococcus faecium | 2 | 0 | |
| Coagulase-negative Staphylococci | 4 | 0 | |
| MSSA | 1 | 0 | |
| MRSA | 0 | 1 | |
| Pseudomonas aerogenes | 7 | 0 | |
| E. coli | 3 | 1 | |
| Proteus mirabilis | 1 | 0 | |
| Enterobacter aerogenes | 1 | 0 | |
| Bacteroides spp. | 1 | 0 | |
| Not isolated | 1 | 3 | |
| Not evaluated | 3 | 1 | |
| Treatment | 0.82 | ||
| Antibiotics only | 10 | 2 | |
| Incisional drainage | 12 | 2 | |
| Percutaneous drainage | 5 | 2 | |
| Debridement | 1 | 0 |
- Note: Data are presented as number or median (interquartile range).
- Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; ORC, open radical cystectomy; POD, postoperative day; RARC, robot-assisted radical cystectomy; SSI, surgical site infection.
Table S1 shows the results of the univariate analysis of SSI incidence before PSM. In addition to open surgical procedures (vs. RARC), SSIs were significantly associated with increased bleeding volume, blood transfusion, and delayed postoperative oral feeding. Multivariate logistic regression analysis revealed that increasing bleeding volume significantly increased the incidence of SSIs (odds ratio (OR), 1.03 [per 100 mL increase]; 95% confidence interval:1.003–1.05; p = 0.027). In contrast, robot-assisted procedures, delayed oral feeding, or blood transfusion were not significant risk factors for SSIs (p = 0.30, 0.35, and 0.56, respectively; Table S2).
Analysis after PSM
The patients' characteristics of the matched cohort (ORC: 34 and RARC: 34) are shown in the right half of Table 1. For PSM, all preoperative variables and simultaneous surgical procedures (type of urinary diversion, simultaneous nephroureterectomy, and urethrectomy) listed in Table 1 were matched.
In the univariate analysis after PSM, SSI incidence was significantly associated with ORC, increased bleeding volume, blood transfusion, and delayed postoperative oral feeding as well as the results before PSM (23.5% vs. 5.9%, p = 0.040, 3125 mL vs. 530 mL, p < 0.001, 80.0% vs. 43.1%, p = 0.03, and 7 days vs. 5 days, p = 0.046, respectively, Table 3).
| SSI event + (n = 10) | SSI event − (n = 58) | p-value | |
|---|---|---|---|
| Age, year | 63.5 (55–72) | 70 (64–75) | 0.15 |
| Male | 5 (50.0%) | 31 (53.5%) | 0.84 |
| BMI, kg/m2 | 24.7 (23.1–25.7) | 22.8 (20.8–24.9) | 0.09 |
| Current smoker | 0 (0%) | 4 (6.9%) | 0.39 |
| Diabetes mellitus | 1 (10.0%) | 13 (22.4%) | 0.37 |
| Steroid use | 1 (10.0%) | 2 (3.5%) | 0.35 |
| ASA-PS | 0.72 | ||
| ≤2 | 8 (14.0%) | 49 (88.0%) | |
| 3 | 2 (18.2%) | 9 (81.8%) | |
| Preoperative hemoglobin, g/dL | 12.3 (10.7–14.2) | 11.9 (10.5–13.1) | 0.31 |
| Preoperative serum albumin, g/dL | 4.2 (3.8–4.4) | 4.0 (3.8–4.2) | 0.11 |
| Preoperative pyuria | 7 (70%) | 36 (62.1%) | 0.63 |
| Preoperative bacteriuria | 2 (28.6%) (n = 7) | 19 (38.8%) (n = 49) | 0.60 |
| Clinical T Stage | 0.96 | ||
| ≤2 | 7 (14.6%) | 41 (85.4%) | |
| ≥3 | 3 (15.0%) | 7 (85.0%) | |
| Neoadjuvant chemotherapy | 4 (40.0%) | 28 (48.3%) | 0.63 |
| Operative method | 0.040 | ||
| ORC | 8 (23.5%) | 26 (76.5%) | |
| RARC | 2 (5.9%) | 32 (94.1%) | |
| Urinary diversion | 0.42 | ||
| Using the intestinal tract | 8 (17.0%) | 39 (83.0%) | |
| Other methods | 2 (9.5%) | 19 (90.5%) | |
| Simultaneous nephroureterectomy | 2 (20.0%) | 10 (17.2%) | 0.83 |
| Simultaneous urethrectomy | 9 (90.0%) | 41 (70.7%) | 0.20 |
| Total operation time, min | 470 (411–517) | 476 (386–542) | 0.89 |
| Bleeding volume, mL | 3125 (1453–4063) | 530 (223–1490) | < 0.001 |
| Blood transfusion | 8 (80.0%) | 25 (43.1%) | 0.03 |
| Length of antimicrobial prophylaxis | 0.78 | ||
| ≤2 days | 2 (12.5%) | 14 (87.5%) | |
| ≥3 days | 8 (87.5%) | 44 (84.6%) | |
| Oral feeding (POD) | 7 (5.8–7.3) | 5 (3–7) | 0.046 |
- Abbreviations: ASA-PS, American Society of Anaesthesiologists Physical Status; BMI, body mass index; ORC, open radical cystectomy; POD, postoperative day; RARC, robot-assisted radical cystectomy; SSI, surgical site infection.
Only bleeding volume also remained as an independent risk factor of SSI by multivariate logistic regression analysis after PSM (OR, 1.13 [per 100 mL increase]; 95% confidence interval:1.02–1.25; p = 0.001), and RARC, delayed postoperative oral feeding or blood transfusion were not remained as significant risk factors for SSIs as well as the results before PSM (p = 0.60, 0.11, and 0.78, respectively; Table 4).
| Odds ratio | 95% confidence interval | p-value | |
|---|---|---|---|
| RARC (conf. ORC) | 0.39 | (0.01–14.5) | 0.60 |
| Oral feeding (by 1-day delay) | 1.18 | (0.96–1.43) | 0.11 |
| Bleeding volume (by 100 mL increase) | 1.13 | (1.02–1.25) | 0.001 |
| Blood transfusion (conf. No transfusion) | 1.65 | (0.05–57.7) | 0.78 |
- Abbreviations: ORC, open radical cystectomy; RARC, robot-assisted radical cystectomy; SSI, surgical site infection.
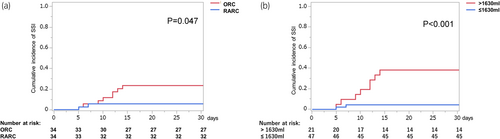
The ROC curve analysis identified 1630 mL as the cutoff bleeding volume for predicting the SSI occurrence after radical cystectomy, with an AUC, sensitivity, and specificity of 0.773, 0.73, and 0.75, respectively (Figure 1). The cumulative incidences of SSI were compared and stratified by the difference in surgical procedures or more or less than the cutoff bleeding volume. They were significantly lower in RARC group (vs. ORC) and in bleeding volume less than 1630 mL group (vs. higher than 1630 mL) (5.9% vs. 23.5%, p = 0.047, 4.3% vs. 38.1%, p < 0.001, respectively) (Figure 2).
DISCUSSION
This study revealed that SSI incidence was significantly lower in patients who underwent RARC than in those who underwent ORC. Higher bleeding volume, even more than that in the ORC group, remained an independent factor for SSI both before and after PSM. In addition, the accuracy of the cutoff bleeding volume of 1630 mL for predicting SSI occurrence was moderate (AUC: 0.773).
Several previous studies have compared the incidence of perioperative complications between ORC and RARC. However, most of these studies either (i) evaluated urinary tract and surgical site infections simultaneously as infectious complications or wound dehiscence and SSI simultaneously as wound complications or (ii) did not evaluate the details of the complications.1, 7, 8, 18 On the contrary, a few studies evaluated the difference in the incidence of SSI in detail. One randomized study comparing the efficacy of ORC and RARC reported that wound infection occurred in 8.3% (13/156) and 3.7% (6/161) of the ORC and RARC groups, respectively.9 A previous large cohort database study in the United States reported that RARC was significantly associated with decreased SSI (OR: 0.72).19 The present study observed a lower SSI incidence rate in the RARC group than in the ORC group, consistent with previous studies. These results suggest that, like colorectal or prostate surgery, robot-assisted, minimally invasive procedures are beneficial for reducing SSIs after radical cystectomy.11, 20, 21
This study observed a more significant decrease in superficial incisional SSIs than in deep incisional or organ/space SSIs was observed in the RARC group (11.7% vs. 2.3%). Differences in the causative bacteria or the prevalence of resistant strains between the ORC and RARC groups were unclear because the number of patients with confirmed causative bacteria in the RARC group was insufficient. A previous randomized trial demonstrated that superficial SSIs occurred in 12% and 7% of the ORC and RARC groups, respectively.10 This report was partly consistent with the present study's results. However, the differences in the incidence of superficial SSIs between the ORC and RARC groups seemed smaller in the previous study's cohort than those in the present cohort. These differences may be partly associated with the urinary diversion methods. The present study utilized ICUD, whereas extracorporeal urinary diversion (ECUD) was utilized in the previous study. ICUD was associated with a shorter skin incision, a shorter operative time, and less blood loss, which may decrease the risk of bacterial adhesion and growth from the resected intestine to the incised wound.22 Hence, RARC with ICUD would be more beneficial; however, further studies comparing the incidence of SSIs between patients that undergo ICUD and those that undergo ECUD are required.
The multivariate analysis showed that only bleeding volume remained an independent risk factor for SSIs after radical cystectomy both before and after PSM. Our study also found that the difference in the cumulative incidence of SSI was more remarkable for stratification by cutoff bleeding volume than for that by ORC versus RARC. These results were consistent with previous studies and seemed rational because increased blood loss was associated with hypoxia induced by anemia and with the decreased concentrations of antibiotic agents and immune cells in the serum or the tissue. Consequently, this may promote the adhesion and growth of pathogenic bacteria to the wound.23-25 In addition, several previous studies suggested blood transfusion, strongly associated with high volume of blood loss increased the risk of SSI by immunosuppressive effect associated with transfusion-related immune modulation, even though that was not remained as independent factor in our study.26, 27 These results suggest that even ORC could reduce the incidence of SSIs to as low as observed with RARC when well-trained surgeons perform the procedure with little blood loss. In contrast, many studies have revealed that RARC significantly and clearly reduces bleeding volume and perioperative transfusions compared with ORC, indicating that robot-assisted procedures are beneficial for reducing SSIs after radical cystectomy by decreasing bleeding volume.1, 8, 10, 19, 28
This study also revealed that the rational cutoff bleeding volume for predicting SSIs after PSM was 1630 mL with a moderate ability of prediction (AUC 0.773). A previous study suggested that antibiotic concentrations for cefazolin in the tissue were decreased by bleeding volume and that the cutoff volume considering additional doses of antibiotics was 1500 mL.25 Our cutoff bleeding volume may be close to the value unable to maintain effective antimicrobial concentrations in the tissue.
The main limitation of this study was its retrospective, single-center design using historical controls. The patients' backgrounds and treatment policies, including the preoperative managements, may differ between the ORC and RARC groups, even though we performed PSM. These differences must have affected SSI incidence. In addition, the length of antimicrobial prophylaxis was quite varied among patients, which may lead to a strong bias for the present results. Hence, further large-scale prospective studies with uniform perioperative and infection control protocols are required.
In conclusion, SSI prevalence after RARC was significantly lower than that after ORC. Although decreased bleeding volume was an independent and more significant factor for reducing SSIs after radical cystectomy compared to the robot-assisted procedure, RARC would be beneficial for reducing SSI incidence because several studies have revealed that RARC significantly reduces bleeding volume.
ACKNOWLEDGMENTS
The authors would like to thank Editage (www.editage.com) for the English language editing.
AUTHOR CONTRIBUTIONS
Jun Kamei: Data curation; Investigation; Writing—original draft. Kaori Endo: Investigation. Masahiro Yamazaki: Data curation. Toru Sugihara: Formal analysis. Ei-Ichiro Takaoka: Supervision. Satoshi Ando: Funding acquisition; Writing—review & editing. Haruki Kume: Supervision; Writing—review & editing. Tetsuya Fujimura: Conceptualization; Funding acquisition; Writing—review & editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
APPROVAL OF THE RESEARCH PROTOCOL BY AN INSTITUTIONAL REVIEWER BOARD
Approval number: A22-023.
INFORMED CONSENT
Written consent was waived because of the study's retrospective nature, and patients provided consent via an opt-out approach.
REGISTRY AND THE REGISTRATION NO. OF THE STUDY/TRIAL
N/A.
ANIMAL STUDIES
N/A.




