Moving toward improved immune checkpoint immunotherapy for advanced prostate cancer
Abstract
Human prostate cancer is a heterogenous malignancy that responds poorly to immunotherapy targeting immune checkpoints. The immunosuppressive tumor microenvironment that is typical of human prostate cancer has been the main obstacle to these treatments. The effectiveness of these therapies is also hindered by acquired resistance, leading to slow progress in prostate cancer immunotherapy. Results from the highly anticipated late-stage clinical trials of PD-1/PD-L1 immune checkpoint blockade in patients with advanced prostate cancer have highlighted some of the obstacles to immunotherapy. Despite the setbacks, there is much that has been learned about the mechanisms that drive resistance, and new strategies are being developed and tested. Here, we review the status of immune checkpoint blockade and the immunosuppressive tumor microenvironment and discuss factors contributing to innate and adaptive resistance to immune checkpoint blockade within the context of prostate cancer. We then examine current strategies aiming to overcome these challenges as well as prospects.
Abbreviations & Acronyms
-
- ADT
-
- androgen deprivation therapy
-
- APCs
-
- antigen-presenting cells
-
- B2M
-
- beta-2-microglobulin
-
- CTLA-4
-
- cytotoxic T lymphocyte antigen-4
-
- CTLs
-
- cytotoxic T lymphocytes
-
- DDR
-
- DNA damage-response
-
- HLA
-
- human leukocyte antigen
-
- ICB
-
- immune checkpoint blockade
-
- ICIs
-
- immune checkpoint inhibitors
-
- IFN-γ
-
- interferon-gamma
-
- Lag-3
-
- lymphocyte-activation gene 3
-
- mCRPC
-
- metastatic castration-resistant prostate cancer
-
- MSI
-
- microsatellite instability
-
- OS
-
- overall survival
-
- PAP
-
- prostatic acid phosphatase
-
- PCa
-
- prostate cancer
-
- PD-1
-
- programmed cell death protein-1
-
- PD-L1
-
- programmed death-ligand-1
-
- PSA
-
- prostate-specific antigen
-
- PSCA
-
- prostate stem cell antigen
-
- PSMA
-
- prostate-specific membrane antigen
-
- TAAs
-
- tumor-associated antigens
-
- TMB
-
- tumor mutation burden
-
- TME
-
- tumor microenvironment
-
- TSAs
-
- tumor-specific antigens
INTRODUCTION
Immunotherapy has transformed the therapeutic landscape for a variety of human cancers, yet some like prostate cancer (PCa) have not yet benefited from any real gains. This lack of success has prompted many researchers to determine the causes that could have rendered these therapies ineffective. However, PCa is a complex, heterogenous disease that progresses slowly and evolves over time in response to metabolic and/or therapeutic stressors to inhibit natural anti-tumor immunity. Consequently, innate and acquired resistance to immune checkpoint inhibitors (ICIs) results from constantly changing interactions between cancer cells and the immune system. These changes are manifested, in part, by alterations to the cellular and non-cellular makeup of tumors. The tumor microenvironment (TME) is a complex ecosystem of diverse cells and structures. Immune cells are an important component of the TME and are hijacked by cancer cells to evade natural immunity and resist immunotherapy. New research has shed light on additional factors that are well beyond the local TME that also contribute to promoting tumor survival by suppressing antitumor immunity. In this review, we discuss recent results from immune checkpoint blockade (ICB) immunotherapies in PCa and discuss the factors that contributed to their failures. We also review current strategies being developed to overcome these obstacles and the current landscape of immunomodulatory therapies and future perspectives.
ICB AND PCa
Immune checkpoints are a class of molecules that serve as regulators of the immune system. These function as inhibitory or stimulatory immunoreceptors and in healthy individuals, maintain immune homeostasis, and protect against autoimmunity. However, cancer cells often dampen antitumor immune responses by activating or regulating a variety of these inhibitory immune receptors. ICB aims to block signals from these surface receptors by binding to the receptor itself or its ligand. ICIs are therapeutic antibodies that are designed to block key immunosuppressive signals. The first ICIs were developed to stimulate or augment the tumor-killing ability of cytotoxic T lymphocytes (CTLs) and fell under two main classes, those that target the cytotoxic T lymphocyte antigen-4 (CTLA-4) and those that target the programmed cell death protein-1 (PD-1) and programmed death-ligand-1 (PD-L1) axis (Figure 1a). CTLA-4 is an inhibitory receptor expressed in T cells and its functions oppose those of the costimulatory receptor CD28. Compared to CD28, CTLA-4 has a higher affinity to the CD80/86 receptors expressed on the surface of antigen-presenting cells (APCs) and upon its interaction suppresses further T-cell activation.1 PD-1 is a costimulatory receptor that regulates T-cell activation through its interaction with the PD-L1 or PD-L2 receptor.2-4 In tumors, PD-L1 is often expressed on the surface of cancer cells and various immune cells.5 Given their surprising efficacy, several of these ICIs have found their way into the clinic and have become the standard of care for several human cancers (Figure 1b). Recently, a new ICI targeting lymphocyte-activation gene 3 (Lag-3) has been added to the arsenal and is being used for patients with melanoma.6
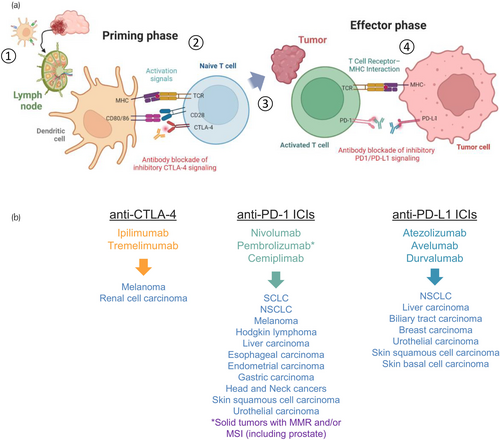
Several studies have been investigating the therapeutic potential of ICB for advanced PCa (see Table 1). These were spurred from early preclinical studies and clinical reports that showed some promise for the therapeutic potential of CTLA-4 and PD-1/PD-L1 inhibition in human PCa.18-20 However, these treatments have largely failed in late-stage clinical development. Two phase-3 trials evaluating ipilimumab (anti-CTLA-4) versus placebo in patients with docetaxel-resistant metastatic castration-resistant prostate cancer (mCRPC) (CA184-043) and chemotherapy-naïve mCRPC (CA184-095) did not improve OS.7, 8 These results were disappointing given the high hopes; however, there were indications of antitumor activity in a subset of patients. Phase 1b KEYNOTE-028 reported favorable activity for the PD-1 inhibitor pembrolizumab in mCRPC patients with PD-L1-positive tumors.18 The phase 2 KEYNOTE-199 trial further evaluated the activity of pembrolizumab in five cohorts from a heavily pretreated patient population with mCRPC. Pembrolizumab monotherapy was administered to patients previously treated with docetaxel (cohorts 1–3) or enzalutamide (cohorts 4 and 5). Results showed some promise with objective response rates in some patients regardless of PD-L1 status. These results prompted further investigations of pembrolizumab with other treatment combinations in phase 3 trials.21 However, the results from three phase 3 trials evaluating various combinations; pembrolizumab plus olaparib (KEYLYNK-010), pembrolizumab plus enzalutamide and androgen deprivation therapy (ADT, KEYNOTE-641), and pembrolizumab plus docetaxel (KEYNOTE-921) were disappointing.15-17 The KEYLYNK-010 trial was halted because the interim results did not show any improvements in radiographic progression-free survival (rPFS) or overall survival (OS) with pembrolizumab plus olaparib versus enzalutamide or abiraterone in an unselected patient population.15 The KEYNOTE-641 trial was also discontinued as the addition of pembrolizumab to enzalutamide and ADT did not show any improvements with its primary endpoints (rPFS and OS) to enzalutamide plus ADT alone.16 While the combination of pembrolizumab plus docetaxel showed slight improvements in median rPFS, these were statistically insignificant.17 Results with the PD-L1 inhibitor, atezolizumab were also disappointing. The phase 3 IMbassador250 trial investigated the efficacy of adding atezolizumab to enzalutamide and was initiated based on findings from the initial phase 1 trial of atezolizumab monotherapy indicating some biological activity and the promising activity of pembrolizumab in the KEYNOTE-199 trial.12, 21, 22 This was the first phase 3 trial to evaluate immunotherapy combinations for mCRPC, unfortunately, the trial was terminated early as this treatment combination did not improve rPFS or OS for mCRPC patients who had progressed after abiraterone therapy.12 CheckMate 7DX (NCT04100018) is an ongoing phase 3 trial evaluating the PD-1 inhibitor, nivolumab, along with docetaxel chemotherapy for mCRPC and is based on the results of the phase 2 CheckMate 9KD trial showing an objective response rate of 40% and median radiographic progression-free survival of 9 months.13 This trial is still recruiting patients and its primary completion is expected in 2026.
| Year | Identifier | Study title | Phase | Target | Treatments | Trial size | Trial result | Outcome measures | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 2014 | NCT01322490 | Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicenter, randomized, double-blind, phase 3 trial | 3 | CTLA-4 | Ipilimumab versus Placebo | 799 | Adding ipilimumab had no significant benefit in OS | Median OS was 11.2 months (95% CI 9.5–12.7) with ipilimumab and 10.0 months (8.3–11.0) with placebo (HR 0.85, 0.72–1.00; p = 0.053) | 7 |
| 2017 | NCT01057810 | Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer (CA184-095) | 3 | CTLA-4 | Ipilimumab versus Placebo | 598 | Adding ipilimumab had no significant benefit in OS, but increased PFS and PSA response in Pts with mCRPC | Median OS was 28.7 months (95% CI, 24.5–32.5 months) with ipilimumab and 29.7 months (95% CI, 26.1–34.2 months) with placebo arm (HR 1.11; 95.87% CI, 0.88–1.39; p = 0.3667). Exploratory analyses showed a higher prostate-specific antigen response rate with ipilimumab (23%) versus placebo (8%) | 8 |
| 2019 | NCT02788773 | Durvalumab with or without tremelimumab in metastatic castration-resistant prostate cancer | 2 | CTLA-4 PD-L1 | Tremelimumab and Durvalumab | 52 | Durvalumab did not show sufficient clinical activity in Pts with mCRPC | ORR was 0% (95% CI, 0–25%) 0/13 for durvalumab and 16% (95% CI, 6–32%) 6/37 for durvalumab + tremelimumab | 9 |
| 2020 | NCT02787005 | Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: Multicohort, open-label phase II KEYNOTE-199 study | 2 | PD1 | Pembrolizumab | 250 | Modest antitumor activity in a subset of Pts with mCRPC | Cohorts 1–3 of pembrolizumab monotherapy in pretreated patients with mCRPC had an ORR rate of 3% to 6%. Cohorts 4 and 5 had pembrolizumab added to enzalutamide. ORR for patients with measurable disease in cohort 4 was 12.3% (95% CI 6–11) | 10 |
| 2021 | NCT03204812 | Durvalumab and tremelimumab in treating chemotherapy naive patients with metastatic castration-resistant prostate cancer | 2 | CTLA-4 PD-L1 | Tremelimumab and Durvalumab | 26 | Modest antitumor activity in a subset of Pts with mCRPC | PSA decline ≥50% occurred in three patients (12%). Six patients (24%) achieved stable disease for >6 months. At a median follow-up of 43.6 months, median rPFS was 3.7 months (95% CI: 1.9–5.7), and median overall survival was 28.1 months (95% CI: 14.5–37.3) | 11 |
| 2022 | NCT03016312 | Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial IMbassador250 trial | 3 | PD-L1 | Atezolizumab + Enzalutamide versus Enzalutamide | 759 | Adding atezolizumab to enzalutamide did not improve OS in unselected Pts with mCRPC | Adding atezolizumab to enzalutamide did not meet the primary endpoint of improved OS in unselected patients (stratified HR 1.12, 95% confidence interval [0.91, 1.37], p = 0.28). Ongoing biomarker studies aim to provide insights into the immunobiology of prostate cancer | 12 |
| 2022 | NCT03338790 | Nivolumab plus docetaxel in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer: results from the phase II CheckMate 9KD trial (NCT03338790) | 2 | PD-1 | Nivolumab plus Docetaxel | 84 | Antitumor activity was observed in chemotherapy-naïve mCRPC Pts | ORR was 40.0% (95% CI, 25.7–55.7) and PSA50-RR was 46.9% (95% CI, 35.7–58.3). Median rPFS and OS were 9.0 (95% CI, 8.0–11.6) and 18.2 (95% CI, 14.6–20.7) months, respectively | 13 |
| 2022 | NCT03179410 | A phase 2 trial of avelumab in men with aggressive-variant or neuroendocrine prostate cancer (NCT03179410) | 2 | PD-L1 | Avelumab | 15 | Avelumab had limited activity in Pts with NEPC/AVPC | ORR was 6.7%, including one patient (6.7%) with a complete remission, 20% with stable disease, and 67% with progressive disease. Median rPFS was 1.8 months (95% CI 1.6–3.6 months), and median OS was 7.4 months (85% CI 2.8–12.6 months) | 14 |
| 2023 | NCT03834519 | Study of pembrolizumab (MK-3475) plus olaparib versus abiraterone acetate or enzalutamide in metastatic castration-resistant prostate cancer (mCRPC) (MK-7339-010/KEYLYNK-010) (KEYLYNK-010) | 3 | PD-1 | Pembrolizumab + Olaparib versus NHA | 529 | Pembrolizumab plus olaparib did not significantly improve rPFS or OS vs NHA in unselected mCRPC Pts | Median rPFS was 4.4 months (95% CI, 4.2–6.0) with pembrolizumab + olaparib and 4.2 months (95% CI, 4.0–6.1) with NHA (HR, 1.02 [95% CI, 0.82–1.25]; p = 0.55). Median OS was 15.8 months (95% CI, 14.617.0) and 14.6 months (95% CI, 12.6–17.3), respectively (HR, 0.94 [95% CI, 0.77–1.14]; p = 0.26) | 15 |
| 2023 | NCT03834493 | Study of pembrolizumab (MK-3475) plus enzalutamide versus placebo plus enzalutamide in participants with metastatic castration-resistant prostate cancer (mCRPC) (MK-3475-641/KEYNOTE-641) | 3 | PD-1 | Pembrolizumab + Enzalutamide versus Enzalutamide | 1244 | Adding pembrolizumab to enzalutamide did not improve OS or rPFS in Pts with mCRPC | Merck discontinued the study after interim analysis showed that adding pembrolizumab plus enzalutamide and ADT did not improve the trial's dual primary endpoints (PFS or OS) and crossed a pre-specified futility boundary for OS | 16 |
| 2023 | NCT03834506 | Study of pembrolizumab (MK-3475) plus docetaxel versus placebo plus docetaxel in chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC) (MK-3475-921/KEYNOTE-921) | 3 | PD-1 | Pembrolizumab + Docetaxel versus Docetaxel | 1030 | Adding pembrolizumab to docetaxel did not improve OS or rPFS in chemotherapy-naïve mCRPC Pts | The primary endpoints of rPFS (median 8.6 months with pembrolizumab + docetaxel vs 8.3 months with placebo + docetaxel; HR 0.85, 95% CI 0.71–1.01; p = 0.0335) and OS (median 19.6 months vs 19.0 months; HR 0.92, 95% CI 0.78–1.09; p = 0.1677) were not met | 17 |
- Abbreviations: ADT, androgen deprivation therapy; AVPC, small cell and aggressive variant prostate cancer; mCRPC, metastatic castration-resistant prostate cancer; mHSPC, metastatic hormone-sensitive prostate cancer; NEPC, neuroendocrine prostate cancer; NHA, next-generation hormonal agent; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PSA, prostate-specific antigen; rPFS, radiographic progression-free survival; RR, response rate.
WHY DO SOME PATIENTS RESPOND TO ICI AND OTHERS DO NOT?
Many factors contribute to the mechanisms that drive resistance to ICIs in human cancers; however, most of the responses to ICB occur in tumors that are T cell-inflamed at the time of treatment.23-25 The immune response to cancer is complex and varies across individuals due to tumor-intrinsic and tumor-extrinsic factors. Moreover, a lack of response can result from innate or acquired resistance. The basic premise for ICB in cancer is that CTL killing ability in tumors is hampered by immune checkpoints and could be restored with ICIs. Tumors that failed to elicit immune responses were termed “cold tumors” compared to “hot tumors”, which are inflamed and immunologically active. Human PCa is considered an immunologically cold tumor.
Despite the therapeutic shortcomings of the atezolizumab + enzalutamide combination in the IMbassador250 trial, the authors performed a planned exploratory biomarker analysis on pretreatment samples to associate responses with PD-L1 blockade.12 The investigators examined various putative biomarkers linked to ICB responses, including alterations to DNA damage-response (DDR) genes, PTEN status, PD-L1 immune cell expression, effector T-cell gene signatures, and AR amplification. Compared to renal cell and urothelial carcinomas, PCa patients exhibited lower effector T cell, MHC-I, and macrophage gene signatures as well as a lower fraction of patients with tumor mutation burden (TMB) ≥10 mutations/Mb and PD-L1-positive immune cells, confirming the low immunogenicity of prostate tumors. However, longer PFS was seen with the addition of atezolizumab in patients whose tumors exhibited ≥5% PD-L1-positive immune cells, CD8A expression, and effector T-cell signatures, which are features that had not yet been firmly established in human PCa. The authors also linked improved rPFS in atezolizumab-treated patients with TAP1, a gene that encodes transporter associated with antigen processing 1 protein (TAP1) that complexes with TAP2 to mediate unidirectional translocation of peptide antigens from the cytosol to the endoplasmic reticulum for loading onto MHC-I and CXCL9, which encodes the inflammatory CXCL9 that is predominantly induced by interferon-gamma (IFN-γ).26, 27 Findings from this study did not find an association between DDR mutations or AR amplifications and rPFS in patients receiving atezolizumab. In addition, a clear connection between microsatellite instability (MSI) and rPFS could not be established due to low patient counts, only two patients exhibited MSI-high tumors, and both were randomized to the enzalutamide-only group. TMB ≥4.5 mutations/Mb, which is the median observed in the Foundation Medicine Inc. database, was associated with a non-significant trend for longer PFS in the atezolizumab + enzalutamide combination. Patients with PTEN loss or TP53 alterations had improved PFS with atezolizumab. This study provided key insights for ICB responses to PD-L1 in mCRPC, (1) it identified features that could be associated with innate resistance to atezolizumab+enzalutamide, (2) it provided evidence that patients benefiting from this treatment combination had pre-existing immunity, and (3) it identified features that have the potential to preselect patients that may benefit from the treatment. From the existing evidence, we can surmise that mCRPC patients with immunogenic tumors are most likely to benefit from ICB.
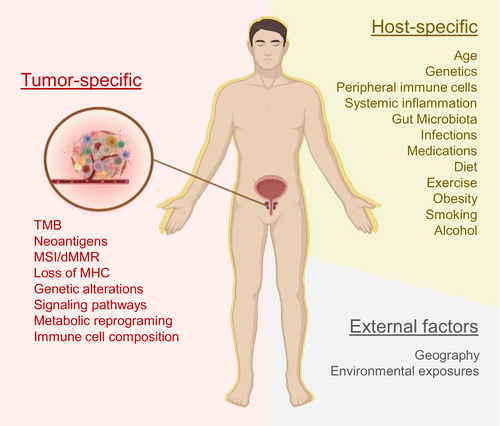
FACTORS AFFECTING ANTITUMOR IMMUNITY
Antitumor immunity requires a coordinated effort by the host immune system. The composition of the TME is the primary determinant of response to ICB and is largely determined by innate or tumor-specific factors—however, several extrinsic factors contribute as well (see Figure 2). Together, these factors not only affect local immune responses but also extend to the systemic immune system, ultimately affecting clinical outcomes. Immunotherapy enhances the ability of the immune system to eradicate cancer cells; however, this would work best with individuals with a strong functional immune system. As individuals age, the immune system changes and is further affected by general health, disease, medications, lifestyles, and environmental exposures. Low-grade systemic chronic inflammation occurs with aging but can be further exacerbated by several factors that include but are not limited to chronic disease, infections, obesity, and cancer.28-30 Patients suffering from cancer typically have weakened or impaired immune systems because of disease perturbations or cancer treatments. Factors that contribute to host immunity to PCa and response to ICB are discussed below.
NEOANTIGENS AND TMB
Undoubtedly, the immune system plays important roles in tumor biology, beginning with the basic premise of immune surveillance, that is, the ability of the immune system to discern and identify malignant transformed cells. Immunogenic tumors can elicit adaptive immune responses. However, tumor immunogenicity depends on its antigenicity and other immunomodulatory factors. Antigenicity refers to the level at which cancer cells express the human leukocyte antigen (HLA)-restricted antigens that are recognized by T cells. Antigenicity in tumors results from mutated or non-mutated antigens expressed by cancer cells.31 However, these cancer antigens are not always immunogenic, and defects in the antigen processing and presentation machinery also impact antigenicity.32, 33 Cancer antigens can be divided into self- or non-self-antigens. Self-antigens, also known as tumor-associated antigens (TAAs), are elevated in cancer cells but may also be present in healthy cells. These molecules are overexpressed in cancer cells or embryonic genes reactivated in differentiated cells. However, these are generally not highly immunogenic and may lack tumor specificity. Conversely, tumor-specific antigens (TSAs) or neoantigens are non-self-antigens that are derived from mutated protein products and are restricted to cancer cells and tend to be more immunogenic. PCa is characterized by several TAAs such as prostate-specific antigen (PSA), prostatic acid phosphatase (PAP), prostate-specific membrane antigen (PSMA), and prostate stem cell antigen (PSCA); however, these tend to be poorly immunogenic.34-37 Cancers with high TMB tumors tend to have higher levels of neoantigens that can be recognized by the immune system and are thus more likely to benefit from ICB.38, 39 However, PCa patients tend to have tumors with a low TMB rate, <3 mutations/Mb.40, 41 At present, mCRPC patients with a TMB rate of ≥10 mutations/Mb, which represents about 6%–7% of cases, have a greater likelihood of receiving benefits from ICB.12, 42
DEFECTS IN ANTIGEN PRESENTATION
The presentation of antigens on MHC-I is crucial for the priming of CD8+ T cells. MHC-I is a complex transmembrane glycoprotein composed of HLA-I protein (A, B, or C), beta-2-microglobulin (B2M), and an intracellular peptide.43 Loss of MHC-I expression contributes to the evasion of immune recognition being both an innate and acquired form of immune resistance.44 A complete loss of MHC-I has been reported in the range of 50%–66% for primary PCa and 80% for lymph node metastases.45, 46 In addition, some patients with high TMB tumors (≥10 mutations/Mb) fail to respond to ICB because of poor presentation of driver mutation neoantigens by MHC-I.47 The mechanisms that drive loss of MHC-I in PCa have not yet been fully revealed; however, a recent study published showed that HLA-A, HLA-B, and HLA-C genes were downregulated in a subset of patients and were negatively correlated to DNA methyltransferase (DNMT) and histone deacetylase (HDAC) genes DNMT3B and HDAC, and revealed repressive epigenetic signatures associated with HLA-I genes in low-HLA-I expressing PCa cell lines.48 Moreover, DNMT and HDAC inhibition enhanced HLA-I gene expression in vitro and in vivo. However, single-agent HDAC inhibitors have not shown much antitumor activity in CRPC and have failed to show a substantial benefit with ICIs in other solid cancers.49-54 The loss of B2M was linked to resistance to ICB.55 B2M is a secreted protein that is regulated by AR and is elevated in patients with advanced disease.56 It remains unclear whether there are functional differences between cellular and free B2M with regard to MHC-I expression; however, studies show that excess B2M can be ingested by macrophages, resulting in immune suppression through upregulated IL-1β and IL-18 secretion and exhibiting a wound-healing-like transcriptional profile.57, 58 It has also been demonstrated through preclinical models that stressors such as hypoxia can limit the recognition of cancer cells by T cells by downregulating MHC-I molecules.59-61 Also, upregulation of PI3K signaling can impair IFN-γ-dependent modulation of MHC-I and MHC-II expression, which can be reversed with PI3K inhibitors.62
MSI AND MISMATCH REPAIR DEFICIENCY
Microsatellite instability is another marker of genomic instability and is prevalent in tumors with mismatch repair deficiency (dMMR).63-66 Additionally, most tumors that are MSI-high also have a high TMB.63 It is estimated that 2%–3% of human PCa cases have an MSI-H/dMMR molecular phenotype.67, 68 Several studies have shown that tumors that are MSI-high and/or have dMMR respond better to ICB.69-71 The phase 2 trial of pembrolizumab in patients with MSI-high tumors, across 12 solid cancers, demonstrated an objective response of 53% and complete responses in 21% of patients.70 This led the U. S. FDA to approve the first tumor-agnostic immunotherapy for solid tumors with dMMR and/or MSI. Subsequent retrospective studies confirmed robust activity of pembrolizumab in patients with the MSI-H/dMMR phenotype.72, 73
GENETIC ALTERATIONS
Genetic changes that drive the malignant transformation of normal cells can also impact mechanisms that modulate the TME and antitumor immune responses. A pan-cancer immunogenomic analysis of 33 tumors from the Cancer Genome Atlas (TCGA) dataset provided key insights into the immune landscape of cancer by identifying distinct molecular subtypes.74 Findings from this analysis revealed that cancers that ranked in the top tertile, in terms of leukocyte composition, were also the most responsive to ICIs. However, PCa ranked low within this scale (<10%) and was characterized by a rich stromal cell fraction. Alterations to AR TP53, PTEN, CHD1, RB1 BRCA2, ATM, and ETS genes are enriched in patients with mCRPC and play various roles in immune modulation that shape the TME.67, 75-79 TP53 is the most frequently mutated gene in cancer and is the most frequently altered tumor suppressor in human PCa.76, 78, 80 Some cancers with TP53 alterations have shown increased immune infiltration likely owing to higher production of neoantigens because of genomic instability and show better responses to immunotherapy, whereas other cancers do not.80-83 PTEN is a tumor suppressor that is inactivated in ~40% of PCa cases and correlates with a higher Gleason score, poorer prognosis, and increased metastasis. Inactivation of PTEN in PCa cells leads to transcriptional reprogramming that promotes immune suppression.84 In preclinical models, prostate-specific deletion of Pten results in prostate carcinogenesis, and concomitant inactivation of Trp53 drives a more aggressive phenotype characterized by immune suppression that is mediated by increased MDSC infiltration.85-88 The immunosuppressive TME resulting from the loss of PTEN has been linked to mediating resistance to anti-PD-1 blockade in other human cancers.89, 90 CHD1 is a tumor suppressor that functions as a chromatin regulator and loss of function contributes to prostate carcinogenesis, DNA repair defects, resistance to AR-targeted therapy, and immune modulation.91-96 Preclinical studies have shown that CHD1 promotes an immunosuppressive TME by promoting MDSC recruitment via IL-6-STAT3 signaling.95 Defects in DNA-repair genes have been identified in 17% of the population, with truncating mutations to BRCA2 and ATM being the most common.97 These alterations occur at a higher frequency in men with mCRPC compared with those with localized disease (11.8% vs. 4.6%, respectively).98 While defects in DNA repair can result in carcinogenesis and genomic instability, tumors with defects in the DNA machinery also become susceptible to therapies such as poly (ADP-ribose) polymerase inhibitors (PARPi). This is because mutations in both alleles of homologous repair genes such as BRCA1 and BRCA2 lead to defects in the DNA repair process, thus the cells become more dependent on non-homologous end joining, which requires PARP.99, 100 Olaparib, rucaparib, niraparib, and talazoparib are PARPis that have been approved for the treatment of men with mCRPC with DDR alterations.101-103 These therapies have various implications for other treatments, such as DNA-damaging radiotherapy, platinum-based chemotherapies, and possibly immunotherapy. Presumably, DNA damage, which leads to genomic instability, could result in neoantigens that stimulate tumor immunogenicity. DNA damage can lead to the accumulation of DNA fragments that could stimulate antitumor immune responses via type I interferon signaling from cyclic GMP–AMP synthase-stimulator of interferon genes (cGAS-STING) activation.104-106 A recent report identified an immunogenic signature of genomic markers in aggressive localized PCa. This tumor phenotype was PD-L1-positive, infiltrated with exhausted progenitor CD8+ T cells, and differentiated effector T cells in close proximity to MHC-II+ APCs.107 The authors also associated this phenotype with the loss of RB1, BRCA2, and CHD1. Studies like this highlight the importance of correlating genomic alterations that contribute to immunogenicity, which could be used to preselect those patients who may benefit from ICB.
ANDROGEN RECEPTOR AND ANDROGENS
Approximately 70%–80% of advanced PCa have aberrant AR signaling, which is mostly attributable to AR amplification.76, 78 AR activating point mutations are present in approximately 7% of PCa cases.77, 79 Defects in AR splicing also occur in mCRPC and are predominantly manifested as truncated AR splice variant 7 (AR-V7) and variant 3 (AR-V3).77, 108, 109 These AR aberrations have been greatly characterized in the progression to CRPC and resistance to AR-targeted therapies; however, less is known about their direct impact on tumor immunogenicity or modulation of the TME.110 pTVG-AR (MVI-118), a DNA vaccine that encodes the AR-ligand binding domain, has shown immunological activity in a preclinical PCa model. Immunization of A2/TRAMP mice resulted in AR LBD peptide-specific immune responses and HLA-A2-restricted CTL activity, which was associated with delayed tumor progression and improved survival times.111 A phase 1 trial of pTVG-AR (NCT02411786) showed that 47% (14/30) of patients developed Th1-type immunity and had significantly longer PSA-PFS versus those patients that did not develop immunity.112 At present, it appears that most of the immunomodulatory effects of AR are not from AR alterations, but instead from the targeting of androgens and AR signaling.
IMMUNOSUPPRESSIVE NICHES OF THE TME OF CRPC
Targeting androgens has been the mainstay therapy for advanced PCa, and AR signal blockade with AR targeting agents (abiraterone, enzalutamide, apalutamide, and darolutamide) is being utilized for the treatment of patients with advanced PCa in hormone-naïve and castration-resistant settings. However, AR is ubiquitously expressed in several immune cell types, including T cells, B cells, macrophages, monocytes, neutrophils, and mast cells.113-117 In addition, AR has also been detected in cancer-associated fibroblasts (CAFs) and endothelial cells.118, 119 Sex hormones like testosterone play a hand in immune modulation and targeting AR impacts not only cancer cells but also the systemic immune system. Sex differences in immune responses have been well documented, and recent data suggested that men receiving ADT for PCa appeared to be more protected from SARS-CoV-2 infections.120-123 ADT has T-cell stimulatory effects, including the reversal of thymic involution and increasing peripheral naïve T cells.124-127 Studies have also shown that androgen withdrawal increases levels of T cells in peripheral lymphoid tissues and potentiates the induction of antigen-specific T-cell activation.128, 129 ADT can also enhance T-cell infiltration in tumors but can be offset by increased Treg differentiation and accumulation.130-132 Early reports have also shown that increased PD-L1 expression could be associated with enzalutamide resistance.20, 133 It has recently been shown that AR can limit CD8 T-cell responses to PD-1/PD-L1 blockade by downregulating IFN-γ, TNF-α, and granzyme B and driving cells into a terminally exhausted state.134, 135 The authors showed that anti-PD-1 blockade with ADT plus enzalutamide could reinvigorate antitumor immunity and improve survival in preclinical PCa models; however, a similar approach failed to yield favorable results in human mCRPC.12, 135
Immune suppression by myeloid niches within the TME contributes to intrinsic resistance to ICB in CRPC. Neutrophils or polymorphonuclear (PMN) leukocytes are the predominant immune cells in the blood and constitute a significant portion of the inflammatory cell infiltrate in tumors. Neutrophils that are recruited to tumors can exert pro-tumor and anti-tumor effects depending on their transcriptional signatures.136-138 Moreover, neutrophil differentiation, recruitment, and function are influenced by androgens.139-141 Polymorphonuclear-myeloid-derived suppressor cells (PMN-MDSCs) are a heterogenous population that is derived from bone marrow and are distinct from neutrophils.142 These cells are a relatively immature population that still shares many morphological and phenotypic features with classical neutrophils.143 As the name implies, MDSCs have immunosuppressive functions and are associated with various pathological conditions, including accumulation in tumors. Peripheral levels of PMN-MDSCs are elevated in patients with mCRPC and are recruited to tumors where they accumulate.144-146 Hypoxia, which is a hallmark of cancer and is also prevalent in human CRPC, can also increase levels of extracellular adenosine to recruit myeloid cells into tumors.147-150 In tumors, PMN-MDSCs produce several suppressive molecules such as IL-23, TGF-β, arginase 1, IL-10, and ROS that dampen effector T-cell function and induce the expansion of Tregs.145, 151 PMN-MDSCs also inhibit T-cell cytotoxic activity by producing CCL4 and CCL5 to recruit Tregs in tumors.152 Thus, tumors that are rich in neutrophil/PMN-MDSC populations are more likely to be innately resistant to PD-1/PD-L1 ICB.153-155
Tumor-associated macrophages (TAMs) have been causally linked to PCa growth and progression—however, these are extremely plastic cells and can have diverse functions based on their stimuli.156-158 M2 macrophages polarized by Th2-derived cytokines like IL-4/10/13, TGF-β, or prostaglandin E2 have a wound-healing response.159, 160 Conversely, M1-polarized macrophages are activated by IFN-γ and TNF-α from Th1-helper cells and produce proinflammatory cytokines, like IL-1β, TNF, IL-12, and IL-18, and engage in direct or indirect tumor-killing activity.161, 162 A study of single-cell RNA sequencing has revealed insights into the landscape of TAMs in human PCa.163 The study revealed that TAMs with an anti-inflammatory or anti-inflammatory/proliferative profile were predominant and accounted for ~75% of the macrophage fraction and those patients whose predominant phenotype coincided with an inflammatory TAM profile were associated with a better prognosis.163 TAMs with a wound-healing–response gene expression profile are also significantly enriched in bone metastases compared with other metastatic sites or primary tumors and contribute to macrophage-induced anti-androgen resistance to enzalutamide.160 Studies have also shown that PD-L1 is expressed in various myeloid cell populations and can negatively impact adaptive T-cell immune responses.164-169 Moreover, mCRPC patients treated with anti-CTLA-4 and anti-PD-L1 ICIs have shown increases in myeloid cell populations in tumors that were expressing PD-L1 and the immune checkpoint V-domain Ig suppressor of T-cell activation (VISTA), suggesting an adaptive response to ICB.11, 170 Upregulation of compensatory checkpoints or other immunosuppressive molecules is among the mechanisms driving adaptive resistance to immune responses after anti-CTLA-4 and anti-PD-1/PD-L1 ICIs. Lag-3, Tim-3, TGIT, and PD-L2 are other immune checkpoints that have been linked to hampering antitumor immunity and contributing to resistance to ICB in prostate tumors.171-176
CAFs are an ever-evolving heterogenous population in the TME that promote proliferation, migration, expansion, and castration resistance of PCa cells.177 The aging prostate is also associated with benign proliferative changes characterized by the proliferation of fibroblasts and myofibroblasts. Transcriptomic analyses have shown that the aging prostate stroma has upregulation of several genes that encode secreted inflammatory mediators, including secreted CXC-type chemokines (CXCL1/2/5/6/12), interleukins (IL-11/33), and transcripts with cytokine homology (CYTL1) which mediate chronic inflammation.178, 179 As tumors grow, fibroblasts along with myofibroblasts begin to replace the smooth muscle cells depositing collagens among other extracellular matrix (ECM) components.177 Functional subsets of fibroblasts were identified by single-cell RNA sequencing in adult mice and a phenotype that was enriched by Sca-1 and CD90 was in close contact with epithelial cells, and expressed growth factors and genes associated with cell motility, developmental process, and androgen biosynthesis, whereas those with a Sca-1+/CD90-low phenotype highly expressed genes associated with the extracellular matrix and cytokine-mediated signaling pathways.177 CAFs alter the organization of the ECM creating a dense and disorganized matrix, which along with a faulty microvasculature, increases hypoxia and alters the migratory ability of T cells into the tumor space.180-184 CAFs can also promote tumor immune escape by interacting with cancer and immune cells to induce PD-L1 expression.185-188 In addition, evidence indicates that CAFs also play a role in recruiting and supporting Tregs.189, 190
TUMOR EXTRINSIC FACTORS THAT CONTRIBUTE TO IMMUNE RESISTANCE
Additional factors beyond the local TME have also been associated with resistance to current ICIs. Host factors that contribute to immunity such as age and genetics are immutable; however, other factors could be modified to improve cancer response to immunotherapy. These factors include the composition of the peripheral immune system, microbiota, chronic conditions, medications, obesity, and lifestyle (diet, exercise, smoking, and alcohol consumption), and these have been extensively reviewed elsewhere.191-195 Moreover, geography and environmental factors can have direct and indirect influences on the fitness of an individual's immune system and should be considered.196, 197 Recently, more focus has been placed on diet, exercise, and gut microbiota as modulators or determinants of outcomes to ICB.175, 198-202 Many of these factors are innate to the host and would constitute the “tumor macroenvironment” and warrant further investigations.
To date, several mechanisms contributing to immune resistance have been uncovered and characterized and more remain to be discovered. In the meantime, we can use the information gained to tailor and improve clinical outcomes for advanced PCa.
WHERE DO WE GO FROM HERE?
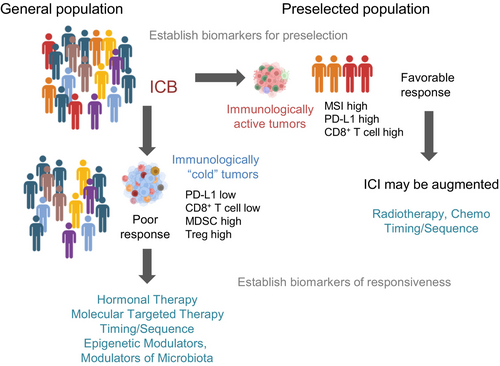
Improving T-cell priming and overcoming T-cell exhaustion have been the backbone of CTLA-4 and PD-1/PD-L1 ICB. While some patients have seen some benefits, these are often short-lived and most fail to experience any gains. We must (1) establish and utilize biomarkers to select patients that are more likely to benefit from standard ICB and (2) expand and develop additional strategies to overcome immune resistance. These include increasing tumor immunogenicity and reprogramming excluded or suppressive TMEs to enhance tumor infiltration of T cells (Figure 3). At this juncture, modulating the TME to a more favorable immunophenotype may be the most promising approach to improve the response to ICB for the majority of patients who fall outside preselection criteria. As such, a vast amount of work has been done in the preclinical stage evaluating new approaches of which some have advanced into the early phases of clinical development and are listed in Tables 2 and 3. Selected trials with preliminary results are briefly discussed below.
| NCT identifier | Status | Study title | Phase | Patient population | Interventions | Combination therapy | Mode of combination therapy | Immune checkpoint inhibitor | Estimated Completion date |
|---|---|---|---|---|---|---|---|---|---|
| NCT03248570 | Active, not recruiting | Pembrolizumab in metastatic castration-resistant prostate cancer (mCRPC) with or without DNA damage repair defects | 2 | mCRPC With or Without DNA DDRD | Pembrolizumab followed by chemo after disease progression | None | - | Pembrolizumab | 2024-07 |
| NCT03506997 | Recruiting | Trial of pembrolizumab in metastatic castration-resistant prostate cancer (PERSEUS1) | 2 | mCRPC with high mutational load, high MSI, or DDRD | Pembrolizumab | None | - | Pembrolizumab | 2025-09 |
| NCT03637543 | Recruiting | Nivolumab in patients with high-risk biochemically recurrent prostate cancer | 2 | PD-L1 positive and negative mCRPC | Nivolumab | None | - | Nivolumab | 2025-03 |
| NCT03651271 | Active, not recruiting | Treatment With nivolumab and ipilimumab or nivolumab alone according to the percentage of tumoral CD8 cells in advanced metastatic cancer (AMADEUS) | 2 | Nivolumab with or without Ipilimumab based on percentage of tumoral CD8 cells | Nivolumab with or without Ipilimumab | None | - | Nivolumab Ipilimumab | 2023-06 |
| NCT04019964 | Recruiting | Nivolumab in biochemically recurrent dMMR prostate cancer | 2 | mCRPC with high mutational load, high MSI, or DDRD | Nivolumab | None | - | Nivolumab | 2025-01 |
| NCT03061539 | Active, not recruiting | Nivolumab and ipilimumab treatment in prostate cancer with an immunogenic signature NEPTUNES | 2 | dMMR, DDRD, or high inflammatory infiltrate | Nivolumab + Ipilimumab | None | - | Nivolumab Ipilimumab | 2027-06 |
| NCT04717154 | Recruiting | Ipilimumab with nivolumab for molecular-selected patients with castration-resistant prostate cancer (INSPIRE) | 2 | mCRPC with dMMR, DDRD, BRCA2 inactivation or BRCAness signature, or CDK12 biallelic inactivation | Nivolumab + Ipilimumab | None | - | Nivolumab Ipilimumab | 2026-06 |
| NCT04126070 | Recruiting | Nivolumab + Docetaxel + ADT in mHSPC patients with DDRD or inflamed tumors | 2 | mHSPC patients with DDRD or inflamed tumors | ADT + Docetaxel + Nivolumab | ADT Docetaxel | AR signal inhibition Cell death | Nivolumab | 2025-06 |
| NCT03810105 | Completed | A study of olaparib and durvalumab in prostate cancer | 2 | Castration Sensitive Biochemically Recurrent Non-Metastatic PCa with DDRD | Durvalumab | Olaparib | DNA Damage Repair | Durvalumab | 2023-07 |
| NCT04336943 | Recruiting | Durvalumab and olaparib for the treatment of prostate cancer in men predicted to have a high neoantigen load | 2 | Patients with CDK12 mutations, dMMR, MSI-high or homologous recombination mutation | Olaparib Durvalumab | Olaparib | DNA Damage Repair | Durvalumab | 2026-04 |
- Abbreviations: ADT, androgen deprivation therapy; AR, androgen receptor; DDRD, DNA damage repair defects; dMMR, defective mismatch repair; mCRPC, metastatic castration-resistant prostate cancer; mHSPC, metastatic hormone sensitive prostate cancer; MSI, microsatellite instability.
| NCT identifier | Status | Study title | Phase | Patient population | Interventions | Modulator | Mode of immune modulation | Immune checkpoint inhibitor | Estimated completion date |
|---|---|---|---|---|---|---|---|---|---|
| NCT05176483 | Recruiting | Study of XL092 in combination with immuno-oncology agents in subjects with solid tumors (STELLAR-002) | 1 | Advanced solid cancers including mCRPC | Combinations of XL092, Ipilimumab, Nivolumab, Relatlimab | XL092 | Multi-TKI | Nivolumab Ipilimumab Nivolumab + Relatlimab | 2026-05 |
| NCT04477512 | Active, not recruiting | Cabozantinib and abiraterone with checkpoint inhibitor immunotherapy in mHSPC (CABIOS Trial) | 1b | Untreated mHSPC | Combination of abiraterone, cabozantinib, and nivolumab with ongoing ADT | Abiraterone acetate Cabozantinib | AR signal inhibition Multi-TKI | Nivolumab | 2026-03 |
| NCT03170960 | active, not recruiting | Study of cabozantinib in combination with atezolizumab to subjects with locally advanced or metastatic solid tumors | 1b | Advanced solid cancers including mCRPC | Cabozantinib + Atezolizumab | Cabozantinib | Multi-TKI | Atezolizumab | 2024-08 |
| NCT03689699 | Active, not recruiting | Nivolumab and BMS-986253 for hormone-sensitive prostate cancer (MAGIC-8) | 1b | HSPC | Nivolumab or Nivolumab + BMS-986253 + ADT | Degarelix BMS-986253 | AR signal inhibition IL-8 signal inhibition | Nivolumab BMS-986253 | 2023-08 |
| NCT04989946 | Recruiting | Phase I/II trial of androgen deprivation, with or without pTVG-AR, and with or without nivolumab, in patients with newly diagnosed, high-risk prostate cancer | 1b | Newly Diagnosed, High-Risk Prostate Cancer | Degarelix + pTVG-AR + Nivolumab prior to prostatectomy | Degarelix pTVG-AR | AR signal inhibition Vaccine | Nivolumab | 2025-04 |
| NCT03543189 | Recruiting | Combination of nivolumab immunotherapy with radiation therapy and androgen deprivation therapy | 1b | Gleason Group 5 Prostate Cancer | Brachytherapy and EBRT + Nivolumab post-ADT | ADT Radiotherapy | AR signal inhibition Cell death DNA damage | Nivolumab | 2024-12 |
| NCT04109729 | Recruiting | Study of nivolumab in combination w radium-223 in men with mCRPC (Rad2Nivo) | 1b | mCRPC | Nivolumab Radium-223 | Radium-223 | Cell death DNA damage | Nivolumab | 2025-04 |
| NCT05445609 | Not yet recruiting | Vidutolimod (CMP-001) in combination with nivolumab for the treatment of metastatic castration-resistant prostate cancer | 2 | mCRPC | Nivolumab + Vidutolimod | Vidutolimod | TLR9 agonist | Nivolumab | 2027-06 |
| NCT04090528 | Recruiting | pTVG-HP DNA vaccine with or without pTVG-AR DNA vaccine and pembrolizumab in patients with mCRPC | 2 | mCRPC | pTVG-AR or pTVG-HP with Pembrolizumab | pTVG-AR pTVG-HP | Vaccine | Pembrolizumab | 2025-12 |
| NCT05655715 | Recruiting | Checkpoint inhibitors and SBRT for mCRPC (CheckPRO) | 2 | mCRPC | Nivolumab with or without stereotactic body radiotherapy | Radiotherapy | Cell death | Ipilimumab + Nivolumab | 2025-01 |
| NCT05168618 | Recruiting | Cabozantinib and atezolizumab for the treatment of mCRPC, The AtezoCab trial | 2 | mCRPC | Cabozantinib + Atezolizumab | Cabozantinib | Multi-TKI | Atezolizumab | 2027-01 |
| NCT05502315 | Recruiting | Study of cabozantinib and nivolumab in mCRPC (CANOPY) | 2 | mCRPC | Cabozantinib + Nivolumab | Cabozantinib | Multi-TKI | Nivolumab | 2024-10 |
| NCT03753243 | Recruiting | Neoadjuvant pembrolizumab plus androgen axis blockade prior to prostatectomy for high-risk localized prostate cancer | 2 | High-risk localized prostate cancer | Pembrolizumab + Enzalutamide prior to prostatectomy | Enzalutamide | AR signal inhibition | Neoadjuvant Pembrolizumab | 2023-04 |
| NCT03338790 | Active, not recruiting | An investigational immunotherapy study of nivolumab in combination with rucaparib, docetaxel, or enzalutamide in mCRPC (CheckMate 9KD) | 2 | mCRPC | Nivolumab + Rucaparib, Enzalutamide, or Docetaxel | Enzalutamide Rucaparib Docetaxel | AR signal inhibition DNA Damage Repair | Nivolumab | 2024-06 |
| NCT04471974 | Recruiting | ZEN-3694, enzalutamide, and pembrolizumab for the treatment of metastatic castration-resistant prostate cancer | 2 | mCRPC that progressed after antiandrogen therapy | ZEN-3694 + Enzalutamide + Pembrolizumab | ZEN-3694 | BET Bromodomain Inhibitor | Enzalutamide + Pembrolizumab | 2025-12 |
| NCT04116775 | Recruiting | Fecal microbiota transplant and pembrolizumab for men with mCRPC | 2 | mCRPC | Enzalutamide + Pembrolizumab + FMT | FMT | Biological | Enzalutamide + Pembrolizumab | 2023-10 |
| NCT05563558 | Recruiting | Pembrolizumab, carboplatin, and cabazitaxel in aggressive mCRPC (PEAPOD_FOS) | 2 | mCRPC characterized with adenocarcinoma and/or neuroendocrine carcinoma | Cabazitaxel + Carboplatin + Pembrolizumab | Cabazitaxel Carboplatin | Cell death | Pembrolizumab | 2026-11 |
| NCT05169684 | Recruiting | A Study of BMS-986218 or BMS-986218 Plus nivolumab in combination with docetaxel in participants with mCRPC | 2 | Chemotherapy-naïve mCRPC that progressed after antiandrogen therapy | BMS-986218 (anti-CTLA-4) Nivolumab | Docetaxel | Cell death | BMS-986218 Nivolumab | 2026-02 |
| NCT04159896 | Unknown | ESK981 and nivolumab for the treatment of metastatic castration-resistant prostate cancer | 2 | mCRPC | ESK981 + Nivolumab | ESK981 | Multi-TKI | Nivolumab | 2022-03 |
| NCT04446117 | Recruiting | Study of cabozantinib in combination with atezolizumab versus second NHT in subjects with mCRPC (CONTACT-02) | 3 | mCRPC has previously been treated with only one NHT | Atezolizumab + Cabozantinib vs. Second NHT | Cabozantinib | Multi-TKI | Atezolizumab | 2024-08 |
| NCT04100018 | Recruiting | A study of nivolumab or placebo in combination with docetaxel in men with advanced castration-resistant prostate cancer (CheckMate 7DX) | 3 | mCRPC that progressed after antiandrogen therapy | Nivolumab + Docetaxel vs. Docetaxel | Docetaxel | Cell death | Nivolumab | 2028-08 |
- Abbreviations: ADT, androgen deprivation therapy; AR, androgen receptor; DDRD, DNA damage repair defects; dMMR, defective mismatch repair; FMT, fecal material transplantation; mCRPC, metastatic castration-resistant prostate cancer; mHSPC, metastatic hormone-sensitive prostate cancer; MSI, microsatellite instability; NHT, novel hormonal therapy.
PRESELECTED PATIENTS
NEPTUNES (NCT03061539) is a phase 2, two-stage biomarker-selected trial of nivolumab plus ipilimumab for previously treated pts with mCRPC. In this study, immunogenic signatures were defined by at least one of the following, dMMR (IHC), DDR deficiency (UW-OncoPlex targeted exome sequencing assay), or high TIL infiltration (multiplexed IHC, CD4, CD8, or FOXP3+ >20% nucleated cells). Initial data from cohort 1 showed significant antitumor activity from the ICI combination with an overall composite response rate of 9/35 (26%, 95% CI 12%–43%).203 The trial has expanded to cohort 2 and is ongoing. AMADEUS (NCT03651271) is a tumor-agnostic phase 2 trial of nivolumab with or without ipilimumab for patients with advanced metastatic cancer based on CD8+ T cell infiltration.204 Patients with CD8-high tumors (>15% CD8 cells) received nivolumab monotherapy whereas those with CD8-low tumors received ipilimumab plus nivolumab. This trial used comprehensive longitudinal biomarker analysis on tumors and blood using multi-omic approaches to identify and correlate pre- and on-treatment biomarkers with treatment outcomes. Of the 79 patients enrolled, 12 (15%) were PCa patients. Meeting results showed that increased tumoral infiltration of CD8+ T cells on-treatment tended to be associated with improved clinical benefit (p = 0.0582), and responders had enrichment in genes relating to IFN-γ and JAK/STAT signaling, whereas non-responders were associated with increased glycolysis pathway genes and MYC targets.204
COMBINATION THERAPY WITH MOLECULAR TARGETING AGENTS
Most molecular targeted therapies aimed at cancer cells to some degree also affect the local TME and the systemic immune system. Cabozantinib is a multiple receptor tyrosine kinase inhibitor (mRTKI) that targets VEGFR2, MET, RET, KIT, TYRO-3, AXL, and MER and has been approved for several indications in advanced renal cell carcinoma, thyroid cancer, and hepatocellular carcinoma.205, 206 Cabozantinib as monotherapy has also shown some clinical activity in patients with mCRPC.207, 208 Additional research shows that cabozantinib has immunomodulatory activity and cabozantinib plus atezolizumab showed promising antitumor activity in patients with mCRPC after novel hormonal therapy.209, 210 CONTACT-02 (NCT04446117) is a phase 3 trial, evaluating cabozantinib plus atezolizumab versus second novel hormone therapy (abiraterone or enzalutamide) in patients with mCRPC and results are eagerly anticipated.211 Additional compounds have shown promise in preclinical studies and are currently being investigated as potential enhancers of ICB in advanced PCa (see Table 3).
CONCLUSIONS
Much has been learned from the lack of success from recent phase 3 studies of ICB for advanced PCa. However, a deeper understanding of the TME and host interactions provides reasons for continued optimism. Undoubtedly, some patients have gained from ICB and more will continue. With a better understanding of predictive biomarkers from several exploratory analyses, and more on the way, we will hopefully be better able to preselect patients who may benefit the most from ICB. It is also the hope that combination therapy strategies will be the way forward for immunotherapy for those patients who fall outside the preselected group.
AUTHOR CONTRIBUTIONS
Marco Antonio De Velasco: Conceptualization; investigation; supervision; writing – original draft; writing – review and editing. Yurie Kura: Data curation; investigation; writing – original draft; writing – review and editing. Kazutoshi Fujita: Conceptualization; investigation; writing – original draft; writing – review and editing. Hirotsugu Uemura: Conceptualization; supervision; writing – original draft; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
M.A.D. has received funding from AstraZeneca. Y.K. has no conflict of interest to declare. K.F. has received honoraria from Jannsen Pharmaceutical and AstraZeneca. H.U. has received research funding from AstraZeneca, Ono Pharmaceutical, and research grants from Astellas, Chugai, MSD, Osaka Urology Research Foundation, Pfizer, and Takeda; consulting fees from BMS/Ono and Bayer; lecture fees from Bayer, BMS, Janssen, MSD, Ono, Sanofi, Takeda and Pfizer Japan; scholarship/encouragement donations from Kissei.




