Clinical guidelines for the diagnosis and treatment of lower urinary tract dysfunction in patients with spinal cord injury
Abstract
The present article is an abridged English translation of the Japanese clinical guidelines for the diagnosis and treatment of lower urinary tract dysfunction in patients with spinal cord injury updated as of July 2019. The patients are adult spinal cord injured patients with lower urinary tract dysfunction; special consideration of pediatric and elderly populations is presented separately. The target audience is healthcare providers who are engaged in the medical care of patients with spinal cord injury. The mandatory assessment includes medical history, physical examination, frequency-volume chart, urinalysis, blood chemistry, transabdominal ultrasonography, measurement of post-void residual urine, uroflowmetry and video-urodynamic study. Optional assessments include questionnaires on the quality of life, renal scintigraphy and cystourethroscopy. The presence or absence of risk factors for renal damage and symptomatic urinary tract infection affects urinary management, as well as pharmacological treatments. Further treatment is recommended if the maximum conservative treatment fails to improve or prevent renal damage and symptomatic urinary tract infection. In addition, management of urinary incontinence should be considered individually in patients with risk factors for urinary incontinence and decreased quality of life.
Abbreviations & Acronyms
-
- AD
-
- autonomic dysreflexia or autonomic hyperreflexia
-
- CIC
-
- clean intermittent catheterization
-
- DO
-
- detrusor overactivity
-
- DSD
-
- detrusor sphincter dyssynergia
-
- GoR
-
- grade of recommendation
-
- ICUD
-
- International Consultation on Urological Diseases
-
- JASCoL
-
- Japanese Society of Spinal Cord Lesion
-
- JCS
-
- Japanese Continence Society
-
- JUA
-
- Japanese Urological Association
-
- LoE
-
- level of evidence
-
- NLUTD
-
- neurogenic lower urinary tract dysfunction
-
- OAB
-
- overactive bladder
-
- QOL
-
- quality of life
-
- RCT
-
- randomized controlled trial
-
- SCI
-
- spinal cord injury or spinal cord injured
-
- SIU
-
- Société Internationale D'Urologie
-
- SNM
-
- sacral neuromodulation
-
- UDS
-
- urodynamic study
-
- UTI
-
- urinary tract infection
-
- VUR
-
- vesicoureteral reflux or vesicoureteric reflux
Introduction
The first and second editions of the Japanese clinical guidelines for the diagnosis and treatment of lower urinary tract dysfunction in patients with SCI were published in 2005 and 2011, respectively, but an abridged English translational version was not published.1, 2 The third edition of the guidelines was published in July 2019. The committee members had planned to publish an abbreviated English translation of the guidelines when the panel started revision. One reason is that Committee 9 (current and future international patterns of care of neurogenic bladder after spinal cord injury) of the International Consultation cited the Chinese, Taiwanese, and Malaysian versions but, regrettably, not the Japanese guidelines, as Asian guidelines for lower urinary tract dysfunction in patients with SCI in a joint SIU-ICUD publication.3 The target individuals are adult SCI patients with lower urinary tract dysfunction, and the target readers are healthcare providers who are engaged in medical care of SCI patients.
Methods
The guidelines were developed by a panel recommended by the JCS, JASCoL and JUA. The panel reviewed relevant references published between 2011 and 2018, and retrieved from the PubMed and Japana Centra Revuo Medicina databases, because the previous edition used thoroughly searched relevant articles published before 2010. The panel also collated other related data, as required (including those written before 2011 and after 2018). We also referred to previous Japanese guidelines1, 2 and a joint SIU-ICUD International Consultation Report entitled “Urologic management of the spinal cord injured patient”.4
In this revised edition, the panel tried to draw up guidelines that would improve critical and important predefined outcomes as follows: prevention of urinary tract complications (renal impairment, symptomatic UTI, urolithiasis, gross hematuria, urethral stricture, urethrocutaneous fistula, bladder cancer and catheter blockage), as well as AD, achievement of social urinary continence and improvement of QOL.
In terms of the study design and scale, we mainly evaluated data dealing with treatment. The data (Table 1), LoE inferred from them (Table 2) and GoR (Table 3) were determined. The recommendation grade comprised the levels of rationale and treatment characteristics, such as the size of the effect, applicability, adverse events and costs, and was determined after discussion and agreement among the panel, who took into consideration the real-world practice for SCI patients in Japan. Therefore, some inconsistency between LoE and GoR is present in these guidelines. A draft was peer reviewed by a reviewing committee consisting of 15 members who were recommended by the JCS, JASCoL and JUA, which include doctors (specializing in urology, orthopedic surgery, rehabilitation medicine, neurology and internal medicine), nurses and SCI patients. Then, a revised draft was peer reviewed by a panel of trustees of the JCS, JASCoL and JUA, and the draft was opened to public opinion before finalizing. Funding was provided by the JCS, JASCoL and JUA. Conflicts of interest were regulated according to the JCS bylaws. The content recommended in these guidelines is based on scientific evidence and was not influenced by the interests of any specific organization, product or technology.
| Level | Description |
|---|---|
| I | Large-scale RCTs (≥100 participants in each group) or RCTs with the number of participants fulfilling the pre-calculated statistical power with definitive results |
| II | Small-scale RCTs or RCTs with the number of participants not fulfilling the pre-calculated statistical power with definitive results. If the results were not definitive, then the level was reduced by one |
| III | Controlled studies carried out without randomized allocation |
| IV | Prospective observational studies with no control |
| V | Retrospective case studies or the opinions of specialists |
| Level | Descriptions |
|---|---|
| 1 | Supported by multiple level I clinical studies |
| 2 | Supported by a single level I clinical study or multiple level II clinical studies |
| 3 | Supported by multiple level III clinical studies |
| 4 | Supported by multiple level IV clinical studies |
| 5 | Supported by multiple level V clinical studies |
| Grade | Description |
|---|---|
| A | This action is strongly recommended |
| B | This action is recommended |
| C | There is no clear evidence for recommending this action |
| C1 | The action can still be performed |
| C2 | Performing the action is not recommended |
| D | Not performing this action is recommended |
| Pending | No decision has been made regarding the GoR |
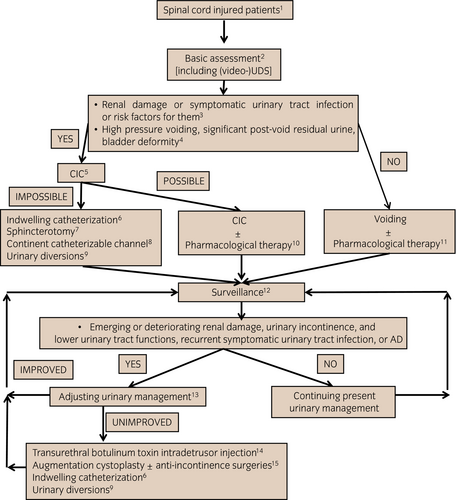
Algorithm
-
SCI patients
This algorithm is applicable to adult SCI patients. Patients with lower urinary tract dysfunction mainly caused by other neurological disorders are excluded. The special considerations in symptomatic UTI (see VI), pediatric (see VII) and elderly (see VIII) populations, as well as cervical SCI patients (see IX) are described separately.
-
Basic assessment (see III)
Diagnostic evaluation consists of basic and optional evaluations (Table 4). All patients should undergo the basic evaluation. The panel believes that video-UDS should be included in the basic evaluation.
-
Risk factors for renal damage (upper urinary tract deterioration or renal impairment) and symptomatic UTI
3-1. Risk factors for renal damage (LoE 5):
- Lower urinary tract dysfunction: decreased maximum bladder capacity, DO, DSD, decreased bladder compliance (<10–20 mL/cmH2O), high detrusor leak point pressure (>40 cmH2O);
- Urinary management: indwelling urethral catheterization, bladder reflex triggering (reflex voiding); and
-
Level and severity of the injury: tetraplegia, complete paralysis.
3-2. Risk factors for symptomatic UTI (LoE 5):
- VUR secondary to lower urinary tract dysfunction (decreased maximum bladder capacity, DO, DSD, decreased bladder compliance, high detrusor leak point pressure);
- Urinary management: indwelling urethral catheterization; and
- Level and severity of injury: cervical cord injury, A or B on American Spinal Injury Association impairment scale.
-
High-pressure voiding, significant post-void residual urine and bladder deformity
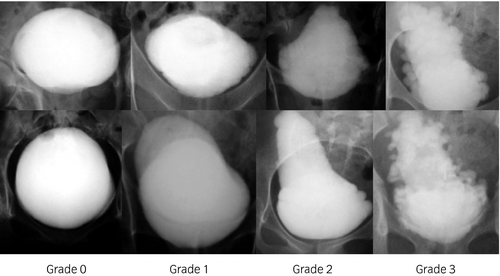
High-pressure voiding is caused by functional bladder outlet obstruction. Significant post-void residual urine (>100 mL) is caused by bladder outlet obstruction, detrusor underactivity or acontractile detrusor. Grade 2 or higher bladder deformity, as defined by Ogawa’s classification (see III), is considered significant.
-
CIC (see IV-1-4)
CIC should be adopted for patients who already show findings of, or have risk factors for, renal damage or symptomatic UTI, and who have high-pressure voiding, significant residual urine and bladder deformity. When CIC is not feasible, the decision on urinary management should be made after careful consideration of the patient’s condition, and benefits and risks of other urinary management techniques.
-
Indwelling catheterization (see IV-1-5)
Indwelling catheterization is mainly indicated for cervical SCI patients who cannot be managed with CIC. Suprapubic catheterization should be better than urethral catheterization.
-
Sphincterotomy (see IV-4-5)
Sphincterotomy is indicated for the male SCI patient who cannot be managed with CIC by themselves or caregivers and can keep a collecting device placed on his penis.
-
Abdominal continent catheterizable channel (see IV-4-3 and 4-4)
A catheterizable channel is indicated for patients who cannot carry out CIC through their native urethra for various reasons. This procedure might be combined with augmentation cystoplasty, anti-incontinence surgeries and construction of continent urinary reservoirs.
-
Urinary diversions (see IV-4-4)
Urinary diversions are the last resort for SCI patients who do not respond to any relevant treatment modality.
-
CIC ± pharmacological therapy (see IV-1-4, IV-3-1 and 2)
Based on the video-UDS findings, physicians should determine the frequency and timing/interval of CIC, as well as pharmacological therapy appropriate for improving or preventing urinary tract complications and urinary incontinence. The intermittent (temporary) use of the specialized indwelling catheter is an option for patients with nocturnal polyuria and/or difficulty in carrying out CIC away from home.
-
Voiding ± pharmacological therapy (see IV-1-3, IV-3-1 to 3-3)
Voiding includes spontaneous voiding, bladder reflex triggering and bladder expression with Crede/Valsalva maneuvers. Of these, spontaneous voiding is recommended. On the contrary, bladder reflex triggering is indicated for carefully selected patients, but bladder expression is not recommended.
-
Surveillance (see V)
It is emphasized that lower urinary tract dysfunction sometimes improves with time. Therefore, CIC might be converted into voiding in some patients.
-
Adjusting urinary management
Video-UDS is strongly recommended when less invasive follow-up examinations show emerging or worsening renal damage and urinary incontinence, recurrent UTI, recurrent urolithiasis, increased post-void residual urine, worsening bladder deformity, and exacerbating AD. Urinary incontinence deteriorates the patient’s QOL. Therefore, when refractory urinary incontinence remains after adjusting urinary management and the patient wants further improvement of incontinence, active treatment to improve urinary incontinence should be considered.
13-1. Risk factors for urinary incontinence (LoE 5)
Risk factors are DO, decreased bladder compliance and intrinsic sphincter deficiency.
Reflex urinary incontinence results from DO causing an intravesical pressure rise and a decreased bladder capacity during the storage phase. Stress urinary incontinence results from an incompetent urethra due to intrinsic sphincter deficiency with or without high storage pressure resulting from decreased bladder compliance when intravesical pressure exceeds maximum urethral closure pressure during increased abdominal pressure.
13-2. Risk factors for deteriorating QOL (LoE 5)
Risk factors are indwelling urethral catheterization and CIC by caregivers.
The health-related QOL in SCI patients improves when they have fewer concerns about problems and constraints due to urinary incontinence, UTI, and exacerbating bladder functions. Independence from elimination difficulties markedly contributes to better QOL after successful rehabilitation.
-
Transurethral botulinum toxin intradetrusor injection (see IV-4-1)
Although at last botulinum toxin has been approved for refractory DO in Japan as of December 2019, this is now considered to be an established treatment worldwide for refractory DO that is resistant to CIC combined with anticholinergic drugs.
-
Augmentation cystoplasty (see IV-4-2) ± anti-incontinence surgeries (see IV-4-3)
After all the maximum conservative and less invasive therapies fail, augmentation cystoplasty with or without anti-incontinence surgeries still plays an important role in managing the uncontrollable upper urinary tract deterioration, recurrent symptomatic UTI and intractable urinary incontinence. Postoperative regular CIC, as well as the possible occurrence of significant peri- and postoperative complications, underscores the importance of thorough preoperative informed consent.
| Basic evaluation | Optional evaluation |
|---|---|
| Present and past medical history | QOL evaluation |
| Physical examination including neurological examination | Renal scintigraphy |
| Frequency–volume chart or bladder diary (a record of CIC in patients with CIC) | Cystourethroscopy |
| Urinalysis | |
| Blood chemistry | |
| Transabdominal ultrasonography | |
| Post-void residual urine in patients with voiding | |
| Uroflowmetry in patients with voiding | |
| Video-urodynamic study | |
| Cystourethrography |
Summary of the guidelines
I. Epidemiology
The incidence of SCI has regional differences, and ranges between 30.8 and 134.1 persons per 1 million people per year.5, 6 Recently, the incidence of cervical SCI without bone injury in elderly patients has markedly increased.
II. Pathophysiology
The pathophysiologies of supra-sacral SCI and sacral SCI differ, although the bladder sensation is reduced or absent regardless of the level of injury. After the spinal shock phase, DO develops through emergence of a pathological spinal micturition reflex in the supra-sacral SCI. Concomitant DSD hampers low pressure and efficient emptying. On the contrary, in the sacral SCI, a non-relaxing sphincter obstruction and intrinsic sphincter deficiency with acontractile detrusor or detrusor underactivity cause emptying dysfunction and urinary incontinence, respectively.
III. Diagnosis
1. General assessment
General assessment consists of medical history, assessment of QOL, focused physical and neurological examinations of the lumbosacral region, urinalysis, blood chemistry, and frequency-volume chart (bladder diary). In terms of assessment of QOL, Qualiveen 30 is the only validated questionnaire in Japanese for NLUTD.7 Recently, a Japanese version of the Intermittent Self-Catheterization Questionnaire for evaluating QOL in patients with intermittent self-catheterization was published, but it is not specific for SCI.8
The panel recommends a cystatin C test for early detection of renal impairment, because cystatin C is less affected by muscle mass than creatinine (LoE 5, GoR C1).9, 10
2. Video-UDS
UDS is an indispensable functional examination for NLUTD in SCI patients, because the neurological levels of injury do not always predict the nature of NLUTD.11 UDS can clarify the risk factors for renal damage (upper urinary tract deterioration or renal impairment), AD during the micturition cycle, and provide convincing evidence for urinary management in SCI patients. When available, video-UDS is preferred, because morphological findings on the urinary tract, including information on complications, such as bladder deformity, VUR and intraprostatic reflux of urine, can be obtained at the same time.12
The panel recommends video-UDS as a basic examination to allow physicians to identify the optimal urinary management for individual patients (LoE 5, GoR B).
3. Imaging and endoscopy
The goal of radiation exposure “as low as reasonably achievable (ALARA)” should be kept in mind.13 Transabdominal ultrasonography should be carried out as a screening test for upper and lower urinary tract abnormalities.14 Cystourethrography is useful for showing VUR and grading bladder deformity according to Ogawa’s classification (Fig. 2).14, 15 Patients having a history of symptomatic UTI as a result of VUR should undergo dimercaptosuccinic acid renal scintigraphy to evaluate renal scarring. Dynamic renal scintigraphy (renogram) is indicated for precisely evaluating split renal function.16 Cystourethroscopy is indicated for investigating bladder stones, anatomical bladder outlet obstruction and bladder tumor. Cystourethrography and cystourethroscopy should be carried out with caution in developing AD during examinations.
IV. Treatment
1. Urinary management
1-1. Summary of urinary management
The terms and descriptions of urinary management used in the guidelines are shown in Table 5.
| Terms | Descriptions |
|---|---|
| Voiding |
|
| Spontaneous voiding (coordinated physiological voluntary voiding) |
|
| Bladder reflex triggering (reflex voiding) |
|
| Bladder expression with Crede/Valsalva maneuvers |
|
| CIC |
|
| Indwelling catheterization |
|
| Indwelling urethral catheterization |
|
| Indwelling suprapubic catheterization |
|
1-2. Urinary management during acute phase
Indwelling urethral catheterization is required as part of the general care immediately after an insult. However, indwelling catheterization should be changed to CIC as soon as the general condition of the patient becomes stable and urine output decreases to approximately 1.5 L per day (LoE 4, GoR B), because long-term indwelling catheterization is associated with catheter-related complications. If voiding seems possible during the recovery period, UDS should be carried out to ensure the safety of voiding.
1-3. Voiding
The ability to void spontaneously (coordinated physiological voluntary voiding) depends on the severity of the paralysis, especially on the residual function of the lower extremities.17, 18 Voiding can be selected as urinary management when patients are free from the risk factors for renal damage (upper urinary tract deterioration or renal impairment) and symptomatic UTI, high pressure emptying, significant post-void residual urine, and bladder deformity. However, significant urinary incontinence is inevitable without the acquisition of spontaneous voiding in this setting, resulting in the continuous need for diapers and/or collecting devices. Therefore, among the various types of voiding, bladder reflex triggering (reflex voiding) and bladder expression by Crede/Valsalva maneuvers are not recommended as the optimal urinary management methods in SCI patients.19, 20
Spontaneous voiding: LoE 4, GoR B
Bladder reflex triggering: LoE 4, GoR C2
Bladder expression with Crede and/or Valsalva maneuvers: LoE 4, GoR C2
1-4. CIC
CIC is the gold standard of urinary management in SCI patients who cannot be permitted to void due to a significant risk of damage to the urinary tract (LoE 4, GoR B).21 When a patient has risk factors for renal damage (upper urinary tract deterioration or renal impairment), CIC should be initiated as soon as possible. Proper education and instruction with adequate follow up are of paramount importance in the long-term compliance and persistence of CIC, as well as avoiding complications, such as urethral trauma and stricture in male patients, gross hematuria, and symptomatic UTI.22
The panel also thoroughly discussed the following three clinical questions.
1-4-1. Is CIC recommended compared with bladder reflex triggering (reflex voiding), bladder expression using Crede/Valsalva maneuvers and indwelling catheterization in a case where CIC is indicated for urinary management?
CIC is strongly recommended compared with bladder reflex triggering (reflex voiding), bladder expression using Crede/Valsalva maneuvers, and indwelling catheterization in terms of preventing and/or improving renal damage (upper urinary tract deterioration or renal impairment), symptomatic UTI and AD (LoE 4, GoR B).23-25 However, all the urinary management techniques listed above, including CIC, are unsatisfactory for achieving urinary continence. Self-CIC is preferred to CIC by caregivers in terms of maintaining QOL.
1-4-2. Are hydrophilic-coated catheters better than non-coated catheters?
Plenty of evidence suggests that hydrophilic-coated catheters decrease the incidence of UTI and gross hematuria, improve QOL, and are cost-effective (LoE 2).26, 27 On the contrary, some reports question their efficacy (LoE 2), and the long-term outcomes remain to be determined (LoE 5).28, 29 Furthermore, the cost of the hydrophilic-coated catheter is a major obstacle to its widespread use (LoE 2). Eventually, the panel considerd GoR as C1 on hydrophilic-coated catheters.
1-4-3. Is the specialized indwelling urethral catheter developed for intermittent (temporary) use recommended for patients with nocturnal polyuria and/or difficulty in implementing CIC away from home?
The specialized indwelling urethral catheter developed for intermittent (temporary) use prevents night-time bladder overdistension that leads to upper urinary tract deterioration (LoE 4). Furthermore, the specialized catheter can maintain QOL when a patient wishes to skip CIC; for example, while working, going out, travelling and playing (LoE 4). Therefore, the use of the specialized catheter is recommended for patients with nocturnal polyuria and/or difficulty in carrying out CIC away from home (GoR C1).30, 31 On the contrary, patients should be well educated about restricting the use of the specialized catheter to a short time period, because the specialized catheter must not be completely substituted for CIC (LoE 5).
1-4-4. Is treatment for asymptomatic pyuria/bacteriuria (asymptomatic UTI) with antibiotics recommended for patients carrying out CIC?
Treatment for asymptomatic pyuria/bacteriuria (asymptomatic UTI) with antibiotics is not recommended (LoE 4, GoR C2), because there is a dearth of clear evidence supporting the use of antibiotics for prevention of symptomatic UTI.32 Rather, the emergence of drug-resistant bacteria is a major concern.33
1-5. Indwelling catheterization (urethral and suprapubic)
Long-term indwelling catheterization, whether urethral or suprapubic, is not recommended for urinary management of SCI patients due to the occurrence of many catheter-related complications (LoE 4). Urethral catheterization frequently causes urethral complications, whereas suprapubic catheterization carries the risk of complications at insertion (LoE 5).34, 35 The urethral catheter should be an adequate caliber (14–16-Fr), and a catheter with a larger caliber should not be used to circumvent the leakage around the catheter (LoE 5).36 In addition, the catheter should be changed at the regular intervals (2–6 weeks) (LoE 5). For insertion of a suprapubic catheterization, spinal or general anesthesia should be considered to prevent complications (LoE 5).
Urethral catheterization: GoR C2
Suprapubic catheterization: GoR C1
The panel also thoroughly discussed the following two clinical questions.
1-5-1. Is indwelling suprapubic catheterization recommended compared with indwelling urethral catheterization for patients who have to be managed with indwelling catheterization?
Patients with suprapubic catheterization seldom suffer from the urethral trauma, urethral diverticula, urethrocutaneous fistula, epididymitis or intrascrotal abscess that are frequently encountered in patients with urethral catheterization.36 Catheter obstruction might occur less frequently with suprapubic catheterization because of its larger caliber. Also, urethral trauma during the change of the catheters cannot occur in suprapubic catheterization. On the contrary, there is a lack of any clear evidence that showed a difference between suprapubic and urethral catheterization in terms of the incidence of symptomatic UTI. Eventually, the panel concluded that suprapubic catheterization is recommended compared with urethral catheterization (LoE 5, GoR C1).37
1-5-2. Is bladder irrigation with normal saline recommended for patients with indwelling catheterization?
Bladder irrigation with saline for the purpose of prevention of catheter blockage is a popular measure in Japan, although there is a distinct lack of evidence supporting its efficacy.38 The panel considered that this measure can be used to deal with unexpected catheter blockage (LoE 5, GoR C1), but that there is no clear evidence that it increases the incidence of symptomatic UTI.
2. Behavioral therapy
2-1. Lifestyle interventions
For OAB and stress urinary incontinence patients, lifestyle interventions have been recommended as the first-line therapy, because many lifestyle factors, such as obesity, smoking and drinking carbonated beverages, are associated with the development and/or worsening of these pathologies. Consequently, physicians routinely suggest weight loss, remedies for severe constipation, avoidance of an excessive intake of caffeine, alcohol and water, information on medications affecting the lower urinary tract function, avoidance of long sitting and chilling of the lower half of the body, and promotion of adequate exercise (LoE 5, GoR C1). However, there is a paucity of evidence for lifestyle interventions in SCI patients.
2-2. Other behavioral therapies
2-2-1. Physical therapy
Physical therapy includes pelvic floor muscle training with or without feedback or biofeedback training. These treatment modalities are probably useful for only the incomplete SCI patients who are able to voluntarily contract the pelvic floor muscle (LoE 3, GoR C1).
2-2-2. Scheduled voiding regimens
The term, “scheduled voiding regimens,” is a generic term that includes bladder training, timed voiding, habit voiding and prompted voiding. Of these regimens, bladder training, defined as holding the urine for some time, is so harmful that this regimen is not indicated for increasing bladder capacity in SCI patients (GoR C2). Timed voiding with regular CIC is generally recommended to prevent urinary incontinence and renal damage (upper urinary tract deterioration or renal impairment) (LoE 5, GoR C1), although a high LoE has not been found.
3. Pharmacological therapy
3-1. Anticholinergic drugs
Anticholinergic drugs inhibit involuntary detrusor contraction and abnormal detrusor tonus, leading to increased bladder capacity, suppressed DO and improved bladder compliance, which achieves a low-pressure reservoir, and to control urinary incontinence.39 This low-pressure storage resolves or reduces VUR and upper urinary tract dilatation, prevents renal impairment, prolongs the interval between catheterizations, and improves urinary incontinence. However, of these issues, only the number of urinary incontinence episodes has been evaluated in RCTs. In Japan, oxybutynin (immediate release and transdermal), propiverine, solifenacin, imidafenacin, tolterodine and fesoterodine are approved for the treatment of OAB and/or NLUTD. The efficacy and safety of oxybutynin immediate release, propiverine, tolterodine and trospium were reported for SCI patients by 2011, when the previous edition of the guidelines was published, whereas small-sized RCTs on oxybutynin immediate release and trospium, and a large-sized RCT on solifenacin were carried out after 2011.40 In SCI patients, higher doses might be required to improve DO and/or bladder compliance, which means that adverse anticholinergic events, such as dry mouth, constipation and blurred vision, might be more problematic. In addition, especially in elderly patients, the total anticholinergic load should be taken into account to prevent cognitive impairment.
Eventually, the panel concluded that anticholinergic drugs are recommended for patients who have the risk factors for renal damage and symptomatic UTI or urinary incontinence (LoE 2, GoR B).
3-2. β3-adrenoceptor agonist
The β3-adrenoceptor is involved in detrusor relaxation during the storage phase. The efficacy and safety of a β3-adrenoceptor agonist (mirabegron) were evaluated in two small RCTs in SCI patients, and showed positive results without safety concerns.41, 42 In Japan, a β3-adrenoceptor agonist is approved only for treatment of OAB.
Finally, the panel concluded that a β3-adrenoceptor agonist is recommended for patients who have risk factors for renal damage and symptomatic UTI or urinary incontinence (LoE 3, GoR C1), although there was insufficient evidence from placebo-controlled RCTs.
3-3. Other drugs
α1 adrenergic receptor antagonists are expected to reduce the resistance at the bladder outlet (bladder neck and prostatic urethra). Some open trials showed the efficacy in patients who were managed with voiding and had DSD or a non-relaxing urethral sphincter despite the lack of RCTs. In Japan, only urapidil was approved for NLUTD, whereas lower urinary tract-selective α1 adrenergic receptor antagonists, such as tamsulosin, naftopidil and silodosin, were approved only for benign prostatic hyperplasia.43 Parasympathomimetic drugs (bethanechol and distigmine) are expected to augment detrusor contractile strength; however, there has been a lack of RCTs with serious concerns for severe side-effects, especially cholinergic crisis.44 These drugs are contraindicated for patients with bladder outlet obstructions, such as DSD. Other oral drugs, for example, phosphodiesterase inhibitors and 5α-reductase inhibitors, have not been investigated for SCI patients.
α1 adrenergic receptor antagonists: LoE 4, GoR C1
Parasympathomimetic drugs: LoE 5, GoR C2
4. Surgical therapy
4-1. Transurethral botulinum toxin intradetrusor injection
Type A botulinum toxin interferes with the fusion mechanisms between a synaptic vesicle containing acetylcholine and a presynaptic membrane, and causes chemical denervation, which leads to relaxation of skeletal as well as smooth muscles. As several level 1 RCTs showed the efficacy and safety of transurethral botulinum toxin intradetrusor injection, this treatment modality is now considered to be an established treatment for refractory DO that is resistant to CIC combined with anticholinergic drugs (LoE 1, GoR A).45, 46 Botulinum toxin significantly decreases the number of urinary incontinence episodes, increases bladder capacity and bladder compliance, and lowers maximum detrusor pressure.47, 48 Frequently encountered complications are UTI, and in patients with voiding, increased post-void residual urine. as well as the need for CIC. As of the publication date (July 2019) of the original guidelines in Japanese, botulinum toxin was approved for blepharospasm and spasmodic torticollis in Japan, but not for refractory DO. However, at last, botulinum toxin was approved for refractory DO as of December 2019, when this abridged English translational version was accepted and final adjustments to publication were made.
4-2. Augmentation cystoplasty (augmentation enterocystoplasty)
Augmentation cystoplasty (augmentation enterocystoplasty) is a time-honored surgical procedure with established efficacy.49 Recent reports showed that the performance of this procedure is decreasing in parallel with the increasing frequency of transurethral botulinum toxin intradetrusor injection. However, after all the maximum conservative and less invasive therapies fail, augmentation cystoplasty still plays an important role in managing the uncontrollable upper urinary tract deterioration, recurrent symptomatic UTI and intractable urinary incontinence that are caused by refractory DO and low bladder compliance (LoE 5, GoR C1).50 On the contrary, augmentation cystoplasty is a major surgical procedure with significant peri- and postoperative complications, and regular CIC is generally required postoperatively, which emphasizes the importance of thorough preoperative informed consent.51 In addition, adequate long-term follow up is mandatory to prevent and recognize complications. Perioperative complications include prolonged ileus, wound infection, thromboembolism and hemorrhage.49, 51 Long-term postoperative complications include urolithiasis, bowel dysfunction, electrolyte–metabolic disturbances, bladder perforation and malignant neoplasm.49, 51 Therefore, the motivation and understanding of the patient and their family are prerequisites for this procedure.
4-3. Anti-incontinence surgeries
There are several anti-incontinence surgical modalities, such as bladder neck reconstruction, bladder neck sling, artificial urinary sphincter and bladder neck closure with abdominal continent catheterizable channel (LoE 5, GoR C1).52-54 The proper procedure depends on physical disabilities, comorbid diseases and characteristics of the lower urinary tract dysfunction, as evaluated by video-UDS. Regular CIC is generally required postoperatively, and significant peri- and postoperative complications can occur, which underscores the importance of thorough preoperative informed consent. In addition, adequate long-term follow up is mandatory to prevent and recognize complications.52 The postoperative complications include persistent urinary incontinence, difficulty in catheterization and urolithiasis, and with an artificial sphincter device, device malfunction and device infection.
4-4. Urinary diversions (incontinent or continent)
Urinary diversions are the last resort for SCI patients who do not respond to any relevant treatment modality (LoE 5, GoR C1), whereas whether the incontinent or continent diversions are chosen depends on their capability to carry out CIC.34 It should be stressed that the procedures are carried out after careful consideration of their acceptability, and the patient needs to undergo careful long-term follow up due to the invasiveness and frequency of complications, which resembles those of augmentation cystoplasty.
4-5. Sphincterotomy
Sphincterotomy is indicated for the male SCI patient who cannot be managed with CIC by themselves or caregivers and can keep a collecting device put on his penis. Sufficient contractile strength/duration of detrusor induced by bladder reflex triggering is also required. Sphincterotomy relieves high intravesical pressure from DSD and lessens the risk of upper urinary tract deterioration.55, 56 This procedure is irreversible in nature and should be carried out after careful consideration, although selected tetraplegic male patients seem to be promising candidates for this procedure. The complications include insufficient relief of DSD resulting in repeated procedures, hemorrhage and urethrocutaneous fistula.
Eventually, the panel concluded that sphincterotomy is recommended for cervical SCI patients who are unable to carry out CIC compared with urethral stents, and suprapubic and urethral catheterization (LoE 5, GoR C1).
4-6. Urethral stents
Urethral stents are reversible and less invasive than sphincterotomy. Permanent stents showed comparable efficacy with sphincterotomy (LoE 3),57 whereas temporary stents that were retained up to 2 years did not show long-term efficacy (LoE 5).58
Permanent stents: GoR pending (not yet approved)
Temporary stents: GoR C2
5. Other treatment modalities
5-1. Intrasphincteric injection of botulinum toxin
Intrasphincteric injection of botulinum toxin has efficacy similar to that of sphincterotomy; however, repeat injections every 3–6 months are required to maintain the efficacy. In Japan, this procedure is very expensive and has not yet been approved (LoE 3, GoR pending (not yet approved)).
5-2. Electrical and magnetic stimulation
SNM might be a treatment of choice for carefully selected incomplete SCI patients with refractory OAB (LoE 5, GoR C1).59
SNM also showed some efficacy in patients with non-obstructive urinary retention; however, this indication has not yet been approved in Japan (LoE 5, GoR pending (not yet approved)).59
Interferential therapy and magnetic stimulation in SCI patients lack evidence of efficacy (LoE 4, GoR pending (lack of relevant evidence for SCI patients)).
Sacral anterior root stimulation with dorsal rhizotomy might be a treatment of choice to achieve voiding and urinary continence in the carefully selected complete suprasacral SCI patients (LoE 4, GoR pending (not yet approved)).60
Electrical stimulation of the posterior tibial nerve or the sensory branch of the pudendal nerve is expected to improve urinary incontinence (LoE 3, GoR pending (not yet approved)).61, 62
V. Surveillance
Several surveillance protocols were reported, but a consensus on these issues has not been reached. The panel considers that the following assessments should be included during follow up of the patients.63
Annual transabdominal ultrasonography is recommended for surveillance of the upper urinary tract (LoE 4, GoR B).
Cystatin C is preferred to creatinine for the early detection of renal impairment (LoE 5, GoR C1).9, 10, 64
Annual transabdominal ultrasonography combined with urinalysis every 3–6 months is recommended for surveillance of the lower urinary tract (LoE 5, GoR C1). The appropriate intervals for video-UDS remain to be determined. Rather, the intervals should be individually tailored according to the risk factors for renal damage and symptomatic UTI, as well as changes in clinical findings during surveillance (LoE 5, GoR C1).65
Whether the regular assessment of QOL during follow up affects urinary tract outcomes requires further studies.66 However, QOL is now considered to be included in primary and/or secondary outcomes as a patient-reported outcome measure (GoR pending (due to limited availabilities of the validated QOL questionnaires in Japanese for patients with NLUTD)).
The panel also thoroughly discussed the following clinical question.
Are urine cytological study and/or cystourethroscopy recommended to screen for bladder cancer?
The sensitivity of urine cytological study and cystourethroscopy is not adequate to screen for bladder cancer, because the prevalence of bladder cancer in this population is not as high as expected. Therefore, regular urine cytological study and/or cystourethroscopy are not recommended to screen for bladder cancer in asymptomatic SCI patients (LoE 5, GoR C2).66
On the contrary, the age at onset of bladder cancer is younger and the prevalence of bladder cancer is higher, respectively, in this population than in the general population. Furthermore, it should be noted that cancer-specific survival is lower because early detection is often difficult, and unfavorable pathological findings are frequently observed, which results in an advanced stage at diagnosis.67 Consequently, it must be emphasized that physicians should assess patients who have not only risk factors, such as chronic indwelling urethral catheterization, but also suspicious symptoms and signs, such as gross hematuria, worsening of AD, refractory recurrent symptomatic UTI and abnormal bladder wall thickening on transabdominal ultrasonography, on an individual basis (LoE 5, GoR C1).
VI. Assessment and management of UTI
Diagnosis of symptomatic UTI consists of significant bacteriuria, pyuria and symptoms suggestive of UTI (Table 6) without any obvious causes for those findings and symptoms.68
| Discomfort and/or pain in renal or suprapubic area in incomplete injury |
| Cloudiness of urine |
| Worsening urine odor |
| Developing or worsening urinary incontinence or leakage around the catheter |
| Pain on micturition (dysuria) |
| Worsening spasticity |
| Discomfort, lethargy, anorexia |
| Autonomic dysreflexia or autonomic hyperreflexia (the level of injury above T6) |
| Fever or chills |
Symptomatic UTI should be differentiated from infectious and non-infectious diseases, such as respiratory and soft tissue infection, and heat retention, as well as venous thromboembolism, respectively.69 Transabdominal ultrasonography should be carried out as a basic evaluation to rule out upper and lower urinary tract obstruction.70
The panel recommended immediate decompression of an obstructed urinary tract and drainage of infectious urine (LoE 5, GoR C1).
In the case of recurrent symptomatic UTI, urinary tract abnormalities, including hydronephrosis, urinary lithiasis and hostile lower urinary tract dysfunction, should be uncovered through further careful urological investigations.
VII. Pediatric SCI
Pediatric SCI differs markedly from adult-onset SCI in causes, complications and phenotypes. Physicians should manage these patients individually, taking into account the patient’s growth and developmental state. Proper urinary management, including CIC and anticholinergic drugs, will increase bladder capacity with growth and prevent upper urinary tract deterioration.71
VIII. Elderly SCI
Elderly SCI is characterized by cervical SCI, incomplete tetraplegia, SCI without bone injury and central SCI. The resultant impairment in hand dexterity makes clean intermittent self-catheterization difficult or impossible.72, 73 It should be noted that aging in SCI patients worsens their activities of daily living, and eventually converts clean intermittent self-catheterization to indwelling catheterization. In addition to this, renal impairment, urolithiasis, and cognitive impairment due to an excessive anticholinergic load should be carefully monitored in these patients.
IX. Cervical SCI
Among SCI patients in Japan, cervical SCI occurs most frequently.74 It has distinct features from other SCIs, because it develops into tetraplegia with the impairment of hand dexterity and is often accompanied by AD. These features prevent patients from carrying out clean intermittent self-catheterization, especially patients with complete injury above C5 and C6 in men and women, respectively.2 When the patient cannot carry out self-catheterization, physicians should seriously consider urinary management, taking into account accompanying functional disabilities and social circumstances. Central cervical SCI affects the upper extremities much more than lower extremities, and the patients generally recover well, except for impaired hand dexterity.75 Although the incidence of lower urinary tract dysfunction was reported to be low, some patients manifest definite urinary tract complications, and therefore, regular urological surveillance is mandatory for patients with central spinal cord injury.76
X. AD
AD is an abnormal autonomic reflex responding to nociceptive stimuli below the level of injury, which develops in patients with high-level SCI (usually above T6).77, 78 A remarkable rise in blood pressure during AD sometimes leads to serious complications, such as intracranial hemorrhage and lethal arrhythmias.77
Physicians should remove the causes of stimuli as soon as possible and deal with the hypertensive crisis (LoE 5, GoR B).
It should be noted that blood pressure should be carefully monitored during examinations that require bladder filling, because bladder and bowel distension are the leading causes of AD (LoE 5, GoR C1).
XI. Economics and Japanese healthcare system
1. Economics
Cost–benefit analysis is based on the evaluation of the incremental cost-effectiveness ratio, and willingness to pay by the Japanese payment authorities ranges from ¥5 million to ¥6 million per one quality-adjusted life year.79 Cost–benefit analyses of botulinum toxin intradetrusor injection and hydrophilic coated catheter were reported in North America and European countries. The letter analysis was carried out in Japan, too, which showed that the incremental cost-effectiveness ratio was ¥3 826 351 per one quality-adjusted life year.80
2. Japanese healthcare system
At claims for medical service fees, NLUTD should be divided into spastic and atonic NLUTD as insurance names of diseases. Otherwise, the claims are subjected to an assessment.
Hospital and clinics are reimbursed ¥18 000 per month, which includes costs of catheters, lubricants and disinfectants, and other related materials, for managing outpatients with CIC. When the patients use disposable catheters, hydrophilic-coated catheters or specialized catheters for intermittent use, ¥6000, ¥9600 or ¥6000, respectively, per month can be added to the ¥18 000 per month, although any of the additional reimbursements is permitted once per month.
In accredited hospitals, medical service fees for managing indwelling catheters or urinary stomas of outpatients can be claimed.
When measuring cystatin C for the early detection of renal impairment, caveats in claiming medical service fees should be obeyed, which requires abnormal values of creatinine or blood urea nitrogen before measuring cystatin C with an interval between measurements of cystatin C at least 3 months apart.
Industrial accident compensation insurance does not reimburse healthcare providers for transabdominal ultrasonography to investigate the urinary tract.
The continence self-management program claims has been approved when a multidisciplinary team assesses the lower urinary tract dysfunction and finds a way to remove an indwelling catheter to achieve independent urination. This epoch-making system highlights the importance of urinary management for all medical providers.
Conflict of interest
Noritoshi Sekido received research grants from Astellas and Ono, and received lecture fees from Astellas and Pfizer. Yasuhiko Igawa received research grants from Astellas, Ono, Integral, Kissei, Kyorin, Lilium Otsuka, Medicon, NipponShinyaku, RaQualia and Tsukada Medical Research, and lecture fees from Astellas. Hidehiro Kakizaki received research grants from Astellas, DaiichiSankyo, Kissei, NipponShinyaku, Taiho and Takeda, and received lecture fees from Astellas, Kyorin, Pfizer and Taiho. Satoru Takahashi received research grants from Astellas, NipponShinyaku and Takeda, and received lecture fees from Astellas, Kissei, NipponShinyaku and Pfizer. Tomonori Yamanishi received research grants from Astellas, DaiichiSankyo, Kyorin, Pfizer and Taiho. Takeya Kitta, Atsushi Sengoku, Ryosuke Takahashi, Katsuyuki Tanaka, Takashige Namima, Masashi Honda, Takahiko Mistui and Toyohiko Watanabe have no conflict of interest.




