Evidenced-based clinical practice guideline for prostate cancer (summary: Japanese Urological Association, 2016 edition)
Abstract
These guidelines cover a wide range of topics from prostate cancer epidemiology to palliative care. Questions arising in daily clinical practice have been extracted and formulated as clinical questions. In the 4 years since the previous edition, there have been major changes – for example, robot-assisted prostatectomy has rapidly come into widespread use, and new hormones and anticancer drugs have been developed for castration-resistant prostate cancer. In response to these developments, the number of fields included in this guideline was increased from 11 in the 2012 edition to 16, and the number of clinical questions was increased from 63 to 70. The number of papers identified in searches of the existing literature increased from 4662 in the first edition, published in 2006, to 10 490 in the 2012 edition. The number of references has reached 29 448 just during this review period, indicating the exponential increase in research on the topic of prostate cancer. Clinical answers have been prepared based on the latest evidence. Recommendation grades for the clinical answers were determined by radiologists, pathologists, and other specialists in addition to urologists in order to reflect the recent advances and diversity of prostate cancer treatment. Here, we present a short English version of the original guideline, and overview its key clinical issues.
Abbreviations & Acronyms
-
- AS
-
- active surveillance
-
- BMA
-
- bone-modifying agent
-
- CA
-
- clinical answer
-
- CAB
-
- combined androgen blockade
-
- CQ
-
- clinical question
-
- CRPC
-
- castration-resistant prostate cancer
-
- CT
-
- computed tomography
-
- EBRT
-
- external-beam irradiation
-
- HDR
-
- high-dose-rate brachytherapy
-
- HIFU
-
- high-intensity focal ultrasound
-
- IDC-P
-
- intraductal carcinoma of the prostate
-
- LDR
-
- low-dose-rate brachytherapy
-
- LH-RH
-
- luteinizing hormone-releasing hormone
-
- LRP
-
- laparoscopic radical prostatectomy
-
- MRI
-
- magnetic resonance imaging
-
- NCCN
-
- National Comprehensive Cancer Network
-
- PDE5
-
- phosphodiesterase type 5
-
- PRIAS
-
- Prostate Cancer Research International Active Surveillance
-
- PSA
-
- prostate-specific antigen
-
- PSA-V
-
- prostate-specific antigen velocity
-
- PSAD
-
- prostate-specific antigen density
-
- PSA-DT
-
- prostate-specific antigen doubling time
-
- QALY
-
- quality-adjusted life years
-
- QOL
-
- quality of life
-
- RANKL
-
- receptor activator of nuclear factor-
 B ligand
B ligand - receptor activator of nuclear factor-
-
- RARP
-
- robot-assisted radical prostatectomy
-
- RG
-
- grade of recommendation
-
- RP
-
- radical prostatectomy
-
- RRP
-
- retropubic radical prostatectomy
-
- SRT
-
- salvage radiotherapy
-
- TURP
-
- transurethral resection of the prostate
-
- UICC
-
- Union for International Cancer Control
Introduction
As the population continues to age and diets change, the number of patients with prostate cancer in Japan is increasing. In 2015, prostate cancer surpassed stomach and lung cancer as the leading type of cancer in men. Prostate cancer care, which has therefore become increasingly important, has also advanced markedly. The pathological features used to diagnose prostate cancer have been revised, and MRI and other types of imaging modalities have proven to be useful. As understanding of the pathology of the disease improves, treatments developed in accordance with this new understanding become increasingly complicated. New drugs have been developed for the treatment of CRPC. Clinical experience with new hormone treatments and anticancer drugs continues to increase, and robot-assisted surgery has spread rapidly, making it now come into common use. The long-term outcomes and safety of AS are also being elucidated. In the field of radiotherapy, advances in the devices used have led to major changes in therapeutic strategy, and the concepts of bone-targeted therapy and bone health have come to be viewed with increasing importance.
This Japanese guideline, which has undergone revision for the first time in 4 years, was released as the Third Edition in October 2016 in order to reflect the rapid changes occurring in the field of prostate cancer treatment. Its goals are: (i) to indicate the appropriate application of prostate cancer therapies; (ii) to improve the therapeutic outcomes and safety of prostate cancer therapy; (iii) to reduce differences in the therapies being provided at different medical facilities; (iv) to do away with ineffective therapies, and thereby alleviate both human and economic burdens; and (v) to enhance mutual understanding between medical professionals and patients. Here, we present the English version of this guideline with a focus on these CQs and CAs. We are confident that this guideline will help in the decision-making related to the treatment of prostate cancer by both patients and medical professionals.
Guideline development process
This guideline was prepared by a committee of 77 people consisting mainly of urologists, but including radiologists and pathologists (Appendix S1). The committee carefully determined 16 categories and 70 CQs essential for daily clinical practice. Specific keywords were identified for each CQ, and searched in PubMed and Japan Medical Abstracts Society to locate articles published between 2011 and 2015. Articles published before 2011 were identified by referring to the references in the 2006 and 2012 editions of this guideline. Based on the findings of the literature search, each member of the guideline committee drafted a review of the topic along with answers to CQs with descriptions. The content of the draft was then polished through several critical readings by multiple committee meetings, opinions solicited through the homepage, and review by the Japanese Urological Association Guideline Committee, the Japan Society of Clinical Oncology and the Japanese Society of Medical Oncology, leading to a unanimous final draft. The guidelines were approved and published by the Japanese Urological Association in October 2016.
The grade of the recommendation and the evidence level in the present manuscript were determined in accordance with the Handbook for Clinical Practice Guideline Development (Table 1). When the evidence was insufficient or of an inappropriate level to make the judgment, the grade of recommendation was determined according to committee discussions and resultant consensus statements.
| RG | |
| A: | Implementation is highly recommended on the basis of robust scientific evidence |
| B: | Implementation is recommended on the basis of some scientific evidence |
| C1: | Implementation is recommended, but no scientific evidence is available |
| C2: | Implementation is not recommended because of lack of scientific evidence |
| D: | Implementation is not recommended on the basis |
Clinical questions and answers addressed in this guideline
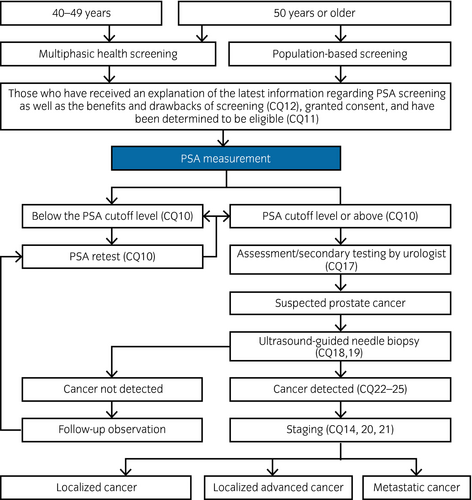
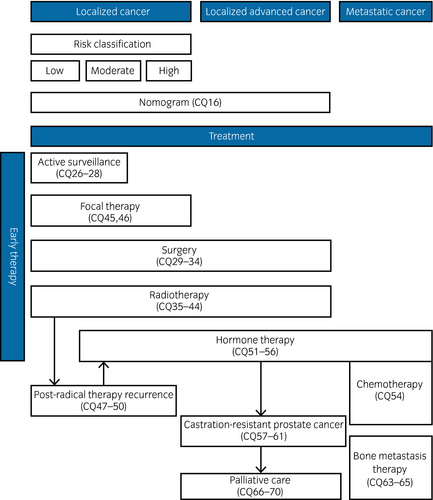
Table 2 shows the 70 CQs and answers divided into 16 categories that are addressed in this guideline. Figures 1 and 2 show the prostate cancer diagnostic and treatment algorithms, and the algorithms by stage with related CQs listed. Local cancers are divided into three risk categories in accordance with the risk categories of D'Amico.1 However, localized advanced cancers include cT3a, T3b and T4, as well as cases with no lymph node or distant metastasis (NCCN category of “very high risk” and cT3a).
| No. | Category | RG | |
|---|---|---|---|
| Clinical question | Clinical answer | ||
| Epidemiology | |||
| 1 | What are the prevalence and mortality rate of prostate cancer? | The number of prostate cancer patients worldwide is estimated as approximately 1.1 million in 2012, accounting for 14.8% (the second largest) of all male cancer. The number of deaths due to prostate cancer occurred in approximately 310 000 (6.6%), the 5th largest figure among cancer death in men. The age-adjusted prevalence and mortality rate was 30.7 and 7.8 per 100 000, respectively. The figures were high in developed nations in Europe and the USA. The mortality rate started on a downward trend after PSA became widely available. In Japan, the prevalence, 78 728 in 2011, is expected to increase to 98 400 in 2015, reaching the most common cancer in men. In 2014, the mortality rate and the age-adjusted mortality rate was 11 507 (the 7th largest) and 7.3 in 100 000 (the 9th largest) in men. | |
| 2 | What are the congenital and genetic risk factors of prostate cancer? | A family history of prostate cancer increases the risk by 2.4–5.6-fold. The increased risk for carriers of a HOXB13 G84E mutation is 3.3–20.1-fold. Those with other genetic mutations and single nucleotide polymorphisms have increased risk as well; the effect is limited, as the odds ratio is under 1.5. | |
| 3 | What acquired and local factors are related to prostate cancer risk? | Acquired factors correlated with prostate cancer are: (i) lifestyle and habits (diet, exercise, tobacco, functional foods, etc); (ii) obesity, diabetes and metabolic syndrome; (iii) inflammation and infection of the prostate; (iv) benign prostatic hyperplasia and lower urinary tract symptoms; and (v) exposure to environmental factors and chemical substances. However, there are conflicting reports for all of these factors, confounding factors were not fully controlled. It is difficult to identify with precision the acquired factors related to prostate cancer. | |
| 4 | What is the natural history of prostate cancer? | Many cases of prostate cancer are thought to develop extremely slowly over dozens of years. Therefore, many men with prostate cancer die of other diseases without ever having been diagnosed with cancer. Some are diagnosed by screening or on examination, while others present with symptoms attributable to prostate cancer. Generally, prostate cancer progresses slowly, but some clinically diagnosed cases progress to death. | |
| Prevention | |||
| 5 | Are lifestyle improvements useful in preventing prostate cancer? | Although there is no clear evidence that making improvements to lifestyle or avoiding certain environmental factors prevents prostate cancer, it is possible that certain factors might have some preventive effects. In terms of diet, it has been reported that intake of DHA and EPA, both of which are contained in large amounts in fish and seafood, or the intake of dairy products, calcium and fat decrease the risk, but there are other studies that have reported the opposite. Thus, the issue is not settled at present. Recently, the link between obesity/metabolic syndrome and prostate cancer has become the focus of attention. It has been suggested that making lifestyle changes might be effective in preventing prostate cancer. | C1 |
| 6 | Do the functional factors contained in soybeans, green tea, tomatoes and other foods contribute to the prevention of prostate cancer? | There is some evidence that diet might play an important role in prostate cancer. Previous studies have focused on functional factors, such as the isoflavones contained in soybeans, catechins in green tea and lycopenes in tomatoes, as having a preventive effect on the development of prostate cancer (RG: C1). However, it is not clear if functional factors, such as the selenium and vitamin D contained in seafood and the vitamin E contained in nuts and fish roe, are effective in the prevention of prostate cancer (RG: C2). There are no epidemiological or clinical studies that have concluded that functional factors are effective, and therefore we look forward to further research in this area. |
C1 C2 |
| 7 | Is a 5-alpha reductase inhibitor effective as a chemopreventive agent? | According to a large-scale randomized controlled trial, a 5-alpha reductase inhibitor was effective in reducing the incidence of prostate cancer, but an association with higher grade prostate tumors could not be completely ruled out. At present, it is not thought to have a significant effect on survival. | C2 |
| 8 | Are there any drugs that are effective chemopreventives for prostate cancer? | Many drugs are being investigated as chemopreventives for prostate cancer, including aspirin, statins and metformin, but there is great variation in the reports on their effectiveness. The issue of whether these drugs are effective chemopreventives is currently unresolved. | C2 |
| Prostate cancer screening | |||
| 9 | Does the incidence of metastatic cancer or mortality decrease as a result of prostate cancer screening? | According to highly-reliable RCTs and practical prospective etiological studies, the implementation of PSA-based prostate cancer screening has led to decreases in the incidence of advanced and metastatic cancer, and thus has led to decreases in the mortality rate of prostate cancer. | B |
| 10 | What are the recommended PSA cut-off levels and screening interval? | The PSA cut-off level is recommended at 4.0 ng/mL for all ages. The alternative recommendation is age-specific PSA cut-offs, which are 0.0–3.9 ng/mL for those aged 50–64, 0.0–3.5 ng/mL for those aged 65–69, and 0.0–4.0 ng/mL for those aged ≥70 years (RG: B). For a PSA level of 0.0–1.0 ng/mL, repeat testing is recommended every 3 years, with annual testing recommended for values between 1.1 ng/mL and the cut-off levels (RG: B). |
B B |
| 11 | How are age and other physical conditions taken into consideration when recommending prostate cancer screening? | It is important to manage the long-term risk of prostate cancer progression, metastasis, and death by confirming changes in the baseline PSA levels for those aged ≤60 years. Deciding whether to continue screening in the elderly is challenging because of the difficulty of accurately predicting life expectancy. Geriatric health assessment using the G8 screening tool might be a useful countermeasure. | B |
| 12 | What are the major benefits and drawbacks of prostate cancer screening? | The major benefit of prostate cancer screening is that early diagnosis might allow treatment before the disease is advanced or has metastasized, thus reducing mortality. Early detection allows appropriate management of individualized treatment from the wide variety of therapies available. In contrast, harms include the fact that screening does not detect all prostate cancers, it increases the number of unnecessary prostate biopsies, it increases the risk of complications associated with prostate biopsies, and it might lead to overdiagnosis and overtreatment of low-grade tumors with a consequent decrease in QOL due to complications associated with treatment. | B |
| 13 | What are the cost-effectiveness ratio and efficiency of prostate cancer screening? | Overdiagnosis and overtreatment reduce the cost-effectiveness of prostate cancer screening. However, a 2014 RCT in Europe with cost-effectiveness analysis reported that the incremental cost-effectiveness ratio for subjects aged 55–59 who underwent screening every 2 years was $73 000 per 1 year increase in QALY. Unlike previous reports, this study found that screening was highly cost effective. However, it also found that for those over the age of 63, screening led to decreased QALYs and cost-effectiveness because of overdiagnosis and overtreatment. | C1 |
| Staging, risk classification and nomograms | |||
| 14 | How is prostate cancer staging performed? | An appropriate staging must be performed based on findings including rectal examination and imaging studies. | B |
| 15 | Are prostate cancer risk classifications useful? | Prostate cancer risk classifications are useful in predicting the therapeutic outcome after radical treatment. | B |
| 16 | Are prostate cancer nomograms useful? | Nomograms are recent evidence-based predictive tools that appear to be accurate. They are recommended for use in prostate cancer treatment and diagnosis. | B |
| Diagnostic methods (markers, imaging, biopsy) | |||
| 17 | What are the recommendations for improving the specificity of PSA tests? | % free PSA, PSAD and nomograms might improve the specificity of PSA tests. | C1 |
| 18 | What are the recommended sites and number of prostate biopsies? | For initial assessment, biopsies in 10–12 sites mainly in the peripheral zone are recommended (RG: A). For subsequent biopsies, multiple biopsies including at the apex and the central zone might improve the cancer detection rate (RG: B). |
A B |
| 19 | Are transrectal, transperineal or combined biopsies recommended? | Although the detection rates of transrectal and transperineal biopsies are the same, transrectal biopsies are associated with a higher risk of complications, such as infection. | A |
| 20 | What tests are recommended for assessment of primary foci (T-staging)? | Local extension (T-staging) is evaluated with diagnostic imaging. Multiparametric MRI, including T2-weighted images, dynamic contrast-enhanced study and diffusion weighted images at 3T, improves diagnostic accuracy. | B |
| 21 | What tests are recommended for the assessment for metastases (N- and M-staging)? | Although lymph node dissection is the most effective method of assessing lymph nodes status, limited lymph node dissection of obturator nodes only is insufficient (RG: B). CT and MRI are insufficient for lymph node assessment due to limited sensitivity and specificity (RG: C1). Bone scintigraphy is useful in untreated cases with a PSA ≥10.0 ng/mL, and positive rectal examination or Gleason score of ≥8, as well as cases with suspicious symptoms of bone metastasis (RG: B). |
B C1 B |
| Pathology | |||
| 22 | What was changed in the revised International Society of Urological Pathology 2014? | New grade group classifications are recommended to replace the Gleason score, which has been generally used to assess the degree of malignancy of prostate cancer. This was determined by consensus at the International Society of Urological Pathology 2014. Although the new classification and the Gleason score are being used in combination, this is expected to shift to exclusive use of the new classification. | |
| 23 | What kind of lesion is an index tumor? | Although multiple tumors caused by different clones are known to occur, only those that might affect prognosis are treated. Such lesions are known as the index tumor (or dominant nodule). In recent years, local treatment of only the index tumor has been attempted. | |
| 24 | What is the diagnostic significance of IDC-P? | Regardless of treatment method, the presence of IDC-P within invasive cancer indicates a poor prognosis, with a greater chance of PSA recurrence, clinical recurrence, cancer-specific death and overall death. The presence of IDC-P in biopsy specimens without invasive cancer components suggests the possibility that highly malignant cancer is present in the background tissue. | |
| 25 | How well are the genetic abnormalities of prostate cancer understood? | Many prostate cancers have TMPRSS2:ERG gene fusion, and therefore diagnosis using ERG protein, a product of this gene fusion, is clinically useful. Genetic abnormalities related to DNA repair have been shown in cases of advanced prostate cancer. Thus, it is conceivable that individualized treatment based on genetic abnormalities will be possible in the future. | |
| Active surveillance | |||
| 26 | AS is indicated for which patients? | Indications are: PSA ≤10 ng/mL, clinical stage ≤pT2, no. positive cores ≤2 (but not limited to this figure in cases of targeted biopsy and saturation biopsy), Gleason score ≤6 and PSAD <0.2 or <0.15 ng/mL. | B |
| 27 | What are the observation methods used for AS and criteria for starting treatment? | Observation during AS requires rectal exam and PSA testing every 3–6 months and prostate biopsy every 1–3 years. The criteria for the start of treatment are findings on repeated prostate biopsy of increased Gleason score or increased number of positive cores (deviation from pathological criteria – reclassification) and confirmation of advanced clinical stage. The significance of PSA doubling time and PSA velocity for this purpose has not been established. | B |
| 28 | Is long-term surveillance safe? | Although there are no long-term study results yet, medium-term results indicate the possibility that in low-risk prostate cancer, there is no difference in outcome for patients who continued AS vs those who underwent radical therapy, which suggests that AS is appropriate. In particular, there is a high probability that prognosis does not differ between patients with a life expectancy of ≤10 fewer years, and those with a life expectancy between 10–20 years. Short-term and medium-term studies have indicated that AS does not have a major effect on patient QOL. | B |
| Radical prostatectomy | |||
| 29 | RP is recommended for which patients? | Patients with low- to intermediate-risk localized prostate cancer who have a life expectancy of ≥10 years (RG: A). It might be appropriate in some cases of high-risk localized prostate cancer as well (RG: B). |
A B |
| 30 | For which patients is lymph node dissection recommended when performing RP? What range of dissection is required? |
It should be performed in cases of intermediate to high risk (RG: B). Extended lymph node dissection should routinely include the external iliac, obturator and internal iliac lymph nodes. Localized lymph node dissection is not recommended (RG: B). |
B B |
| 31 | What factors are related to postoperative incontinence recovery? What treatments are effective for postoperative incontinence? | Preoperative factors include age, obesity (BMI), comorbidities, erectile function and pelvic floor muscle anatomy (RG: C1). Nerve preservation as well as preservation of the urethral sphincter is effective in maintaining urinary continence (RG: A). Effective treatments of postoperative urinary incontinence include pelvic floor muscle manipulation and implantation of an artificial urinary sphincter (RG: B). |
C1 A B |
| 32 | Is nerve preservation recommended as an effective way to maintain sexual function when performing RP? What are the effective treatments for postoperative sexual dysfunction? | Intraoperative nerve preservation is effective in maintaining sexual function (RG: B). Administration of PDE5 inhibitor is effective after nerve preservation surgery (RG: B). |
B B |
| 33 | What are the differences in the therapeutic outcomes of RARP, LRP and RRP? | RARP and LRP have the same carcinostatic effect as RRP (RG: B). Compared with RRP, RARP and LRP are less invasive, cause less blood loss, and allow faster postoperative QOL recovery (e.g. urinary incontinence and sexual function; RG: B). |
B B |
| 34 | For which patients is postoperative adjuvant therapy recommended? What therapies are recommended? | Postoperative adjuvant radiotherapy is recommended in cases of pT3N0M0 with a life expectancy of ≥15 years, especially in cases of seminal vesicle invasion (RG: B). Hormone therapy (androgen deprivation therapy) is recommended in cases of lymph node metastasis (RG: B). Pelvic irradiation and hormone therapy (androgen deprivation therapy) is recommended in cases of lymph node metastasis (RG: C1). |
B B C1 |
| Radiotherapy (external irradiation) | |||
| 35 | What are the optimum dose, fractionation methods and range for radical external irradiation? | At BED 1.5, the recommended dose is 170–190 Gy (equivalent of 72 Gy/36 Fr. or 80 Gy/40 Fr. at normal fractionated irradiation; RG: A). Normal fractionated irradiation is recommended (RG: A). For low- to moderate-risk cases, moderate hypofractionated irradiation is recommended as a substitute for normal fractionated irradiation (RG: B). For moderate- to high-risk cases, uniform preventive irradiation (full pelvic irradiation) of the pelvic lymph node region is not recommended (RG: D). |
A A B D |
| 36 | For which patients is proton beam or heavy ion radiotherapy recommended? | Although clinical trials have reported that proton beam and heavy ion radiotherapy produce satisfactory results in cases of localized and localized advanced prostate cancer, further high-level clinical trials are required to establish that these treatments are superior to existing treatments. | C1 |
| 37 | Is the outcome improved when hormone therapy is used in conjunction with radical external irradiation? What are the optimum timing, drugs and duration of combined therapy? | For moderate-risk cases, hormone therapy for approximately 4–6 months (pre-irradiation or concurrently) is recommended (RG: B). For high-risk cases, adjuvant therapy is recommended, but the effectiveness of high-dose irradiation of approximately 80 Gy remains unclear (RG: C1). When hormone therapy is used in combination with radiotherapy, it is begun before irradiation (RG: A). The recommended combined use drugs are an LH-RH agonist (or antagonist) ± an anti-androgen drug (RG: B). There is no evidence that clearly shows that therapeutic outcome improves when adjuvant hormone therapy is continued for >6 months (RG: C2). |
B C1 A B C2 |
| 38 | Is radiotherapy effective in localized relapse of hormone therapy-resistant cancer? Is localized radiotherapy effective in cases of N1 or M1 prostate cancer? | Radiotherapy is indicated in cases of localized recurrence of hormone therapy-resistant cancer (RG: C1). In cases of N1 prostate cancer, combined use of hormone therapy and radiotherapy improves prognosis (RG: C1). In cases of M1 prostate cancer, the effectiveness of localized radiotherapy is not clear (RG: C2). |
C1 C1 C2 |
| 39 | Does the risk of post-radiotherapy secondary cancer (bladder or rectal cancer) differ by treatment method? | Each treatment method has a different risk for development of secondary cancers. The past use of wide-ranging two-dimensional irradiation and postoperative irradiation had a higher risk of secondary cancers than the highly-accurate external irradiation and interstitial irradiation techniques currently in use. | |
| 40 | What adverse events are associated with external irradiation, and what countermeasures can be used? | The main adverse events associated with external irradiation are damage to the digestive and urinary tracts, and sexual dysfunction. They become more likely as the radiation dose increases (RG: A). Prevention of adverse events associated with external irradiation includes the use of intensity-modulated radiation therapy, an important way to reduce the radiation dose to the rectum, bladder and urethral bulb (RG: B). | A, B |
| Radiotherapy (interstitial irradiation) | |||
| 41 | What aspects of the therapeutic outcome of LDR are superior to those of other therapies? | LDR has the same biochemical recurrence-free rate as RP in low-risk cases. In addition, as the risk increases, it can be expected to have a better biochemical recurrence-free rate than RP, although there is no clear evidence indicating that it leads to improved overall survival (RG: C1). LDR has a better long-term biochemical recurrence-free rate than high-dose EBRT, but there is no clear evidence indicating that it leads to improved overall survival (RG: C1). |
C1 C1 |
| 42 | For which patients is the combined use of LDR, external irradiation and hormone therapy recommended? | It is recommended for high-risk patients. However, there are some patient groups for which hormone therapy is not necessary. In addition, although it is indicated for some moderate-risk cases, there is no clear standard regarding its use. | C1 |
| 43 | Is LDR recommended for the purpose of maintaining QOL? | It is superior to RP in terms of preserving urination function, and it is the same as EBRT (RG: B). For maintenance of sexual function, it is superior to RP when implemented immediately after treatment, and it is the same as EBRT (RG: C1). |
B C1 |
| 44 | How is HDR monotherapy performed? For which patients is it recommended? | There is no consensus on uniform dose fractionation for HDR as monotherapy, but there are reports of single doses of 6–29 Gy, total doses of 19–54 Gy, in 1–9 fractions, 1–5 days of treatment and 1–3 implant sessions. The global trend is toward hypofractionation, large single doses and short-term duration (RG: C1). HDR monotherapy is considered by some to be indicated in low- to intermediate-risk cases, and by others to be indicated as an aggressive treatment in intermediate- to high-risk cases. It is recommended for patients for whom it is desirable to complete treatment in a shorter period of time than possible with EBRT and in intermediate- to high-risk patients for whom the dose will be increased and for whom EBRT would likely be necessary in addition to LDR (RG: C1). |
C1 C1 |
| Focal therapy (cryotherapy, HIFU) | |||
| 45 | Is focal therapy recommended for cases of low-risk localized prostate cancer? | Focal therapy is one possible treatment option for low-risk localized prostate cancer cases diagnosed by biopsy or template biopsy based on MRI findings. | C1 |
| 46 | Is focal therapy recommended for the purpose of maintaining QOL in cases of localized prostate cancer? | In cases of localized prostate cancer in which radical surgery is not indicated or not desired, and when the limitations of the treatment are fully understood by the patient, focal therapy is recommended as a way to maintain QOL. | C1 |
| Salvage therapy: Treatment of post-radical therapy (surgery, radiotherapy) recurrence | |||
| 47 | What are the types and definitions of post-RP recurrence? Is regular PSA monitoring recommended for the purpose of early discovery of post-RP recurrence? | Types of post-RP recurrence include localized recurrence and distant metastasis, that is advanced cancer. PSA level monitoring is the most important method for the early discovery of recurrence. The PSA cut-off level for biochemical recurrence is 0.2 ng/mL. | B |
| 48 | Is SRT recommended for post-RP recurrence? | SRT is an effective treatment option for post-RP biochemical recurrence. It should be started at a PSA level of <0.5 ng/mL. | B |
| 49 | What is the definition of post-radical radiotherapy recurrence? | The definition of post-radical radiotherapy biochemical recurrence is a minimum PSA level of >2.0 ng/mL. | A |
| 50 | What salvage therapy is recommended for post-radical radiotherapy recurrence? | For biochemical recurrence (elevation of PSA), AS or hormone therapy are recommended (RG: C1). For localized recurrence, in addition to AS and hormone therapy, radical localized salvage therapy (RP, cryotherapy, interstitial irradiation, or HIFU) are recommended (RG: C1). For clinical recurrence of distant metastasis, hormone therapy is recommended (RG: A). |
C1 C1 A |
| Hormone therapy | |||
| 51 | Is CAB therapy as primary hormone therapy superior to castration monotherapy in cases of metastatic prostate cancer? | Compared with castration monotherapy, CAB therapy using non-steroidal anti-androgen drugs as primary hormone therapy is more effective and has the similar or allowable adverse event profiles, QOL and costs. CAB is recommended in Japan as one of the standard therapies. However, it is necessary to keep in mind the fact that the superiority of CAB therapy in cases of metastatic prostate cancer has not been clearly proven. | B |
| 52 | Is an LH-RH antagonist recommended as primary hormone therapy? | An LH-RH antagonist (degarelix) is recommended as primary hormone therapy for prostate cancer. | B |
| 53 | Is intermittent hormone therapy recommended as primary hormone therapy for metastatic prostate cancer? | Intermittent hormone therapy yields the same overall survival as continuous hormone therapy. When adverse events, QOL and costs are considered, it is the preferred alternative to continuous hormone therapy. However, it must be kept in mind that the optimum protocol and which patients are likely to benefit have not yet been identified. | C1 |
| 54 | Is combined use of docetaxel chemotherapy with initial hormone therapy recommended in cases of metastatic prostate cancer? | A large-scale clinical trial carried out overseas reported that the use of docetaxel chemotherapy in combination with initial hormone therapy improved the prognosis of patients with metastatic prostate cancer. However, consideration must be given to adverse events when selecting Japanese patients for this treatment. | B |
| 55 | Can an improved prognosis be expected from the use of hormone monotherapy in cases of localized prostate cancer in which radical therapy is not indicated? | The usefulness of primary hormone therapy in cases for which radical therapy not indicated has been investigated in studies conducted around the world. Based on their data, there seems to be little effect on long-term survival or disease-specific survival. However, as the circumstances under which hormone therapy is used in Japan and other countries differ, there is no clear consensus on this situation in Japan. In elderly patients for whom radical therapy is not indicated, therapeutic effectiveness can be expected as long as patients are carefully selected after evaluating the risk of adverse effects. | C1 |
| 56 | What adverse events are associated with hormone therapy and what are the countermeasures? | The adverse events associated with hormone therapy are decreased bone mass and increased risk of bone fracture. Combined use of injected or oral bisphosphonates or anti-RANKL antibody prevents decreases in bone mass and lowers the risk of fractures (RG: B). The adverse events caused by hormone therapy lead to decreased QOL during treatment. Therefore, appropriate measures are recommended for patients who complain of QOL-related problems. Hormone therapy might lead to abnormalities of sugar and fat metabolism, and thus might contribute to cardiovascular disease. Although there is no clear evidence that hormone therapy increases the risk of death in patients with cardiovascular disease, careful therapeutic intervention after appropriate testing is recommended (RG: C1). |
B C1 |
| Castration-resistant prostate cancer (novel hormone agents, chemotherapy drugs) | |||
| 57 | Is docetaxel recommended for the treatment of CRPC? What are the optimum dose and adverse events that require attention? | Docetaxel in doses of 70–75 mg/m2 every 3 weeks + prednisolone 10 mg continuously is the recommended treatment for metastatic castration-resistant prostate cancer. Adverse events include hematological conditions, such as neutropenia and anemia, and non-hematological conditions, such as hair loss, loss of appetite, general malaise, peripheral nerve damage, changes in finger and toe nails, dysgeusia, and edema. Adverse events are dose-dependent, and therefore careful observation is required. | A |
| 58 | Is enzalutamide recommended for the treatment of castration-resistant prostate cancer? What adverse events are associated with the drug? | In comparison with a placebo control, enzalutamide provided significantly better overall survival in patients previously treated with docetaxel. It also significantly extended the period until worsening was detected on imaging in patients not yet on docetaxel, as well as their overall survival rate. Therefore, it is recommended for treatment of CRPC. Adverse events include fatigue, loss of appetite and weakness, although most are grade 1–2, which are relatively safe. However, in rare cases, severe adverse events, such as thrombocytopenia and seizures, can occur. Careful observation is required during the first 4 weeks of treatment. | A |
| 59 | Is abiraterone recommended for the treatment of castration-resistant prostate cancer? What adverse events are associated with the drug? | For metastatic CRPC, combined therapy with abiraterone + prednisolone either before or after chemotherapy is effective in extending overall survival and progression-free survival on imaging, and therefore it is recommended. Adverse events that require attention include liver dysfunction, fluid retention and cardiovascular damage. | A |
| 60 | Is cabazitaxel recommended for the treatment of post-docetaxel recurrence of CRPC? What are the optimum dose and adverse events associated with cabazitaxel? | Cabazitaxel in a dose of 25 mg/m2 every 3 weeks for the treatment of post-docetaxel recurrence of CRPC extends overall survival and is therefore recommended. The dose can be adjusted depending on the patient's comorbidities, age and adverse events. Adverse events associated with cabazitaxel include hematological conditions, such as neutropenia. In order to prevent febrile neutropenia, G-CSF is recommended as a primary prophylactic for patients with risk factors. Non-hematological conditions include diarrhea, liver dysfunction and interstitial pneumonia. Cabazitaxel administration should be started only after preparing countermeasures for these adverse events. | A |
| 61 | What assessment methods are recommended (biomarkers, diagnostic imaging, etc) in order to determine when to start administration of docetaxel, cabazitaxel or novel androgen receptor signal inhibitors (enzalutamide, abiraterone), and evaluate the effectiveness of these treatments? | There are no assessment methods (biomarkers, diagnostic imaging, etc) that can be used to determine when to start administration of docetaxel, cabazitaxel or novel androgen receptor signal inhibitors (enzalutamide, abiraterone) or to evaluate the effectiveness of these treatments when used in cases of CRPC. Currently, PSA levels, blood markers, general imaging findings and the patient's condition (general condition, pain, metastasis to other organs, etc) are used to make an individualized determination in each case. In the future, we look forward to the development of novel biomarkers and imaging methods. | C1 |
| 62 | What is the optimum order of successive treatments for CRPC? | There is no evidence-based information defining the order of successive treatments for CRPC. Successive treatments should be considered based on the patient's situation (symptoms, metastasis, general condition), adverse effects and drug tolerability. | C1 |
| Treatment of bone metastasis (bone targeted therapy, bone health) | |||
| 63 | What diagnostic imaging modalities are recommended for assessing prostate cancer metastasis to bones? | Bone scintigraphy, 18F-FDG-PET (including PET/CT) and MRI are effective in the diagnosis of bone metastases. | B |
| 64 | What monitoring methods other than imaging are recommended for prostate cancer bone metastases? | Chronological changes in bone metabolism markers are effective for monitoring bone metastases as a supplement to imaging. | C1 |
| 65 | When is the use of BMAs for prostate cancer bone metastases recommended? | BMAs are strongly recommended in cases of CRPC with bone metastasis (RG: B), but there is debate regarding their use in cases of hormone therapy-sensitive prostate cancer (RG: C2). |
B C2 |
| Cancer emergencies, palliative care | |||
| 66 | How is the pain associated with prostate cancer bone metastases managed? | The types and doses of analgesics used are determined in accordance with the basic three-step approach to pain therapy advocated by the World Health Organization. When the sites of metastases are localized, external irradiation is effective. | A |
| 67 | How is spinal cord compression due to prostate cancer spinal metastases handled? | Steroid administration is immediately started. If the neurological deficits are still reversible, radiotherapy or surgery (laminectomy) is immediately performed. | A |
| 68 | What palliative radiotherapy is recommended for hematuria caused by advanced prostate cancer? | Palliative radiotherapy is effective for cases of advanced hematuria that causes blockages. | C1 |
| 69 | Is palliative TURP recommended for dysuria caused by advanced prostate cancer? | Palliative TURP is one treatment option for dysuria in patients with advanced prostate cancer. | C1 |
| 70 | Is percutaneous nephrostomy recommended to relieve renal failure caused by hydronephrosis in cases of advanced prostate cancer? | In cases in which untreated prostate cancer has caused obstructive renal dysfunction due to ureteral compression, ultrasound-guided percutaneous nephrostomy is performed. | C1 |
Epidemiology
The number of prostate cancer cases worldwide in 2012 was approximately 1.1 million, making it the second most common cancer among men (14.8%). The age-adjusted prevalence rate was 30.7 per 100 000 (world population base), making it the second most common type of cancer globally.2 In Japan, there were 78 728 men with prostate cancer in 2011, and the age-adjusted prevalence rate of 66.8 (1985 population model) placed it as the third most common cancer among men. The number of deaths in 2014 was 11 507, and the age-adjusted mortality rate was 7.3 per 100 000, making it the ninth most common cause of death. After a peak of 8.6 in 2000, the rate started gradually trending downward.3 Data for races show that the lifetime prevalence is 1 in 13 for Asians, 1 in 8 for white people and 1 in 4 for black people.4 The frequency of latent cancer is 19.9%, 26.7% and 26.2%, respectively, showing that the differences between races are not as large as those for clinically apparent prostate cancers.5
A family history of prostate cancer increases the risk by approximately two- to sixfold, thus suggesting the involvement of genetic factors.6 However, although genome-wide association studies have identified approximately 60 genetic loci including the 8q24 region that are related to the development of prostate cancer, the odds ratio of each is under 1.5, indicating low penetration.7 Acquired factors are thought to include lifestyle, metabolic syndrome, inflammation and infection of the prostate gland, lower urinary tract symptoms, environmental factors, and exposure to chemical substances.
The natural history of prostate cancer differs between latent and clinical cancers, and before and after the introduction of the PSA test. Although prostate cancer generally progresses gradually, clinically diagnosed prostate cancers can be fatal.8
Prevention (chemoprevention)
Efforts to prevent (and implement chemoprevention for) prostate cancer include lifestyle, functional foods and 5-alpha-reductase inhibitors or other chemopreventive agents. Studies of diet have reported that the intake of dairy products, calcium and fats increase the risk of prostate cancer. In contrast, docosahexaenoic acid and eicosapentaenoic acid, which are found in large amounts in fish, have been reported to decrease the risk of prostate cancer.9 However, as conflicting reports also exist, there is no consensus at present.10 Recently, a link between obesity, metabolic syndrome and prostate cancer was identified, suggesting that lifestyle changes might be effective in preventing the disease, among which diet could play an especially important role.11 Previous research has focused the preventive effects of isoflavones in soybeans,12 catechin in green tea,13 lycopene in tomatoes14 and other functional factors. However, the effects of functional factors, such as selenium and vitamin D, found in seafood, or of vitamin E, found in nuts and fish roe, remains unclear.15 As studies have yet to make definitive conclusions on the efficacy of functional factors, further research into this issue is required.
Chemopreventive drugs, such as 5-alpha-reductase inhibitors, have been shown in large-scale randomized studies to significantly reduce the incidence of prostate cancer. However, the possibility that they increase the risk of higher-grade lesions cannot be completely ruled out.16, 17 At present, they do not seem to have a significant effect on survival. Other chemopreventive drugs that are being investigated include aspirin, statins and metformin, but as there is wide variation in the reported effects of these drugs, their effectiveness currently remains unclear.18
Screening
PSA-based prostate cancer screening has been verified by the European Randomized Study of Screening for Prostate Cancer to be effective in reducing the mortality rate of the disease.19 A randomized study carried out in Gothenburg, Sweden, invited men assigned to the screening arm to PSA screening every 2 years. In that study, PSA screening resulted in a 44% decrease in the mortality rate during a median follow up of 14 years.20 A study carried out in the Tyrol region of Austria over the course of a 20-year follow-up period found that when the exposure rate to PSA screening increased to 86.6%, and the actual mortality rate decreased to 64% of the estimated rate.21
In Japan, prostate cancer screening was adopted in 83.0% of municipalities according to a 2015 annual survey carried out by the Japanese Foundation for Prostate Research.22 A survey carried out by the Japanese Foundation for Prostate Research and the Japan Society of Ningen Dock in 2005 found that 88.9% of health screening centers adopted PSA testing as an optional examination.23 Although opportunities to undergo screening are gradually increasing, approximately 10% of prostate cancer patients already have bone metastasis at diagnosis.24 Thus, the exposure rate to PSA screening is expected to remain low. It is important to increase the opportunities to undergo PSA screening, provide appropriate information regarding the disease and the screening process, and establish a more accurate screening system, as recommended by this guideline with the PSA cut-off levels at 4.0 ng/mL for all ages or age-specific PSA cut-off levels.25 It is also important to stratify the risk for morbidity and mortality according to baseline PSA levels. We therefore recommend that individuals undergo PSA screening every 3 years if their baseline level is ≤1.0 ng/mL, or every year if their PSA level is between 1.1 ng/mL and the cut-off level.26-28
The most important benefit of PSA screening has been shown to be a decrease in the mortality rate. However, overdiagnosis, overtreatment and decreased QOL from the treatment are all potential drawbacks of screening. These drawbacks can be reduced by implementing AS, use of less invasive therapies to maintain good QOL and the clinical use of new biomarkers. These treatment modalities will help increase the benefits of screening. It is important to establish the optimum system for those who want to undergo prostate cancer screening based on an accurate understanding of both the benefits and drawbacks of screening.
Disease stages, risk classifications, nomograms
After the histopathological diagnosis of prostate cancer, it is important to determine a clinical stage by a digital rectal examination and the imaging. The most widely used classification is the 2009 UICC classification (7th edn).29 In Japan, the Fourth Edition of the Prostate Cancer Guidelines adapted the UICC classifications.30 In addition, other risk classifications are available to estimate the recurrence rate and the prognosis. The risk classification for prostate cancer is useful for predicting the outcome (recurrence rate) after curative treatment. The D'Amico classification1 and the NCCN classification31 are based on three factors (clinical T stage, PSA level and Gleason score), and they are widely used, particularly for localized prostate cancer. However, the intermediate- and high-risk groups in these classifications include patients with a wide variety of prognoses.32, 33 In addition, as these classification schemes are based on data from patients outside Japan, there remains a possibility that they are not applicable to Japanese patients. Therefore, caution is required when using these classifications.
The nomogram is a mathematical model that estimates the outcome of therapy and the prognosis for individual patients based on more factors than are used in the risk classifications. In recent years, a variety of nomograms have been developed in many fields – including prostate cancer – to predict various end-points. Nomogram is currently the most accurate evidence-based prediction tool, and is recommended for use in daily practice. In 1993, Partin et al. created a flagship nomogram to predict the pathological stages after RP, and this Partin Table is now widely used by urologists.34 In Japan, the Japan PC Table was developed using a Japanese cohort based on the same concept as the Partin Table.35 In the use of nomograms, the physicians need to pay attention to the background data used to determine the nomogram, including a variety of examinations, classifications and treatment modalities, which could be different from the latest ones. In future, more accurate and comprehensive new biomarkers will be developed, based on genetic changes36 and assessment of the patients’ physical condition (age, complications, cognitive function, etc).37
Diagnostic methods (markers, imaging, biopsy)
Diagnosis of prostate cancer is made in three steps: PSA screening indicates the possibility of cancer, the definitive diagnosis is made by biopsy and disease staging is carried out with diagnostic imaging.
Although the importance of PSA measurement as a screening test has been established, the specificity of this test is still not satisfactory.38 As a result, attempts at improving the specificity have been made, so as to reduce the number of unnecessary biopsies. One representative attempt was the introduction of PSA-related parameters, such as PSAD.39 The effectiveness of assessments that combine these with rectal examination and transrectal ultrasound findings, as well as with nomograms, has been shown, as they allow a more comprehensive analysis of the risk of prostate cancer.40
The standard and most widely used method of carrying out prostate biopsies is the six-location biopsy.41 As a result of a variety of improvements in the procedure, biopsies from a total of 10–12 locations, including four to six locations in the peripheral zone in addition to the standard six locations, are now being recommended.42 The fact that cancer can be detected with a high degree of accuracy by carrying out a biopsy on the apex and central zone in patients in which the rectal examination was negative suggests that it would be meaningful to re-biopsy such cases in multiple locations, including the abovementioned apex and central zone.43 There are two biopsy approaches – transrectal and transperineal. Although both have approximately the same detection rates,44 the transrectal has a higher risk of severe infection.45 Nevertheless, because the transperineal method requires anesthesia, a comprehensive evaluation of the relative risks and benefits that includes consideration of the time required for each approach, as well as the cost, should be made.
Staging has a major effect on determining the treatment strategy for prostate cancer. MRI is highly reliable in the evaluation of tumor (“T”)-staging. The importance of multiparametric MRI, including T2-weighted imaging, dynamic contrast-enhanced study and diffusion-weighted imaging, is now appreciated.46 In contrast, there are few breakthrough advances in the evaluation of metastatic disease. Lymph node dissection remains the most reliable method of assessing lymph node (“N”)-staging, as the ability of CT and MRI to diagnose lymph node metastasis is unsatisfactory.47 In the evaluation of distant metastases (“M”-staging), 99mTc-bone scans has been applied for detecting bone metastasis, whereas extraskeletal metastases are assessed with either CT or MRI.48
Pathology
Diagnosis of prostate cancer requires a prostate biopsy and a pathological diagnosis of the presence of cancer cells in the specimen. The histopathological grading is made most commonly using the Gleason score, a system that is unique to prostate cancer.49 It is based on classification of histological patterns labeled 1 through 5. However, with the increasingly widespread use of PSA screening, the classic Gleason score is no longer appropriate in evaluating localized tumors and has therefore been revised.50, 51 For example, even after repeated revisions, it became clear that problems with the Gleason score remained, as shown by recent clinical research and studies in molecular biology. One problem is the fact that needle tissue biopsies rarely produce results showing Gleason patterns 1 or 2, so that in actual clinical practice, a Gleason score of 2–5 is almost never encountered. Although Gleason score 7, 3 + 4 and 4 + 3, is a heterologous disease entity, it has been rarely reported, separately. For this reason, the Gleason score underwent a major revision in 2015 based on the use of grade groups.51, 52 Also, in cases of prostate cancer with considerable heterogeneity, the Gleason score alone is insufficient for determining which is the index tumor that should be the therapeutic target.53, 54 Highly malignant tissue might be present in the background tissue. The importance of IDC-P, which it thought to predict a poor prognosis,55 and the diagnostic significance of genetic changes, such as a fusion gene,56, 57 in prostate cancer going forward are garnering attention.
Active surveillance
AS is an option for patients for whom it has been determined that not starting treatment immediately will not adversely affect their prognosis. They are closely monitored with periodic testing, and radical therapy is then started if the cancer appears to be progressing.
There are two major problems with the current AS program. One is the uncertainty of inclusion criteria. The other is the reliability of follow-up protocol. The criteria for inclusion commonly in use are a PSA level of ≤10 ng/mL, clinical stage of ≤T2, two or fewer positive biopsy cores (exclude in case of targeted biopsies or saturation biopsies), Gleason score of ≤6 and a PSAD of 0.2 or 0.15 ng/mL or lower. Rendering the protocol more stringent will lead to an increase in the number of patients who would lose the opportunity to undergo AS. In contrast, there is a life-threatening risk with less stringent criteria. Currently, there are several prospective studies of AS under way, including the PRIAS study.58, 59 The results of these studies are expected to reflect an amendment of the inclusion criteria for AS, which will be shifting to less stringent one.
Of the indices used to monitor the worsening of prostate cancer during AS, PSA kinetics, such as PSA-DT and PSA-V, were initially viewed as important triggers for initiation of aggressive treatment. However, the reliability of these indicators is becoming lower, because PSA is subject to irregular variations, such as with chronic inflammation of the gland in early prostate cancer.60 The methods used for monitoring during AS are rectal examinations and PSA tests every 3–6 months, as well as prostate biopsy every 1–3 years. The standard for starting treatment is an increase in the Gleason score or in the number of positive cores on biopsy; that is, pathological reclassification, and a determination that the clinical stage is advancing. Partly because of the low reliability of PSA kinetics, re-biopsy (every 1–3 years) is considered indispensable for detecting pathological reclassification. Frequent biopsy during AS, however, is stressful and substantial patients reject re-biopsy. Prospective studies are now designed to clarify whether the multiparametric MRI can be an alternative to detect pathological reclassification and disease progression during AS.
Concerns regarding AS include its effect on QOL, mainly in terms of long-term safety and psychological issues. Several large-scale trials (PRIAS, University of Toronto, Johns Hopkins University, etc) are identifying data related to long-term safety. If these trials show that the overall risk is low, this would suggest that there are no problems with long-term safety.61-63 No results from long-term longitudinal studies of the effect on QOL after AS are currently available, but many short-term studies of 6 months to 1 year have shown that, in general, QOL is not adversely affected.64, 65 A systematic review of cross-sectional and cohort studies showed that the health-related QOL of patients undergoing AS was satisfactory.66 However, as that review did not include randomized studies, the effects of selection bias and the long-term outcomes of studies including Japanese patients remain unclear. Further investigation of these issues is required.
Radical prostatectomy
RP is the only radical therapy that has been shown to improve overall survival and cancer-specific survival in randomized controlled trials compared with watchful waiting. RP is recommended for patients in whom expected survival is ≥10 years and who have low- to intermediate-risk localized cancer. For patients with high-risk localized cancer, RP is one option.67, 68 Preoperative diagnosis of lymph node metastasis by CT and MRI is insufficient. Lymph node dissection is the most reliable diagnostic method. Because of its diagnostic and therapeutic significance, extended lymph node dissection should be carried out in all cases unless there is a low risk of positive nodes.69 Limited node dissection is not recommended.
Preoperative factors that contribute to postoperative urinary incontinence include age, body mass index, comorbidities, erectile function and pelvic floor muscle anatomy.70 Nerve preservation, as well as preservation of the urethra sphincter muscle, is effective in maintaining urinary continence.71 Techniques designed to preserve the neurovascular bundle can be expected to aid in recovery of sexual function. The administration of PDE5 inhibitor after nerve-preserving surgery is effective in treating postoperative erectile dysfunction.72 However, there is still no consensus on the prophylactic use, either continuously or on demand, of PDE5 inhibitors to prevent erectile dysfunction.
RARP, LRP and RRP have nearly the same rates of positive margins and biochemical recurrence.73 Investigation of postoperative incontinence comparing RARP with RRP and LRP showed that RARP had a superior recovery rate.74 Postoperative adjuvant radiation therapy is recommended in patients with pT3N0M0 tumors and who have an expected survival of ≥15 years, particularly in cases of seminal vesicle invasion.75 Androgen deprivation therapy is recommended in cases of pN1.76
Radiotherapy (external-beam irradiation)
The following six CQs discuss radiotherapy with EBRT. (i) What is the optimum dose, dose fractionation, and radiation field for radical X-ray therapy? (ii) For which patients are proton beam and heavy ion radiotherapy recommended? (iii) Are the therapeutic outcomes of EBRT improved when combined with hormone therapy? If so, what are the optimum timing, medications and treatment duration? (iv) Is radiotherapy effective for the local progression of cancers refractory to hormone therapy? Is local radiotherapy to the primary site effective for N1 or M1 prostate cancers? (v) Does the risk of radiation-induced cancers (bladder or rectal cancer) vary by treatment method? (vi) What are the adverse events of EBRT and the measures used to deal with them?
Although advances in radiation technology have allowed higher doses, adverse events and dose-related phenomena still occur. As long-term survival has become possible, the relative risk of secondary cancers cannot be ignored. Long-term conventional hormone therapy causes loss of bone mineral, and is toxic to the cardiovascular system. This might in turn have negative effects on QOL; for example, reduced cognitive ability, depression and decreased sexual function. In the guidelines used in Western countries, hormone therapy is recommended only in cases in which it is absolutely required. Currently, as high radiation doses are possible, both the need for combined hormone therapy and the selection of ideal patients for whom it is indicated have been called into question. There is also the issue of what merits are offered by proton beam, which has better dose distribution, and heavy ion radiotherapy, which has additionally high biological effectiveness in comparison with standard X-ray therapy. Furthermore, there is the question of the biological implication of local control with irradiation in cases of castration-refractory endocrine cancer with lymph node or bone metastasis, which have conventionally been considered difficult to control. This illustrates the fact that the solution to one problem seems to lead to further clinical challenges, an apparently endless chain.
This document has been created based on a review of the latest knowledge on EBRT currently available, but attention must be paid to new evidence as it emerges, including further advances in EBRT techniques and the concomitant use of newly developed drugs.77-86
Radiotherapy (interstitial irradiation)
LDR using low radiation doses with iodine-125 seeds was introduced in Japan in 2003. It is effective and has good long-term outcomes.87 In low-risk cases, LDR is as effective as RP and EBRT, but external irradiation combined with hormone therapy is more effective for moderate- to high-risk cases.88 The therapeutic indications for LDR are being expanded. Combined treatment using LDR, EBRT and hormone therapy has shown satisfactory outcomes, particularly in high-risk cases.89
As LDR consists of radiation localized to the prostate gland, the incidence of adverse effects is low, and post-therapy QOL is good and can be expected to remain so. LDR might adversely affect QOL by causing urinary symptoms, such as urgency and frequency,90 but there are relatively few problems related to decreased QOL in terms of decreased sexual function.91
HDR for prostate cancer using high doses of iridium-192 was first used in Japan in 1994. HDR has recently been included in the USA and European guidelines, and is being aggressively used. It is often used in high-risk cases, generally in combination with external irradiation and hormone therapy; furthermore, HDR monotherapy is also being aggressively used.92 In order to reduce patient burden and increase therapeutic efficacy, the recent tendency has been to increase the amount of each dose, but reduce the total number of doses.93
As the combined use of small radioactive sources (LDR) and EBRT aims at a higher biological effective dose than monotherapy, there is concern that adverse events might increase. A prospective, multicenter study of long-term, serious late adverse events reported a larger number than expected,94 but multiple phase II trials have reported the combination to be safe.95 Recently, intensity-modulated radiation therapy and image-guided radiotherapy have been used in combination therapy. Reports show a reduction in adverse events, because the exposure of healthy organs to radiation is tightly controlled.96 Combined use of LDR and external irradiation is limited to high-risk and some moderate-risk cases. Intensity-modulated radiation therapy and image-guided radiotherapy are likely to be used in such cases as a means of reducing adverse events.
Focal therapy (cryotherapy, HIFU)
Focal therapy is a non-invasive method of treating clinically significant cancer foci within the prostate gland.97 It differs from the conventional view that the entire prostate must be treated to control the cancer. The therapeutic concept lies in the middle ground between radical therapy and AS, treating the cancer foci that are believed to have the greatest effect on the patient's prognosis, while preserving as much normal tissue as possible. It is designed to optimize adequate treatment of the cancer while preserving function. As there are some areas of the prostrate in which focal therapy cannot be used, if there is significant cancer in one of those areas, treatment might be incomplete.98 If there is recurrence or progression, more aggressive treatment might be necessary. Currently, focal therapy could be a treatment option for low-risk localized prostate cancer as diagnosed by biopsy or template-guided biopsy based on MRI findings.99 Focal therapy is also recommended to maintain QOL while providing some treatment for localized prostate cancer cases in which radical therapy is not indicated or not desired by the patient. In such cases, the patient must fully understand the limitations of the treatment. Hendez et al. indicated in their review that clinical trials of focal therapy have been increasing annually since 2006.100 The perception that focal therapy is useful in the prevention of overtreatment is becoming more widespread, and initiatives designed to resolve limitations, including the development of new medical devices, is progressing.
Salvage therapy: Treatment of recurrence after radical therapy (surgery, radiotherapy)
Regular monitoring of PSA levels is the most important method for the early detection of recurrence after RP. A PSA >0.2 g/mL is considered to show biochemical recurrence. However, higher cut-off values of 0.4 or 0.5 ng/mL, which have a better correlation with the onset of metastasis, might be reasonable because clinical recurrence is not apparent in some cases after biochemical recurrence is diagnosed.101 Waiting until that level is reached produces results that do not differ from adjuvant radiation therapy begun earlier.102 Therefore, secondary therapy is begun in many cases once PSA levels of 0.4–0.5 ng/mL are reached.
In cases of biochemical recurrence, comprehensive diagnosis of whether foci are localized or in distant organs is carried out based on PSA levels and PSA-DT measured preoperatively or at the time of biochemical recurrence. Additional points to consider include the Gleason score and operative results, including whether the margins were positive or if seminal vesicle invasion or lymph node metastases were found.103 SRT for biochemical recurrence is an effective treatment option, and it is advisable to start treatment when PSA is <0.5 ng/mL. If there is only a single focus of local recurrence, a second cure might be anticipated with SRT. In cases in which SRT is not expected to be effective or cases in which decreases in the PSA level are not observed after SRT, salvage hormone therapy is considered.
The widely used Phoenix definition of biochemical recurrence after radical radiotherapy is post-treatment levels rising at least 2.0 ng/mL over the minimum PSA level.104 However, as approximately 5% of patients experience a PSA bounce after radical radiotherapy, careful follow up is required. Diagnostic methods for local recurrence include MRI and prostate biopsy, but there is no consensus on the issue at present.
For biochemical recurrence, either continuing close observation or instituting hormone therapy are options. Many studies have reported that starting salvage hormone therapy when PSA levels are <10 ng/mL extends overall survival.105 However, continued observation is preferred in those who do not have the following risk factors: (i) the biopsy Gleason score is 8–10; (ii) the clinical stage was T3b–4 at diagnosis; (iii) PSA-DT at the time of biochemical recurrence is >3 months; and (iv) the length of time from treatment to recurrence is >3 years.106 For local recurrence, in addition to continued observation and hormone therapy, salvage local therapies including RP, cryotherapy, interstitial irradiation or HIFU therapy can be options. At present, none of these salvage local therapies is carried out widely in the clinical setting. For clinical recurrence with distant metastasis, hormone therapy is recommended, as in most cases of advanced prostate cancer.
Hormone therapy
CAB as primary hormone therapy for metastatic prostate cancer: CAB, which uses bicalutamide for advanced prostate cancer, is more effective than castration monotherapy, and leads to either the same level of adverse events, QOL and costs as castration therapy, or is at least within an acceptable range.107 Retrospective analysis using the Japan Study Group of Prostate Cancer database has also shown that CAB is effective in cases of metastatic prostate cancer.108 However, the Advanced Prostate Cancer Consensus Conference did not reach a consensus among specialists regarding whether or not CAB therapy should be used as a primary hormone therapy.109
An LH-RH antagonist as primary hormone therapy: Degarelix, an LH-RH antagonist, has been shown to significantly improve overall survival and the biochemical recurrence-free rate compared with LH-RH agonists.110 It reduces the frequency of adverse events in the joints, musculoskeletal system and urinary tract.110 In patients with a history of heart disease, it might cut the number of cardiovascular events in half.111
Intermittent hormone therapy as a primary hormone therapy for metastatic prostate cancer: The SWOG9346 trial did not prove the non-inferiority of overall survival with intermittent hormone therapy as compared with continuous hormone therapy.112 However, a recently carried out meta-analysis concluded that intermittent hormone therapy could become a substitute for continuous hormone therapy.113
Primary hormone therapy combined with docetaxel for metastatic prostate cancer: According to a meta-analysis of three clinical trials carried out in Europe and the USA, standard hormone therapy combined with docetaxel was shown to be more effective than standard monotherapy.114 However, the use of docetaxel as an initial therapy is not currently covered by insurance in Japan.
Hormone monotherapy for localized prostate cancer: A retrospective analysis using the Japan Study Group of Prostate Cancer database showed that the expected survival with hormone monotherapy for locally advanced or localized prostate cancer is the same as for the general population.115 However, an analysis carried out overseas did not show this effect.116 In elderly patients for whom radical therapy is not indicated, hormone monotherapy can be expected to be an effective treatment in carefully selected cases with assessment of the risk of adverse drug effects.
Adverse events associated with hormone therapy: Adverse events, such as sexual dysfunction, hot flashes, fatigue, gynecomastia and mastalgia, have long been known. Tamoxifen is effective in treating gynecomastia and mastalgia, but it is not covered by insurance in Japan.117 Bisphosphonates118 and anti-RANKL antibody119 are effective in the prevention of osteoporosis and fractures during hormone therapy, but they are not covered by insurance in Japan when used prophylactically. It is not clear if therapeutic exercise is effective in addressing abnormalities of sugar and fat metabolism induced by hormone therapy.120 In addition, opinions are still divided over whether hormone therapy has an effect on cardiovascular events and cognitive function.
Castration-resistant prostate cancer (novel hormones and chemotherapies)
In Japan, treatment for CRPC is undergoing major changes as a result of the availability since 2014 of enzalutamide, a novel androgen receptor signal inhibitor, abiraterone (androgen synthetic inhibitor) and cabazitaxel, a novel taxane-type anticancer drug. Before 2014 the question was “How long can docetaxel be used?” With the newer agents, questions that have arisen are “Which drug should be used before and after docetexel?”
Initial large-scale phase III clinical trials of these three novel drugs were carried out in patients with CRPC who became resistant to docetaxel.121-123 The results of subsequent clinical trials of enzalutamide and abiraterone administered before docetaxel led to expansion of the indications for these drugs, and their use before docetaxel administration is becoming widespread.124, 125 The evidence for the efficacy of the three novel drugs is grade A, based on phase III trials carried out around the world, and on phase I and II trials in Japan. Cabazitaxel is recommended only for use after docetaxel. For each of these drugs, the grounds for efficacy and the side-effects caused in Japanese patients, which was not previously focused on, are now garnering attention. Specifically, the adverse effects of enzalutamide include fatigue and thrombocytopenia, those of abiraterone include liver dysfunction and fluid retention, and those of cabazitaxel include febrile neutropenia and interstitial pneumonia. When cabazitaxel is used, prophylactic administration of sustained G-CSF (peg-filgrastim) is recommended.
Worsening of metastatic foci or the appearance of new lesions on diagnostic imaging independent of PSA levels have been added to the definition of CRPC.126-128 This was done because prostate cancer progression cannot be ascertained based on PSA changes alone. There might be heterogeneity, in which androgen-dependent and -independent cancers are both present.129, 130 One attempt to solve the problem of heterogeneity is the search for biomarkers. As this links basic research with the clinical setting, it has the potential to change clinical practice in the near future. A focus has been placed on methods of assessment. The detection of circulating tumor cells and cell-free DNA, the concept of a liquid biopsy, is an attempt at real-time pathology assessment of constantly changing cancers.131 AR-V7, a splice variant of the androgen receptor, is being investigated as a biomarker for treatment resistance, but it is a long way from becoming accepted for the clinical setting.132, 133
As treatment options increase, the possibility of successive or combined therapy understandably becomes a focus of discussion. Problems associated with combination therapy include adverse drug effects and costs. Their ability to cure or to prolong survival must be shown to outweigh the drawbacks before they can be considered standard treatment. There is still no evidence supporting the use of sequential therapy for CRPC. Taking into account the heterogeneity of prostate cancer, we would like to emphasize the need to consider how to use medications based on specific tumor characteristics. The discovery of simple, clinically useful biomarkers that will help in deciding on treatment is highly anticipated.
Bone metastasis therapies (bone targeted therapy, bone health)
In many cases, prostate cancer bone metastasis is detected with bone scintigraphy.134 Meta-analyses have reported that bone scintigraphy has a sensitivity of 86% and a specificity of 81% for bone metastasis. Recently, it has been reported that 18F-FDG-PET is highly sensitive and highly specific for this purpose.135 In addition, it is now possible to carry out full-body MRI to prevent radiation exposure.136-138
To monitor the condition during treatment in cases of metastases, it is important to carry out regular imaging in addition to monitoring of changes in PSA levels.139 In general, it is thought that carrying out imaging every 3–6 months is effective.140, 141 The bone scan index used when carrying out bone scintigraphy is viewed as a useful periodic marker.142
In addition to imaging studies, bone metabolism markers are an effective way to monitor bone metastasis. Serum type I C-telopeptide, procollagen type I N-terminal propeptide and bone alkaline phosphatase are the main markers used.143-145
Treatment of prostate cancer bone metastases includes general and local treatment designed to alleviate pain and improve the patient's QOL. Medical treatment consists of zoledronic acid, denosumab and other BMAs that target osteoclasts, and the inhibitory effects of these drugs on bone-related events in cases of CRPC have been verified.146, 147 Local therapy consists of external irradiation, surgery and cryotherapy. Meta-analyses have shown that external irradiation alleviates pain associated with bone metastases in 59–73% of cases, and completely relieves pain in 23–34%. Studies of therapies using radioactive isotopes have shown that strontium 89 is effective in alleviating pain.148-150 Radium 223, which was recently approved by the Japanese government, has been reported to extend overall survival of patients with CRPC patients.151
Oncological emergencies and palliation
Palliative care of prostate cancer includes addressing the following: (i) pain from bone metastases; (ii) spinal paralysis as a result of spinal metastasis; (iii) hematuria; (iv) lower urinary tract obstruction; and (v) renal failure associated with obstructive uropathy.
Countermeasures for pain: The three-step approach for pain therapy advocated by the World Health Organization is in wide use.152 External irradiation is useful when the bone metastases are localized. Single-dose irradiation and fractionated irradiation are often compared, but a systematic review carried out in 2014 recommended single-dose irradiation.153
Spinal paralysis: If neurological deficits due to spinal metastases are left untreated, the result can be irreversible paralysis and neurogenic bladder; thus, immediate measures are required. Steroid administration is useful as an adjunct therapy along with radiation or surgery.154 Radiotherapy often consists of 30 Gy/10 fractions.
Countermeasures for hematuria: Palliative radiotherapy for advanced hematuria has been reported to be effective.155, 156 A systematic review carried out in 2014 reported that palliative pelvic irradiation resulted in a 73% rate of improvement of hematuria.157
Countermeasures for lower urinary tract obstruction: TURP for patients with advanced prostate cancer who have dysuria is useful in the improvement of symptoms. Although it more frequently results in postoperative recurrence of urinary retention and the need for repeat surgery than TURP for prostate enlargement, its effects in improving symptoms are adequate.158
Countermeasures for hydronephrosis: In cases of ureteral compression by prostate cancer, with resulting hydronephrosis causing decreased renal function, ultrasound-guided percutaneous nephrostomy should be considered the first-line therapy to treat the hydronephrosis and improve renal function. A ureteral stent is highly convenient, but restenosis often recurs.159
Conflict of interest
None declared.




