How I investigate dysgranulopoiesis
Abstract
Dysgranulopoiesis is a condition in which granulocytic production is defective and is most often described in neoplastic conditions. However, it can also be frequently seen in non-neoplastic conditions. Early suspicion and detection of these non-neoplastic causes may prevent further invasive and expensive interventions. In this review, we take a look at the various causes of dysgranulopoiesis with an emphasis on non-neoplastic etiologies, followed by a detailed outline of the laboratory approach for determining its many causes.
1 INTRODUCTION
Dysgranulopoiesis has been traditionally associated with neoplastic conditions of the bone marrow with ≥10% dysgranulopoiesis being one of the criteria used to diagnose dysplasia in myelodysplastic syndrome (MDS).1 However, dysgranulopoiesis can also be seen outside the context of hematological malignancies, and it is important to be aware of the various non-neoplastic causes. In this review, we address the different causes of dysgranulopoiesis and their diagnosis with particular emphasis on non-neoplastic etiologies.
2 WHAT IS DYSGRANULOPOIEIS?
Dysgranulopoiesis is a condition in which production of granulocytes and their resulting morphologic structure is defective. Dysplasia primarily results from disturbances in maturation caused by chromosomal/genetic aberrations in hematopoietic stem cells and is one of the diagnostic features of myelodysplastic syndrome.2 However, neutrophil dysplasia/dysgranulopoiesis may also result from secondary/non-neoplastic etiologies. These causes are thought to precipitate dysplasia by causing disturbances in the marrow microenvironment affecting neutrophil maturation.3
Dysgranulopoiesis can be detected in both the peripheral blood and in the bone marrow. The morphologic features of dysgranulopoiesis seen in the peripheral blood and bone marrow include neutrophil anisocytosis, nuclear hyposegmentation (pseudo-Pelger-Huët anomaly) or hypersegmentation, reduced cytoplasmic granularity (Figure 1A-F), pseudo-Chédiak-Higashi granules, Döhle bodies and Auer rods.4 Hyposegmented neutrophils or pseudo-Pelger-Huët anomaly is commonly used to diagnose dysplasia and is characterized by “Pelger”-like cells that were described as cells having “a short and compact nucleus with mosaic-like clumping of the chromatin that is especially eye catching” originally by Pelger in the 1920s.5, 6 Huët later observed that “the nucleus consisted of two very distinct segments connected by a very thin filament” in these cells.5, 7
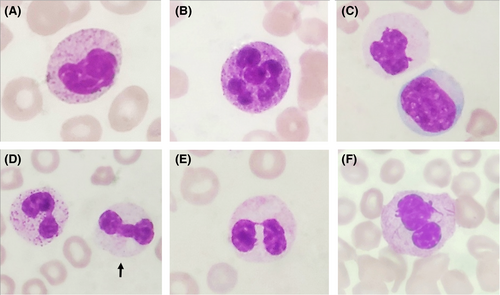
Dysgranulopoiesis is an important criterion for the diagnosis of dysplasia in both MDS and acute myeloid leukemia (AML). The WHO has proposed that ≥10% of myeloid lineage cells showing dysgranulopoiesis as one of the criteria for the diagnosis of MDS and that ≥50% of cells of at least two lineages showing dysplasia as one of the criteria for AML with myelodysplasia-related changes (AML-MRC).1, 8
As the WHO morphological criteria for diagnosing dysgranulopoiesis were only qualitative, the International Working Group on Morphology of MDS (IWGM-MDS) undertook a study to establish standardized criteria for defining morphological granulocytic dysplasia.5 They analyzed six morphologic features in bone marrow smears of myelodysplastic patients: cytoplasmic hypogranularity, Pelger-like cells, other abnormal nuclear shape, abnormally clumped chromatin, macropolycytes (neutrophils at least twice the normal size with an appropriately large nucleus),9 and nuclear projections.
The group found that the following features could be considered as dysplastic in the bone marrow: two-thirds or more reduction in cytoplasmic granules, Pelger-like neutrophils, abnormal neutrophil chromatin clumping, and macropolycytes. They also noticed a significant difference between the number of nuclear projections in healthy controls and myelodysplastic patients, with no healthy controls having any cells containing more than 4 nuclear projections.5 Apart from the IWGM-MDS, other studies have also developed morphologic scoring systems for a more objective assessment of dysplasia.10
3 CAUSES
3.1 Non-neoplastic
Dysgranulopoiesis or dysplasia in itself is not definitive evidence of a clonal process. Many non-neoplastic conditions can also exhibit significant morphologic dysplasia. It is important to distinguish between the neoplastic/clonal and non-neoplastic etiologies as MDS is preleukemic, while dysgranulopoiesis secondary to a non-neoplastic condition may be reversible if the underlying causative factor is removed.
3.1.1 Nutritional
One of the most common causes of non-clonal dysgranulopoiesis is deficiency of vitamin B12 and/or folic acid, especially in developing countries. It can be mistaken for MDS as it may cause marked pancytopenia and megaloblastoid maturational changes like nuclear-cytoplasmic dys-synchrony, large hypersegmented neutrophils (“macropolycytes”), and neutrophil anisocytosis. Megaloblastic and dysplastic changes (multinuclearity, nuclear budding, and karyorrhexis) can also be noted in the erythroid series. Estimation of vitamin B12 and folate levels should always be done before making a final diagnosis of myelodysplastic syndrome in order to exclude an easily remediable cause.11-14
Copper deficiency may cause anemia, neutropenia, and dysplastic changes like cytoplasmic vacuolation of erythroid and myeloid precursors, as well as ring sideroblasts in the bone marrow. Cytoplasmic vacuolation of myeloid precursors is uncommon in MDS and could be used to differentiate the entities. These changes can be corrected by copper supplementation; therefore, evaluation of serum copper levels should be considered in young patients with gastrointestinal disorders or neurologic deficits and also in patients with normal cytogenetics.15-18 Zinc excess may also lead to copper deficiency; in these cases, removal of the source of excess zinc should resolve the cytopenias.19, 20
3.1.2 Infections
Human immunodeficiency virus (HIV) can induce cytopenias and may cause pseudo-Pelger-Huët anomaly, coarse granulation, and degranulation of myeloid cells. Megaloblastic changes in the erythroid series as well as dysmegakaryopoiesis with nuclear hyperlobation or hypolobation may also be seen.21-23 Diagnosis and appropriate treatment with antiretroviral therapy may reverse these changes.
Parvovirus B19 (B19V) in addition to red cell aplasia may also cause trilineage bone marrow dysplasia including myeloid hypogranulation, increase in myeloid blasts and monocytosis.24, 25 Examination for giant erythroblasts with prominent intranuclear inclusions along with immunohistochemistry and anti-parvovirus B19 antibody levels can help with accurate diagnosis.24, 25
Other viral infections that may cause dyspoiesis include Hepatitis C virus (HCV) and human herpesvirus-6 (HHV6) infections. Dyserythopoiesis is more commonly seen in these infections; however, abnormal nuclear segmentation and cytoplasmic hypogranularity can also be noted in the myeloid series.26, 27 Leishmaniasis may cause dysgranulopoiesis in the form of multinucleation and pseudo-Pelger-Huët anomaly.28
3.1.3 Autoimmune disease
Various autoimmune diseases like systemic lupus erythematosus (SLE) and others may show evidence of dysgranulopoiesis.29 The dysplasia may be associated with immunosuppressive therapy or related to the underlying autoimmune process. The diagnosis of an autoimmune disorder in patients may precede development of MDS. Immune dysregulation is thought to be a common pathogenetic mechanism for both autoimmunity and MDS. Evaluation for autoimmune disorders in cases with dysgranulopoiesis might lead to a quicker diagnosis of MDS.30
VEXAS (Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic) syndrome is a late-onset treatment-refractory inflammatory syndrome with associated hematologic abnormalities. It is a newly identified condition seen in men with recurrent and inactivating acquired mutations in UBA1, a gene encoding the ubiquitin-activating enzyme-1. These patients show macrocytic anemia, hematopoietic dyspoiesis, and bone marrow vacuolization restricted to myeloid and erythroid precursor cells.31
3.1.4 Medications and toxins
A plethora of drugs can cause dysplastic changes in hematopoietic cells. Granulocyte colony-stimulating factor therapy (G-CSF/GM-CSF) causes alterations in neutrophil morphology, including a significant left-shifted myelopoiesis, marked hypergranularity, nuclear hyposegmentation and transient increase in blasts (usually <5%) in peripheral blood and sometimes in bone marrow.32 Chemotherapeutic agents may result in transient marked dysplasia of all lineages. Methotrexate and other drugs that affect deoxyribonucleotides synthesis can cause dysgranulopoiesis specifically hypersegmentation.33 Cotrimoxazole and immunosuppressants such as tacrolimus and mycophenolate mofetil can cause marked neutrophil hyposegmentation (pseudo-Pelger-Huët anomaly). This may often be indistinguishable from changes seen in MDS.34, 35 This pseudo-Pelger-Huët anomaly seen after post-transplant immunosuppressive medications may be associated with abnormal chromatin clumping and is reversible, with neutrophils resuming segmentation after dose reduction or discontinuation of the medication. Other features of dysgranulopoiesis like hypogranulation are not commonly seen.36 Exposure to heavy metals such as arsenic, lead can cause cytopenias and dysplastic changes.37 Chronic smoking is also associated with leukocytosis and right-shifted myelopoiesis (neutrophilia). Both smoking and chronic alcoholism are known to cause dysgranulopoiesis and are putative risk factors for the development of MDS.38
3.1.5 Hereditary
One of the dysplastic changes in neutrophils, that is, Pelger-Huët anomaly can be hereditary. Hereditary Pelger-Huët anomaly is an autosomal dominant condition associated with mutations in lamin B receptor (LBR) gene. Although the neutrophils are morphologically abnormal, the neutrophil function is unaffected.32, 39
3.1.6 Normal individuals
There is limited literature regarding the presence of dyspoiesis in peripheral blood and/or bone marrow of healthy individuals. A study that examined bone marrow aspirate smears of 50 healthy volunteers did not find significant morphological dyspoiesis. Dysgranulopoiesis in the form of only one Pelger-Huët like neutrophil was seen in a single patient while mild dyserythropoiesis and occasional non-lobulated megakaryocytes were also noted.40 A study by another group which examined both peripheral blood and bone marrow samples of 89 normal individuals did not note significant dyspoiesis in any of the cell lineages.41 However, another study which examined bone marrow squash slides of 120 healthy bone marrow samples found that the median proportion of cells with changes of dysgranulopoiesis was 10%, with cytoplasmic hypogranularity being the most common form of dyspoiesis (93%). They also noted a strong correlation of age and frequency of dysgranulopoiesis with younger donors showing more frequent granulopoietic dysplasia compared with older donors.42
3.1.7 Pseudo-dysplasia
Ethylenediaminetetraacetic acid (EDTA) commonly used as an anticoagulant in blood collection tubes may also induce dysplasia-like changes in neutrophils. These changes become more pronounced in cells exposed to EDTA for longer durations (>2 h). It is therefore best to examine hematology samples (peripheral blood/bone marrow) collected in vacutainers as soon as possible to avoid these artifacts.43, 44 The preparation of high-quality slides is also essential for the accurate diagnosis of dysgranulopoiesis as poorly stained slides may result in misinterpretation of dysplasia, particularly when assessing neutrophil granulation.1
In summary, there are multiple non-neoplastic causes of dysplasia (Table 1) and thorough clinical history, physical examination, and laboratory investigations are necessary to eliminate them from the differential diagnosis. Many of the non-neoplastic etiologies are easier to correct (especially nutritional deficiencies) and need to be excluded before embarking on a more invasive approach to a diagnosis of MDS. This includes bone marrow examination, cytogenetics, and molecular genetics analysis which may be time-consuming and the costs involved are usually prohibitive in the resource-constrained setting of low-income countries.
| Causes | Morphology findings | Recommended testing/workup |
|---|---|---|
| Nutritional | ||
| Vitamin B12 deficiency | Nuclear-cytoplasmic dys-synchrony, hypersegmented granulocytes (“right shift”), giant myelocytes and metamyelocytes | Serum vitamin B12 levels, homocysteine and methylmalonic acid levels |
| Folic Acid deficiency | Red cell/serum folate levels, serum homocysteine | |
| Copper deficiency | Dysplasia, cytoplasmic vacuolation of myeloid & erythoid precursors, ring sideroblasts | Serum copper levels |
| Infections | ||
| Human immunodeficiency virus (HIV) | Megaloblastic changes, pseudo-Pelger-Huët anomaly, coarse granulation and degranulation of myeloid cells | HIV testing |
| Parvovirus | Myeloid hypogranulation, increase in myeloid blasts and monocytosis | Parvovirus IgG, IgM antibody levels, bone marrow smear examination for parvovirus inclusions and immunohistochemistry |
| Hepatitis C virus (HCV), Human herpesvirus-6 (HHV-6) | Dyserythropoiesis (more common), abnormal neutrophil lobation and/or cytoplasmic granulation | Testing for HCV, HHV-6 serological markers |
| Leishmaniasis | Multinucleation and pseudo-Pelger-Huët anomaly | rK39 antigen test, Antibody levels, bone marrow examination for L-D bodies |
| Autoimmune | ||
| Systemic Lupus Erythematosus (SLE), Rheumatoid Arthritis (RA) | Mild dysgranulopoiesis | Rheumatoid Factor, Autoimmune antibody profile including ANA, dsDNA, and anti-CCP |
| Drugs | ||
| G-CSF/GM-CSF | Significant left-shifted myelopoiesis,marked hypergranularity, nuclear hyposegmentation and increase in blasts | History of G-CSF/GM-CSF treatment |
|
Cotrimoxazole Tacrolimus Mycophenolate mofetil |
Neutrophil hyposegmentation | History of drug therapy |
| Toxins | ||
|
Alcohol Arsenic Smoking |
Dysgranulopoiesis Cytopenias and dyspoiesis Leucocytosis and right-shifted myelopoiesis |
History of alcohol use, smoking Serum arsenic levels |
| Hereditary | ||
| Hereditary autosomal dominant Pelger-Huët anomaly | Pelger-Huët anomaly and dysplasia | Peripheral blood smear examination of family members, Lamin B receptor (LBR) gene mutation testing |
| Pseudo-dysplasia | ||
| EDTA effect, Poor preparation of slides | Hypogranulation of myeloid series |
Examine slides as soon as possible; use of alternate anti-coagulants (heparin/citrate) Proper technique during slide preparation and staining |
3.2 Neoplastic
3.2.1 Myelodysplastic syndromes (MDS)
The myelodysplastic syndromes are a group of clonal hematopoietic stem cell diseases with dysplasia in one or more myeloid lineages along with cytopenias and recurrent genetic abnormalities. The diagnosis of MDS should preferably be considered after a thorough clinical history (including drug history), physical examination, and laboratory investigations especially nutritional levels have been assessed.1
Childhood MDS: Dysgranulopoiesis can also be seen in children and could be due to the various non-neoplastic etiologies described earlier. These need to be excluded before considering the diagnosis of childhood MDS, which is very uncommon. Refractory cytopenia of childhood (RCC) is a provisional childhood MDS entity with persistent cytopenia and dysplasia in children. Neutropenia, pseudo-Pelger-Huët nuclei, and hypogranular neutrophils may be seen in the peripheral blood. Bone marrow may show similar findings along with giant band forms and asynchronous nuclear-cytoplasmic maturation.45
3.2.2 Myeloproliferative neoplasms
Chronic myeloid leukemia (CML)
While dysgranulopoiesis is not commonly seen in chronic phase CML, it may be seen in cases of CML in accelerated phase. Studies have shown that dysgranulopoiesis in CML may correlate with chromosome 17p abnormalities.46, 47
3.2.3 Acute myeloid leukemia (AML)
Dysplastic changes in the granulocytic series may be seen in various AMLs. These changes are characteristically seen in a few AML types with recurrent genetic abnormalities. In AML with t(8;21)(q22;q22.1);(RUNX1-RUNXT1), the promyelocytes, myelocytes, and mature neutrophils show varying degrees of dysplasia in the bone marrow with abnormal nuclear segmentation and cytoplasmic abnormalities like homogenous eosinophilic agranular cytoplasm (“salmon-pink”) in neutrophils.48 AML with t(6;9)(p23;q34.1);(DEK-NUP214) may show granulocytic, megakaryocytic, or multilineage dysplasia.49
AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2);(GATA2, MECOM) typically shows dysmegakaryopoiesis with small hypolobated megakaryocytes. However, dysgranulopoiesis in the form of pseudo-Pelger-Huët anomaly can also be noted.50
Acute myeloid leukemia with myelodysplasia-related changes (AML-MRC) is an AML subtype with morphological features of myelodysplasia in ≥50% of cells in at least two hematopoietic cell lineages. These cases should have absence of prior history of cytotoxic or radiation therapy and presence of recurrent genetic abnormalities. AML-MRC have worse prognosis with lower remission rates compared to other AML subtypes; hence, identification of this entity has clinical significance.51
3.2.4 Myelodysplastic/myeloproliferative neoplasms (MDS/MPNs)
Chronic myelomonocytic leukemia (CMML)
CMML shows features of both a myeloproliferative neoplasm and myelodysplastic syndrome with both peripheral blood monocytosis and dysplasia. Dysgranulopoiesis in the form of hyposegmented neutrophils or abnormal cytoplasmic granulation is commonly seen.52
Atypical chronic myeloid leukemia BCR-ABL1 negative (aCML)
aCML is characterized principally by leucocytosis with morphologically dysplastic neutrophils and their precursors. The dysgranulopoiesis may be quite striking with nuclear abnormalities, abnormal cytoplasmic granulation, and multiple nuclear projections in neutrophils.53
Juvenile myelomonocytic leukemia (JMML)
JMML is a clonal hematopoietic disorder of childhood with proliferation of predominantly granulocytic and monocytic lineages. Dysplasia is usually minimal; however, pseudo-Pelger-Huët neutrophils and hypogranularity can be noted.54
3.2.5 Idiopathic bone marrow dysplasia of unknown significance (IDUS)
Idiopathic bone marrow dysplasia of unknown significance (IDUS) IDUS is defined as the absence of peripheral cytopenia along with dysplasia in ≥10% of neutrophilic, erythroid, and/or megakaryocytes, blast cells are <5%, criteria for MDS are not fulfilled and no MDS-related mutations are present. Both non-clonal conditions (drug-induced dysplasia, nutritional causes, toxins, autoimmune disorders, etc) and clonal disorders (hematological malignancies and clonal hematopoiesis of indeterminate potential) need to be excluded before a diagnosis of IDUS is established.55
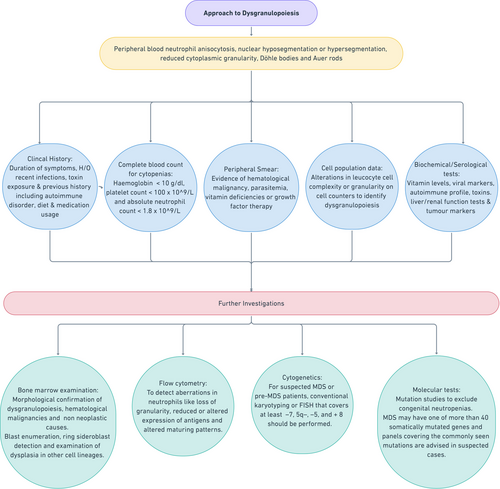
4 APPROACHING A CASE WITH DYSGRANULOPOIESIS
(i) Clinical History and Examination: When investigating a patient with dysgranulopoiesis, clinical history should include age of the patient, details of symptoms and their duration, history of recent infections, history of exposure to toxins (eg, arsenic, alcohol, and smoking), and previous medical history including presence of any autoimmune disorder, dietary habits, or medication usage. A thorough clinical examination should be performed to detect signs of any of the non-neoplastic causes of dysgranulopoiesis (eg, macroglossia in vitamin B12 deficiency, nail and skin changes in chronic arsenic poisoning and alcoholism).
(ii) Complete blood count (CBC): Complete blood count is usually among the first line of investigations carried out in the hematology laboratory to detect the presence of cytopenias. For the diagnosis of MDS, the International Prognostic Scoring System (IPSS) for risk stratification has defined cytopenias as hemoglobin concentration <10 g/dL, platelet count <100 × 109/L, and absolute neutrophil count <1.8 × 109/L.56
(iii) Peripheral smear: The CBC is followed by a peripheral blood smear examination which may reveal various morphological evidence of dysgranulopoiesis as discussed earlier. In addition, it may provide clues to other causes of dysplasia, eg. parasitemia, vitamin deficiencies (macropolycytes, macroovalocytes), or growth factor therapy (granulocytic left-shift with increased coarse cytoplasmic granules).
(iv) Cell population data (CPD): Most automated cell counters today have extended leucocyte differential panels that contain additional CPD parameters that are based on a number of leucocyte cellular characteristics like cell complexity or granularity. It is possible to use alterations in these cellular characteristics to detect dysgranulopoiesis. Various groups have proposed predictive scoring systems utilizing these parameters for the diagnosis of MDS.57-63
(v) Additional laboratory investigations to rule out secondary causes: Biochemical parameters to exclude or detect vitamin deficiency, tumor markers for neoplasms as well as serum testing for toxins, viral infections, and autoimmune conditions should be done.
(vi) Bone marrow examination: Bone marrow aspiration with biopsy can be considered in patients with persistent cytopenia(s) for at least 4 months after common non-neoplastic causes have been excluded.64 In addition to morphological detection/confirmation of dysgranulopoiesis as described earlier, bone marrow examination may also provide further clues to the etiology of the dysgranulopoiesis. Non-neoplastic causes like megaloblastic anemia, leishmaniasis, and parvovirus may be identified on bone marrow slides. It also enables blast enumeration, ring sideroblast detection, and examination of dysplasia in other cell lineages.
A minimum of 500 nucleated cells should be counted in bone marrow smears for assessment of dysplasia and blast enumeration. Additionally, at least 100 nucleated red cells should be examined for presence of ring sideroblasts (Perls’ stain). Apart from MDS, non-neoplastic conditions such as copper deficiency, zinc excess, alcoholism, and drugs (eg. isoniazid) also produce ring sideroblasts, in addition to dysgranulopoiesis.65 Bone marrow biopsy along with immunohistochemistry (CD34, CD117) can provide information regarding marrow cellularity, fibrosis, and enumeration of CD34+ progenitor cells.64
(vii) Flow cytometry: Flow cytometry can detect aberrations in neutrophils like loss of granularity, reduced or altered expression of antigens and altered maturation patterns in bone marrow precursors.66 Both peripheral blood and bone marrow samples may be analyzed. The proposed recommendations include detection of multiple flow cytometric aberrancies (at least 3) in maturation patterns on testing at least two cell lineages, as highly indicative of MDS.67, 68 Some of the most commonly encountered flow cytometric aberrancies encountered in neutrophils are listed in Table 2.69, 70 While no single parameter is said to be diagnostic for MDS, a combination of the above parameters along with other data such as the number of CD34+ cells and cellular side scatter may be used to discriminate MDS from other causes of dyspoiesis and cytopenia.67
| Common flow cytometry abnormalities in Neutrophils | |
|---|---|
| CD11b | Loss or altered expression |
| CD13 | Loss or altered expression |
| CD33 | Loss or altered expression |
| CD16 | Delayed expression |
| CD10 | Loss/reduced expression |
| CD56 | Aberrant expression |
| CD13/CD11b | Altered patterns |
| CD13/CD16 | Altered patterns |
For sample preparation, the ELN (European LeukemiaNet) guidelines favor collecting bone marrow in tubes with heparin anticoagulant, as EDTA is known to influence surface marker expression. They also recommend that bone marrow samples should be processed quickly (preferentially <24 h after aspiration) as an increase in side scatter on neutrophils has been observed in older samples.68
(viii) Cytogenetics: Myelodysplastic syndromes show a multitude of recurrent unbalanced and balanced chromosomal abnormalities. The most frequent are unbalanced aberrations such as monosomy 7/del(7q), del(5q), trisomy 8, and isochromosome 17q. Loss of Y chromosome, trisomy 8, and del(20q) in the absence of morphological dysplasia is not considered definitive evidence of MDS, as they also have been described in non-neoplastic conditions also.67 In patients with suspected MDS or pre-MDS, conventional karyotyping is recommended. If there is no growth or if the karyotype is incomplete (<20 metaphases) then, fluorescent in situ hybridization (FISH) with probes covering at least the common abnormalities should be performed.64, 71
(ix) Molecular: Molecular studies for the ELANE2 gene or the CSF3R gene may be considered to exclude congenital neutropenias, especially in children with persistent cytopenias. Cases of MDS may possess any number of more than 40 somatically mutated genes and next-generation sequencing (NGS) panels covering the commonly seen mutations are advised in suspected cases (Figure 2).64, 72-76
5 SUMMARY
In conclusion, following the identification of dysgranulopoiesis, correlation with complete blood count, underlying medical conditions, biochemical and other ancillary investigations is necessary to rule out non-neoplastic causes. It is necessary to exclude non-neoplastic etiologies of dysplasia before approaching a diagnosis of clonal etiologies/hematological malignancies.
CONFLICT OF INTEREST
The authors have no competing interests to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable – no new data generated.




