Effects of circadian variation, lifestyle and environment on hematological parameters: A narrative review
Abstract
The complete blood count (CBC) is the most widely prescribed laboratory test. It plays a key role in screening, diagnosing, and monitoring a variety of medical disorders. Preanalytical and analytical variables are responsible for more than 50% of laboratory errors that may lead to spurious CBC results. The effects of blood sampling, transport, storage, and analytical errors on hematological parameters have been well described. Circadian variation and changes in lifestyle and environment can also affect blood cells. It has been extensively studied in the past, but highly variable methodology and the presence of confounding factors have provided scattered and inconsistent results. We have investigated the literature to define the impact of circadian variation, modification of the sleep-wake cycle, acute and chronic exercise, eating habits, alcohol, tobacco, drugs of abuse, high-altitude, heat/cold exposure, and air pollution on CBC results. The affected cell type along with the intensity and duration of changes are detailed for each condition. We aim at providing a comprehensive overview of which situations may induce clinically significant changes and have to be taken into account by healthcare professionals before considering a hematological parameter as pathological and requesting complementary tests.
1 INTRODUCTION
The complete blood count (CBC) is the most widely prescribed laboratory test. It plays a key role in screening, diagnosing, and monitoring a variety of medical disorders. Preanalytical and analytical variables are responsible for more than 50% of laboratory errors that may lead to spurious CBC results.1 Numerous good-quality review articles are available on these topics. The “European federation of clinical chemistry and laboratory medicine (EFLM)” provides within-subject biological variation estimates of hematological parameters.2 Variation in hematological parameters linked to ethnic factors and pregnancy is well established. Circadian variation and changes in lifestyle and environment can also affect blood cells. It has been extensively studied in the past, but highly variable methodology and the presence of confounding factors have provided scattered and inconsistent results. Moreover, no up-to-date review article to the best of our knowledge has been published.
In this narrative review, we will define which factors, apart from blood sampling, transport, storage, and analytical errors, can affect hematological parameters to help healthcare professionals to interpret correctly a variation or a value outside the reference range. We will successively explore the impact on CBC results of circadian variation, exercise, diet, drug, and environment. We aim at providing a comprehensive overview of these factors, by specifying which are responsible for short- and long-term modifications and by establishing the intensity and duration of each change.
2 CIRCADIAN VARIATION
2.1 Day/night cycle
The extent of changes for most parameters appears to be smaller in recent literature in comparison with older papers, probably due to the analytical performance improvement of hematology analyzers over time.
Some red blood cell (RBC) parameters have small amplitude rhythms while other fluctuate significantly throughout the day. Red cell distribution width (RDW) and mean cell volume (MCV) are the most stable. Circadian variation of hemoglobin (HGB), hematocrit (HCT), RBC, reticulocyte, and platelet counts are posture-dependent.3 Their highest levels are measured in the morning when getting up, due to gravitational force that shifts plasma to the interstitial space, and the nadir is measured in the evening after the transition from upright position to sleep position, due to the return of plasma in blood vessels. Platelet and reticulocyte counts remain stable during the day after the subject has got up while HGB, HCT, and RBC gradually decline. In the investigation of Hilderink et al, amplitudes of variation were approximately 0.5 g/dL for HGB, 4% (absolute value) for HCT, 0.4 × 1012/L for RBC count, 8 × 109/L for reticulocyte count, and 20 × 109 /L for platelet count. Other recent studies claim smaller fluctuations for these parameters4; standardization of the patient's posture during blood collection may be an explanation.
WBCs exhibit the highest fluctuation over a 24-hour period with a gradual increase throughout the day.5 Neutrophils follow the same evolution as NK cells with a peak in the late afternoon. T cells, B cells, and monocytes hit the highest point at around midnight. The amplitude of variations is approximately 0.8 × 109/L for WBC with values ranging from less than 0.1 × 109 /L for eosinophils and monocytes to 0.5 × 109 /L for lymphocytes.4 A study by Souto Filho et al evaluated the impact of circadian variations of neutrophil count in benign constitutional neutropenia.6 Their data reveal a greater increase of the absolute neutrophil count between the morning and the afternoon when compared with controls, with an increase of 83% and 27%, respectively. This increase is all the more important as the morning neutrophil count was low, allowing 73% of patients to exceed the threshold of 1.5 × 109 /L in the afternoon. In addition to showing a greater within-day variation of the neutrophil count in benign constitutional neutropenia, this study offers an interesting approach for the complex diagnosis of this condition. Data analysis of more than 300 000 participants from the UK Biobank cohort highlights a negative correlation between neutrophil count and day length, unrelated to vitamin D levels.7 Amplitude of variation was 0.8 × 109 /L with a peak in January and a nadir in July given the location of the study.
2.2 Modification of the sleep-wake cycle
Modification of the sleep-wake cycle alters circadian rhythmicity, especially the immune system.
Loef et al conducted a study on 254 night-shift workers and 57 nonshift workers employed in hospital to determine whether disruption of the circadian rhythm can influence WBC and differential counts.8 Data show disturbance on monocytes and lymphocytes. Night-shift workers had a monocyte count 14% higher than their day counterpart, regardless of duration and frequency of night-shift work. Elevated levels of lymphocytes concern only night-shift workers who worked night shifts in the past three days. Disturbance of lymphocytes subsets affects mainly T cells, with mean number of T cells being 12% higher and mean number of CD8 T cells being 23% higher. Changes in the monocyte count seem persistent while changes in the lymphocyte count seem reversible after few days without night-shift work.8
One night of sleep deprivation is enough to induce changes on WBC count. Unlike night-shift work, it affects mainly neutrophils with a loss of rhythmicity shifting toward high values5 and significantly higher neutrophil counts at one point.9
3 PHYSICAL EXERCISE
Data on the effects of exercise on hematological parameters are inconsistent because of methodology (size and timing of the sample), exercise regimen (type, duration, and intensity), environmental condition (weather, altitude), degree of fitness, fluid intake but could also reflect individual responses to the stress of exercise. Despite these discrepancies, a trend emerges for certain settings.
3.1 Short-term effects
The intensity and length of exercise influence the duration and amplitude of the change in the affected cell types.
A study pursued in 2005 on 25 male athletes demonstrates that all blood cell counts increase after one minute of acute aerobic or anaerobic exercise with a return to baseline levels after one hour. The increase was about 5% for HGB, 8% for HCT, 15% for platelet count, 60% for WBC count, 125% for lymphocytes, 70% for monocytes, and 30% for neutrophils and eosinophils counts.10
The earliest changes can be explained by the decrease in plasma volume (hemoconcentration) and the secretion of epinephrine caused by exercise. Decrease in plasma volume is due to shift of fluid into the tissues and loss during sweating and respiration. Secretion of epinephrine results in spleen contraction and demargination of WBCs from the vascular wall into the main circulation.
In their investigation of the impact of an acute high-intensity interval training session, Wradyn et al found similar pattern for WBC with elevation still being observable up to 6 hours after training.11 Kratz et al examined the effects of marathon running on CBC results at 4h and 24h after a race. Significant differences between premarathon and postmarathon samples are observed for almost all parameters. WBC count was increased by 300% at 4 hours after the race and remains 70% higher than premarathon values after 24 hours. Values were above the reference range for all runners at the first time point and 18% for the second one. Leukocytosis is mainly due to an increase of neutrophils and monocytes, which are both increased by more than 300% at 4 hours after the race, followed by a gradual return to normal for neutrophils while monocytes remain stable after 24 hours. Conversely, lymphocytes and eosinophils are decreased within 4 hours after the race and return to baseline levels after 24 hours. HGB and platelet count have the same evolution with a mid-increase at 4 hours after the marathon followed by normalization after one day. A slight decrease of HCT is observed at both time points.12
Longer and more intense efforts are associated with changes that can last for several days. Main hypotheses explaining the persistence of leukocytosis are secretion of cortisol, which has the same effects as epinephrine, and inflammatory reaction to muscle and airway damages.12 Regarding RBC changes, initial plasma losses are followed by plasma volume expansion beginning few hours after cessation of exercise and lasting for several days. Athletes’ hyper-hydration, secretion of albumin by the liver and renal retention of water and salt may contribute to the hemodilution. Exercise-induced hemolysis has been described in long-distance runners. Mechanical damage to RBC induced by repeated foot strike seems to be the main cause.13 Oxidative stress and osmotic changes may also contribute to hemolysis during exercise given that it has been described in other sports, such as swimming and cycling.14
3.2 Long-term effects
Aerobic training is characterized by an early elevation in plasma volume followed by gradual RBC volume expansion, which is proportional to intensity and duration of training. A study15 evaluating the effect of endurance training on untrained volunteers reported a 14% increase in plasma volume and a 12% increase in RBC volume at week 8. The changes were detectable as of week 2 for the former and week 4 for the latter, resulting in a transient decrease in HCT and HGB up to 8% in the first weeks. Regular training lacks effect on platelet and WBC lineages apart from a fleeting decline linked to the initial hemodilution.
3.3 Blood doping
Use of anabolic androgenic steroids (AAS), erythropoiesis-stimulating agents (ESA), and autologous RBC transfusion by athletes should also be considered to interpret CBC changes.
Doping with AAS is quite common in recreational and professional bodybuilding but also in other sports to improve muscle mass. Elevated levels of HCT and HGB due to an increase of EPO production is one of the numerous effects of AAS abuse. These changes are visible from the fourth day after the injection and are sustained for two weeks.16 Repeated use of AAS may lead to very high values of HGB and HCT. Long-term abuse may also increase platelet and WBC count by about 35%.17 Reversibility of blood cells changes due to AAS abuse can take over a year.
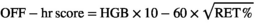
Values over 133 are considered to be evidence of doping.19 Artificial hemodilution is used by athlete to mask suspicious fluctuations on OFF-hr score related to blood doping. The use of desmopressin seems effective while hyper-hydration with water, sport drinks, or solutions containing glycerol lack effect on OFF-hr score.20 An increase in platelet count, in the magnitude of 10%-20%, has also been described after using multiple dose of EPO.21 Autologous RBC transfusion is another type of blood doping used to increase stamina. Following blood withdrawal, reticulocytes increase by more than 200% between days 7 and 14 and return to baseline values together with HGB after a month. Following blood reinfusion, HGB returns to baseline levels within two days, and reticulocytes appear 30% lower than initial values from days 7 to 21.22 OFF-hr score fails to detect autologous blood transfusion and requires using other biomarkers, such as serum EPO and soluble transferrin receptors.
4 DIET AND DRUGS
4.1 Eating habits
Traditionally, fasting is optional for CBC blood sampling. Lippi et al23 investigated the effect of a light meal on CBC in 17 healthy volunteers and found clinically significant changes up to 4h after meal. Fluctuation of WBC count during the 4-hour interval is linked to changes in neutrophils and lymphocytes. Neutrophil count increases from 1h after meal and peaks at 11% after 4 hours. Lymphocyte count decreases from 1h after meal, falls at 19% after 2 hours, and returns to baseline values after 4 hours. Local immune activation related to intestinal exposure to Ag ingested during the meal may be an explanation. Decrease of HCT and RBC count was detectable from 2h after meal and reach almost 4% after 4 hours. Hemodilution induced by food and fluid intake is likely the cause.
Vegetarian and vegan diets are becoming increasingly popular due to their alleged health benefits. But, exclusion of meat diet may also reduce intake of some essential nutrients (notably iron, vitamin B12, and zinc) and affect blood cells. Cross-sectional analyses of hematological parameters by diet group were conducted on a large cohort of 450 000 participants to define whether there is any difference between meat eaters, fish eaters, vegetarians, and vegans.24 People with low or no red meat intake had lower HGB levels; the difference of which was up to 0.5 g/dL when vegans were compared with regular meat eaters. Platelet count was lower for vegans and higher for vegetarians, varying by a maximum of 8% in comparison with meat and fish eaters. WBC count across the different diet groups appeared similar in Indians and declined along with animal-protein consumption in Caucasians. The decline was up to 10% when vegans were compared with regular meat eaters and the affected cells included neutrophils, lymphocytes, monocytes, and eosinophils. In healthy individuals, reducing red and processed meat consumption for 6 weeks seems long enough to reduce HGB levels by 5%, platelet count by 10%, and WBC count by 27%.25
Obesity affects more than two billion people worldwide. Body mass index (BMI) is the prevalent indicator for obesity (BMI≥30); waist circumference has also been correlated with obesity and metabolic syndrome. In a study carried out by Vuong et al,26 waist circumference increase was associated with concomitant increase in RBC, platelet, WBC, neutrophil, and lymphocyte count and decrease in MCV. Such modifications became clinically significant with waist circumference above 80 cm for females, 90 cm for Asian males, and 94 cm for non-Asian males. The underlying mechanism for the positive correlation between waist circumference with WBC and platelets is likely chronic inflammation. RBC increase may be due to insulin resistance, especially in metabolic syndrome. Obese and nonobese people following a caloric restriction diet for a year demonstrate weight loss with decreased WBC count concomitantly with other inflammation markers.27, 28
4.2 Alcohol
Acute alcohol exposure alters blood cells from 30 or 40 grams of pure alcohol (two or three standard drinks in most countries). The osmotic pressure changes are responsible for a slight decrease of MCV from 1 to 4 hours post ingestion.29 A retrospective study of trauma patients shows a higher admission HCT in patients with blood alcohol levels above 0.8 g/L compared with the nonintoxicated group.30 Decrease of circulating neutrophils and increase in circulating lymphocytes, both B cells and T cells, were reported in acutely intoxicated patients with blood alcohol concentration above 1 g/L.31
Habitual alcohol intake affects RBCs depending on the frequency and amount of drinking. Macrocytosis is observed in up to 89% of alcoholic with a daily alcohol consumption above 80g.32 The increase in MCV of occasional alcohol users is usually mid and nonassociated with clinically relevant macrocytosis. A study published in 2006 reported a 2 fL above the MCV upper normal limit in drinkers with a daily alcohol consumption below 40 g/day compared with abstainers.33 Ethanol-induced effects in erythropoiesis appear mainly related to acetaldehyde, which is the first metabolite of ethanol.34 A MCV above 110 fL or associated with an anemia should prompt investigation for concomitant folate deficiency, the frequency of which is higher in heavy drinkers. The MCV returns to normal levels after few months of abstinence.
A study by Wakabayashi et al carried out in 6 508 men found no link between chronic alcohol consumption and platelet count, whatever their average alcohol intake per day.35 In contrast, Cho et al36 found a significant decline in platelet count for patients with carbohydrate-deficient transferrin (CDT) level above the reference range compared with controls, with respective mean platelet count being 200 × 109/L and 258 × 109 /L. Differences in methodology might explain such discrepancies. A slight decrease in WBC count is observed for habitual alcohol drinking, which is not clinically significant given that the WBC count decrease was below 0.1 × 109 /L.37
The human alcohol metabolizing aldehyde dehydrogenase (ALDH) enzymes are involved in alcohol metabolism pathway by converting aldehydes to acetate; they include, among others, ALDH1B1 and ALDH2 as main representatives. Genetic polymorphisms of ALDH1B and ALDH2 influence the degree of changes on WBCs and RBCs of alcoholics. Variants leading to increased acetaldehyde levels, notably ALDH2*2 and ALDH1B*2 alleles, are associated with lower WBC, granulocyte and monocyte counts, lower HGB levels and higher lymphocyte count and MCV. This finding is important because 40% of East Asians have at least one ALDH2*2 allele and 90% at least one ALDH1B*2 allele.38 The ALDH2*2 genotype might increase the risk of sporadic aplastic anemia, which may explain the twofold to threefold higher incidence of this condition in East Asia.39
4.3 Tobacco
Acute smoking affects hematological parameters depending on the time of blood collection after cigarette smoking. CBC parameters are similar when comparing values before and immediately after exposure to cigarette smoke, except for neutrophils that were reported slightly elevated by some authors. When a CBC is analyzed one hour after smoking two cigarettes within 15 minutes, significant changes are observed for lymphocytes, neutrophils, and eosinophils.40, 41 Evolution of the lymphocyte count one hour after exposure remains unclear because it has been said decreased or increased depending on the study.40, 41 One to four hours postsmoking, neutrophil count significantly increases while eosinophil count slightly declines.40 Monocyte count follows the same trend as eosinophils for patient with a short smoking history (< 25 years).40 Passive cigarette smoking has similar effects on blood cells while e-cigarette smoking does not influence the CBC.41
An investigation including more than 100 000 individuals from the Copenhagen general population studying and using a Mendelian randomization approach revealed that continuous cigarette smoking has short-term effects on RBCs and long-term effects on platelets and WBCs.42 Compared with never smokers, being a current smoker was associated with up to 17% increase for WBCs, 5% increase for platelets, and approximately 2% for HGB, HCT, and MCV. Higher daily cigarettes consumption prompted higher increases of WBC count. Cholinergic receptor nicotinic α3 (CHRNA3) gene coding for nicotinic acetylcholine receptor subunits harbors a variant, rs1051730, associated with cigarette per day (CPD) and lung cancer in African Americans.43 Higher values of WBC, neutrophils, and MCV are also observed for smokers with the CHRNA3 rs1051730 genotype. Cessation of smoking enable normalization of HCT and HGB in less than one year while normalization of other parameters may take several years. This finding is in conflict with a previous study demonstrating that smoking cessation leads to recovery of WBC and neutrophil count in one year.44
The impact of smoking on WBCs may be explained by the irritating effect of cigarette smoke on the respiratory system, which induces an inflammatory response stimulating the production and release of WBC from the bone marrow, and the secretion of epinephrine and cortisol induced by nicotine, which mobilizes WBCs from the marginal pool and the spleen. Smoking habits should be considered as a potential cause for mild chronic leukocytosis and neutrophilia given that approximately 20% of smokers have a WBC count above 11 × 109 /L and 10% have a neutrophil count above 7.7 × 109 /L.45
Cigarette smoke mainly affects RBCs in two ways. High levels of HGB and HCT may be linked with the chronic exposure to carbon monoxide that increases the permeability of the capillaries, leading to plasma volume reduction, and that produces a chronic hypoxemia, stimulating secretion of erythropoietin (EPO). The fact that RBC count remains stable may be explained by hemolysis resulting from oxidative damage induced by cigarette smoking.46 Tobacco smoke contains acetaldehyde that may impair erythropoiesis and explain the slight elevation of MCV.47 HGB threshold to define anemia and polycythemia should be adjusted depending on the daily cigarette consumption. Sharma et al suggest adjusting HGB threshold by adding 0.2 g/dL for light smokers (1 - 5 CPD), 0.4 to 0.6 g/dL for moderate smokers (6 - 20 CPD), and 0.7 g/dL for heavy smokers (> 20 CPD).48 This adjustment is additive with the effects of altitude.
Cigarette smoking is known to promote certain cancers. Chronic smokers have a higher percentage of B cells than nonsmokers. This increase may rarely evolve toward a persistent polyclonal B-cell lymphocytosis (PPBL). Results of a long-term follow-up of 111 patients with PPBL give a global view of this condition. Patients are mostly middle-aged women, with no symptoms and a history of tobacco consumption in 98% of cases. At the time of diagnosis, 83 patients had a lymphocyte count above 4 × 109 /L with a median percentage of binucleated lymphocytes at 4%. Updated results on 150 patients published few years later demonstrated the strong association between PPBL and the presence of supernumerary isochromosome +I(3)(q10) and/or chromosomal instability. The occurrence of subsequent malignancies was observed in 12% of patients, with an equal distribution of solid tumors, NHL, and monoclonal gammopathy of undetermined significance (MGUS).49
4.4 Drugs of abuse
Psychostimulant such as cocaine, amphetamine, and methamphetamine are widely used recreational drugs. Acute cocaine exposure increases HCT, HGB, and RBC count. Hematological changes temporally correlate with transient splenic contraction induced by cocaine. The increase in HCT and HGB peaks at approximately 5% over baseline within 10 minutes of the IV administration and 30 minutes of the intranasal administration. 60 minutes after the administration, the two parameters return to baseline for IV administration and remain high for intranasal administration. There are no changes in platelets and WBCs.50
Agranulocytosis is a rare but well-known adverse event linked to chronic use of cocaine. It is due to levamisole, an additive found in 70% of cocaine seized in the United States in 2009, used to increase the bulk and potentiate the stimulatory and dependence effects of the drug. Genetic predisposition is likely to play a role in the onset of this complication, notably HLA-B27 and variants in the ATP-binding cassette subfamily member 12 (ARBCC12) and cytochrome P450 family 11 subfamily A member 1 (CYP11A1) genes. Conversely, variants in the adrenoceptor beta 1 (ARDB1) gene have been recently described as a protective variant.51
Richards et al reported results of a retrospective review of all psychiatric patients presenting for medical clearance of the emergency department of their institution from January 2009 to December 2011. During this period, 1 206 patients were identified, 72.6% of whom were negative for amphetamine and cocaine, 19.9% were positive for amphetamine alone and 6% were positive for cocaine alone. Mean WBC count was decreased by 15% for cocaine users and increased by 12% for amphetamine users in comparison with controls. Moreover, 23.8% of amphetamine-positive patients had a WBC count above 11 × 109 /L.52 The opposite effects of cocaine and amphetamine on WBCs may be explained by two main factors. First, previous studies have proved that amphetamines stimulate pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, whereas cocaine inhibits them and increases levels of anti-inflammatory cytokines, such as IL-10. Second, the short half-life of cocaine may not be sufficient to induce glucocorticoid-induced leukocytosis described with amphetamines. Some studies have also reported an increase in platelet count for methamphetamine addicts, which can be related to the pro-inflammatory effect of amphetamines.
Opioids are highly addictive drugs that often lead to chronic abuse. A cross-sectional study conducted in 180 patients with such heroin or opium found that chronic use of opioids could affect blood cells and that some of these changes can be normalized after a one-month withdrawal period. The main difference in WBC lineage between opioid dependents and controls was an increase in absolute neutrophil count, peaking at 44% increase for heroin dependents, which return to baseline values after the withdrawal period. The RBC count remained unchanged but MCV and HCT were significantly higher in opioid dependents in comparison with controls, peaking at 13% increase for both parameters in heroin dependents, which begin to normalize after one-month withdrawal period in heroin dependents but not in opium dependents.53 In his analysis, Mei-Hing Ng shows that macrocytosis may concern 20% of patients and is due to concurrent use of alcohol in most cases.54 Platelet count slightly increases in both cases.
The effects of marijuana smoking on hematological parameters are difficult to highlight due to the concomitant use of tobacco in most studies. Review of hematological studies taking into account this confounding factor shows no change in HGB, HCT, and platelet count and possibly a slight increase in WBC and neutrophil count for heavy cannabis users.55 Such impact on WBC is confirmed in a publication focusing on synthetic cannabinoid users showing a 15% increase in WBC and 22% increase in neutrophils when compared with healthy individuals.56
5 ENVIRONMENT
5.1 High-altitude
Approximately 140 million people worldwide live at high-altitude above 2 500m, the largest population being in the Andes, Tibet, and Ethiopia. Exposure to high-altitude stimulates erythropoiesis and increases HCT and HGB levels. Such elevation is due to early plasma volume reduction, related to fluid shift from intravascular to interstitial spaces, followed few days after by new RBC production, related to hypoxia-induced erythropoiesis. Several authors have coined that a variable degree of HGB is required to fulfill physiological requirements in the different regions of the world.57, 58 We distinguish three patterns of adaptation to high-altitude hypoxia.
In their meta-analysis, Gassman et al57 found that the highest increase in HGB with altitude affected residents of the Andes (1 g/dL/1 000m) compared to other regions of the world (0.6 g/dL/1 000m). Andean high-altitude natives follow the classic pattern characterized by a HGB increase correlated with the degree of arterial hypoxemia, and so to the altitude level. Several formulas for HGB adjustment have been proposed to define anemia and polycythemia in high-altitude populations.48, 57 Among those, the World Health Organization (WHO) recommends correcting the cutoff point of HGB to define anemia in high-altitude populations, by adding up 0.2 g/dL to the cutoff for people living at 1000 m to 4.5 g/dL to 3.5 g/dL for those living at >4500m.48 The classic pattern is suitable for the greatest number, except the Tibetan and Ethiopian populations that are associated with the two other patterns of adaptation to high-altitude hypoxia.
Tibetans are adapted to have normal HGB concentrations, despite arterial hypoxemia, up to 3 999m.57 In addition, the average HGB concentration slightly increases and remains constant at approximately 16 g/dL (males) and 15 g/dL (females) between 4000 and 5000 m. They are genetically protected from hypoxia-induced polycythemia given that 70% of them carry a Tibetan-specific mutation on the Egl-9 Family Hypoxia Inducible Factor 1 gene (EGLN1) favoring low HGB concentration at high-altitude.59
The natives of the Semien mountains, part of the Ethiopian Highlands, have another pattern of adaptation to high-altitude that enable them to have HGB concentrations and arterial oxygen saturation within the ranges of the ones of sea-level populations. A field study by Beall et al58 examined 236 Ethiopian natives at 3530 m (14 - 86 years of age) and found an average HGB concentration of 15.9 g/dL in males and 15.0 g/dL in females. Four candidate genes, including calcium-binding atopy-related autoantigen 1 (CBARA1), Vav Guanine Nucleotide Exchange Factor 3 (VAV3), thyroid hormone receptor beta (THRB), and aryl hydrocarbon receptor nuclear translocator 2 (ARNT2), were highlighted recently for involvement in high-altitude adaptation in Ethiopia.60
Extreme erythrocytosis may be seen in people with chronic mountain sickness, a medical condition affecting 5 to 30% of people who resides permanently at high-altitude (> 2 500m) and that can be associated with HGB levels 25% higher than healthy highlanders.61
There are conflicting data on the impact of high-altitude on platelet count. A detailed analysis of study methodology revealed great variability in speed of ascent, maximum altitude, length of stay, health status, and ethnicity of participants. Native residents and people on prolonged stay at high-altitude exhibit a platelet count ranging from 30% lower to 75% higher in comparison with sea-level residents.62, 63
Acute altitude exposure is known to increase WBC count, which can attain 20% after 30h exposure to 4 300m altitude.64 This elevation is mainly related to lymphocyte and neutrophil changes and return to baseline values following few weeks of altitude acclimatization.
5.2 Heat and cold exposure
Modification of outside temperature affects body temperature. It is associated with definite effects on erythrocytes while a trend is difficult to establish for WBCs, as data are inconsistent.
Acute heat exposure can cause dehydration if not compensated by sufficient fluid intake; this is highlighted by increased HCT and HGB. After few days of heat exposure, a natural heat acclimation that translates into plasma volume expansion induces a progressive decrease in HCT and HGB. The relative change can be up to 15% in normal healthy adults.65 Cold exposure is associated with hemoconcentration resulting in increased HGB and HCT levels. It appears that two hours at 6°C is enough to induce an increase in RBC count by 7%.66
5.3 Air pollution
Air pollution exposure is known to harm human health and is generally expressed as exposure to particles smaller than 10 µm (PM10) or 2.5 µm (PM2.5). Daily and annual targets given by WHO air quality guidelines are, respectively, 50 and 20 µg/m3 for PM10 and 25 and 10 µg/m3 for PM2.5.
Saton67 suggested that a 100 µg/m3 increase in mean daily PM10 over three days may decrease HGB levels by 0.37 g/dL. The fall in RBC count caused by the sequestration of red cells in capillaries may be an explanation.
More recently, data of a German cohort of 4 814 participants were used to determine whether long-term exposure to PM2.5, so-called fine particulate matter, had an impact on inflammation markers.68 An increase in the platelet count, 2.3% for every 2.4 µg/m3 increase in long-term exposure to PM2.5, was reported while WBC count remained unchanged.
6 CONCLUSION
It can be challenging for healthcare professionals to interpret correctly a variation or a value outside the reference range. In addition to rule out preanalytical and analytical errors, modifications of lifestyle and environment have to be taken into account before considering a hematological parameter as pathological and requesting complementary tests. Table 1 summarizes long-term effects of lifestyle and environment on hematologic parameters described in this publication. Available data on circadian variation, lifestyle, and environment factors that may induce clinically significant changes in hematological parameters are compiled in Table 2.
| Parameter | CVi (%) | Diurnal variation (#) | Elevation | Diminution |
|---|---|---|---|---|
|
HCT or HGB |
2.8 2.7 |
4% 0.5 g/dL |
|
|
| MCV | 0.8 | < 1 fL |
|
|
|
Platelet count |
5.6 | 20 × 109 /L |
|
|
|
WBC count |
10.8 | 0.8 × 109 /L |
|
|
| Neutrophil count | 14.0 | 0.5 × 109 /L |
|
|
| Lymphocyte count | 10.8 | 0.3 × 109 /L |
|
|
| Monocyte count | 13.3 | <0.1 × 109 /L |
|
|
| Eosinophil count | 15.0 | < 0.1 × 109 /L |
|
|
- Abbreviations: AAS, anabolic androgenic steroids; BAC, blood alcohol concentration.; CVi, intra-individual biological variation expressed as coefficient of variation (%); ESA, erythropoiesis-stimulating agents; HCT, hematocrit; HGB, hemoglobin; MCV, mean cell volume; PM, Particulate Matter.
| Factor | WBCs | RBCs | Platelets |
|---|---|---|---|
| Night-shift work | ↑ (WBC, NE ±LY) | Unclear | Unclear |
| Physical exercise | |||
| Endurance training | = | = or ↓ (HCT) | = |
| Use of AAS | ↑ | ↑ (HCT, HGB) | = or ↑ |
| Use of ESA | = | ↑ (HCT, HGB) | ↑ |
| Eating habits | |||
| Vegans | = or ↓ | ↓ (HCT, HGB) | ↓ |
| Obesity | ↑ (WBC, NE, LY) |
↑ (HCT, HGB) ↓ (MCV) |
↑ |
| Use of drugs | |||
| Alcohol | ↓ | ↑ (MCV) | = or ↓ |
| Tobacco | ↑ (WBC, NE ±LY) | ↑ (HCT, HGB ±RBC, MCV) | ↑ |
| Cocaine | ↓ (WBC, NE) | Unclear | Unclear |
| Amphetamines | ↑ (WBC, NE) | Unclear | = or ↑ |
| Opium | ↑ (WBC, NE) | ↑ (HCT ±MCV) | ↑ |
| Heroine | ↑ (WBC, NE) | ↑ (HCT ±MCV) | = or ↑ |
| Cannabis | ↑ (WBC, NE) | = | = |
| Environment | |||
| High-altitude | = | ↑ (HCT, HGB) | Unclear |
| Heat exposure | Unclear | ↓ (HCT, HGB) | Unclear |
| Cold exposure | Unclear | ↑ (HCT, HGB) | Unclear |
| Air pollution | = | ↑ (HCT, HGB) | ↑ |
- Abbreviations: AAS, anabolic androgenic steroids; ESA, erythropoiesis-stimulating agents; HCT, hematocrit; HGB, hemoglobin; LY, lymphocytes; MCV, mean cell volume; NE, neutrophils; RBC, red blood cell; WBC, white blood cell.
CONFLICT OF INTEREST
Joffrey Feriel, Draga Tchipeva and François Depasse are Diagnostica Stago employees.
AUTHOR CONTRIBUTIONS
All authors contributed to the design of the review article and the interpretation of the relevant literature. JF and DT wrote the first draft, and FD revised it critically. All authors approved the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




